Research Progress on the Application of Graphene Quantum Dots
Abstract
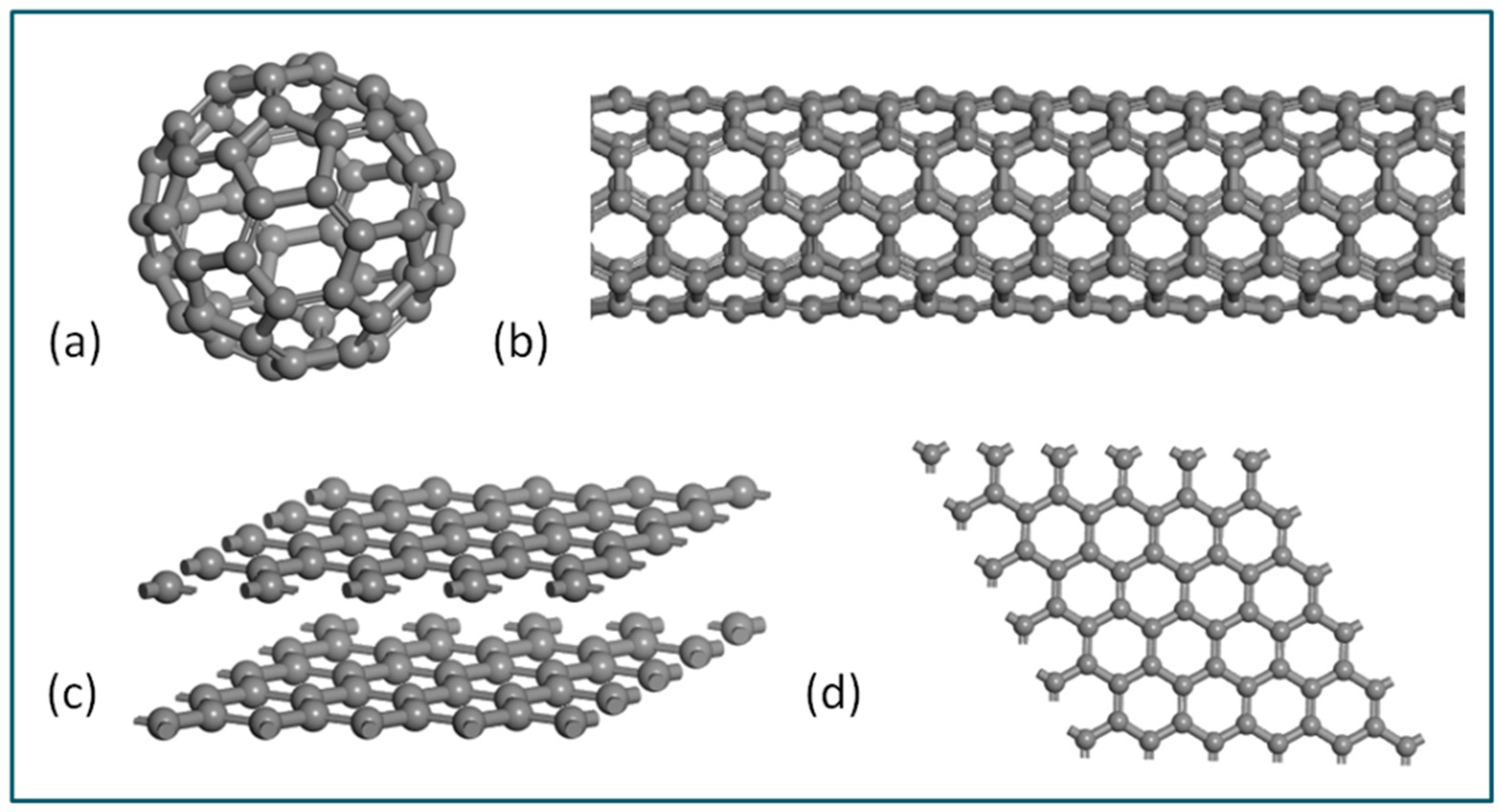
:1. Introduction
2. Synthesis Strategy
2.1. Top-Down Process
2.1.1. Chemical Stripping Method
2.1.2. Electrochemical Exfoliation Process
3. Physical and Chemical Properties
3.1. Size, Chemical Composition and Crystal Structure
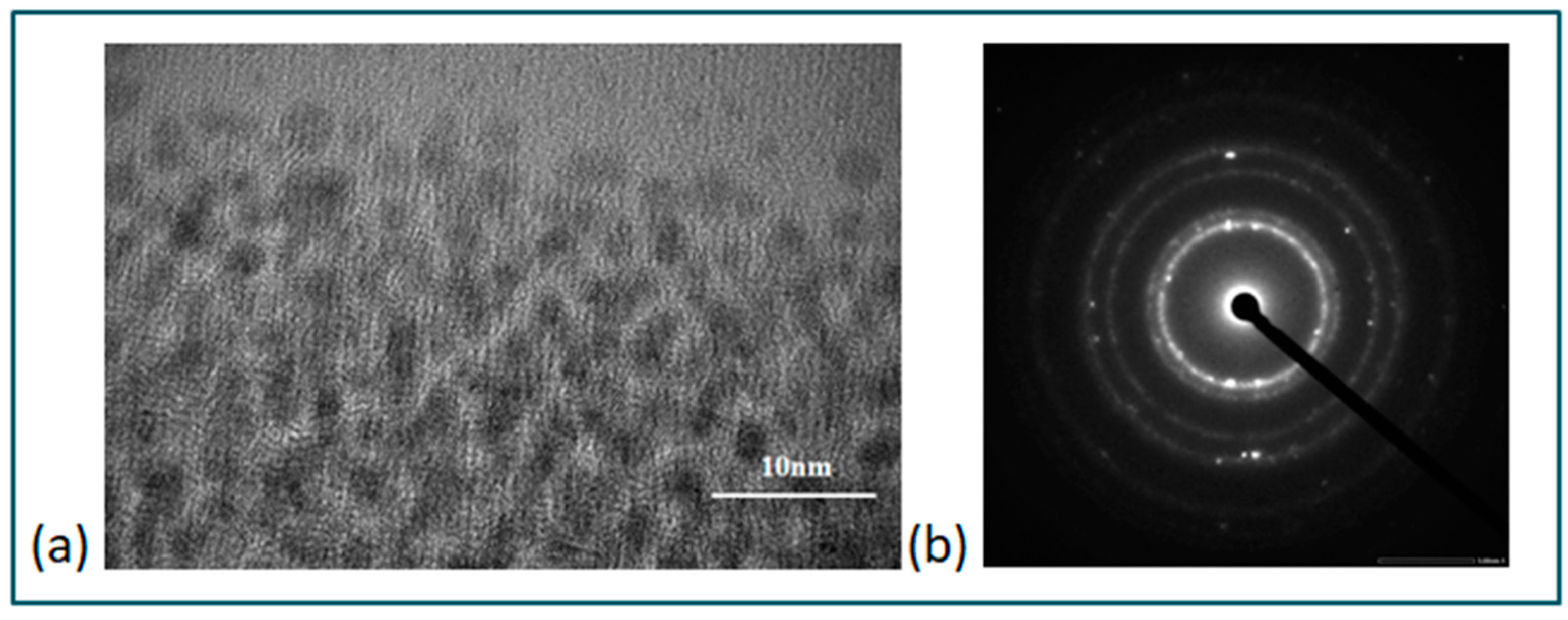
3.1.1. Size
3.1.2. Chemical Composition
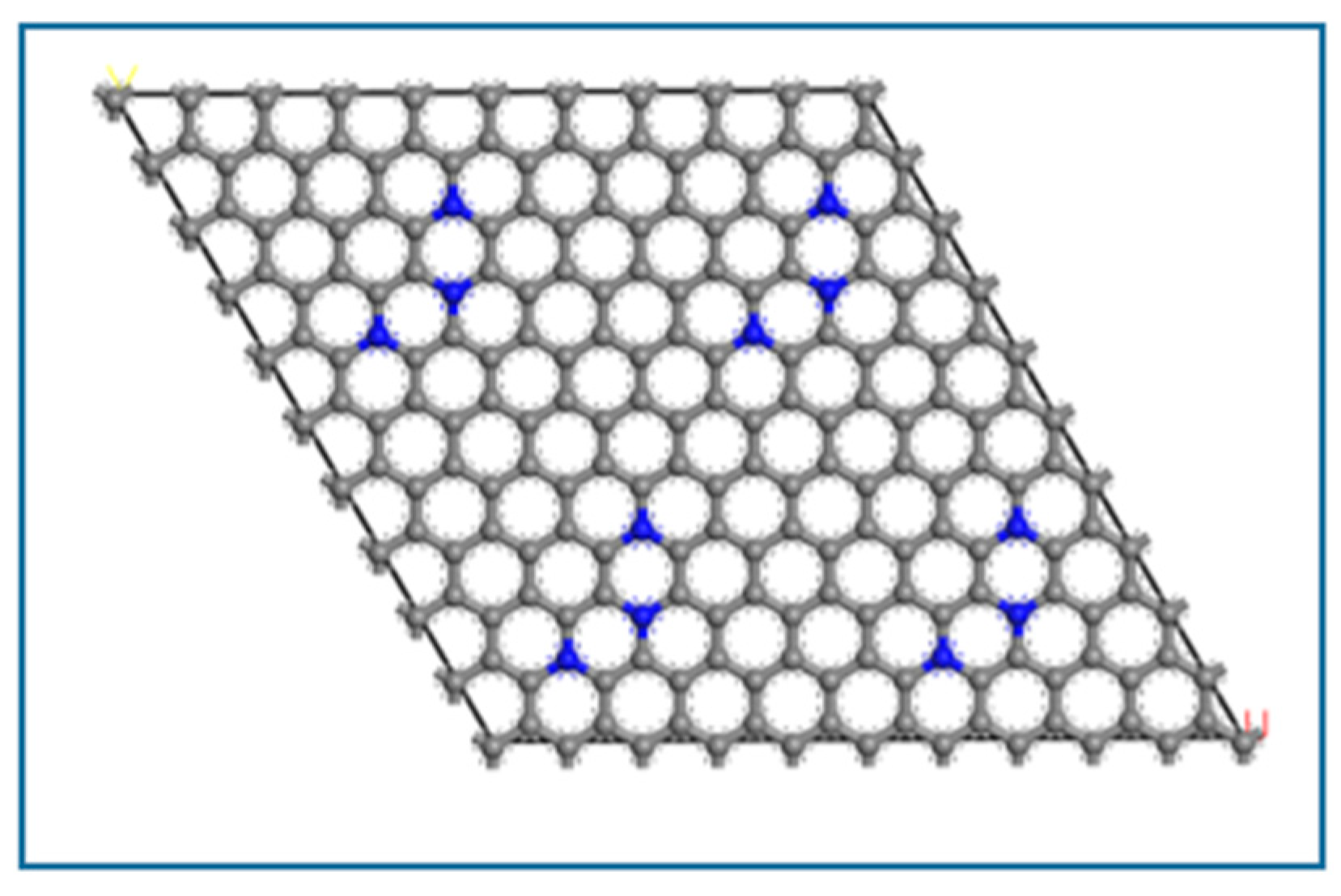
3.1.3. Crystal Structure
3.2. Optical Properties
3.2.1. Photoluminescence Properties
3.2.2. Electrochemical Luminescence Performance (ECL)
4. Applications in the Field of Sensing
4.1. Ion Detection Sensor
4.2. Biosensor
4.2.1. Fluorescent Biosensor
4.2.2. Electrochemical Biosensor
4.2.3. Electronic Sensor
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Wu, G.; Yang, G.; Peng, J.; Zhu, J.J. Focusing on luminescent graphene quantum dots: Current status and future perspectives. Nanoscale 2013, 5, 4015–4039. [Google Scholar] [CrossRef] [PubMed]
- Lijima, S. Helical microtubules of graphic carbon. Nature 1991, 354, 56–58. [Google Scholar]
- Kroto, H.W.; Heath, J.R.; O’brien, S.C.; Curl, R.F.; Smalley, R.E. Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Zhang, W.B.; Li, L.; Li, S.C.; Ma, J.Z.; Liu, C.; Bao, Y. Modification and application of graphene quantum dots. Acta Mater. Compos. Sin. 2022, 39, 3104–3120. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Li, L.S.; Yan, X. Colloidal Graphene Quantum Dots. J. Phys. Chem. Lett. 2010, 1, 2572–2576. [Google Scholar] [CrossRef]
- Li, L.L.; Jing, J.; Fei, R.; Wang, C.Z.; Qian, L.; Zhang, J.R.; Zhu, J.J. A Facile Microwave Avenue to Electrochemiluminescent Two-Color Graphene Quantum Dots. Adv. Funct. Mater. 2012, 22, 2971–2979. [Google Scholar] [CrossRef]
- Yan, X.; Cui, X.; Li, L.S. Synthesis of Large, Stable Colloidal Graphene Quantum Dots with Tunable Size. J. Am. Chem. Soc. 2010, 132, 5944–5945. [Google Scholar] [CrossRef]
- Yan, X.; Cui, X.; Li, B.; Li, L.S. Large, solution-processable graphene quantum dots as light absorbers for photovoltaics. Nano Lett. 2010, 10, 1869–1873. [Google Scholar] [CrossRef]
- Ding, Y.; Diao, Q.; Liu, D. Synthesis of graphene quantum dots and its application in gas sensing. Chin. J. Anal. Chem. 2022, 50, 495–505. [Google Scholar]
- Li, Y.Q.; Xiao, Y.; Tao, Q.; Yu, M.M.; Zheng, L.; Yang, S.W.; Ding, G.Q.; Dong, H.; Xie, X.M. Selective coordination and localized polarization in graphene quantum dots: Detection of fluoride anions using ultra-low-field NMR relaxometry. Chin. Chem. Lett. 2021, 32, 3921–3926. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M.H. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.R.; Zhu, W.; Zhao, M.H.; Wang, G.; Yang, S.W.; Liu, Z.D.; Zheng, L.; Guo, Q.J. Graphene quantum dots promoted the synthesis of heavily n-type graphene for near-infrared photodetectors. J. Phys. Chem. C 2020, 124, 1674–1680. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, Y.Y.; Jiang, H.X.; Li, X.M.; Teng, K.S.; Luk, C.K.; Zeng, S.J.; Hao, J.H.; et al. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef]
- Lu, F.; Yang, S.W.; Song, Y.X.; Zhai, C.; Wang, Q.; Ding, G.; Kang, Z. Hydroxyl functionalized carbon dots with strong radical scavenging ability promote cell proliferation. Mater. Res. Express 2019, 6, 065030. [Google Scholar] [CrossRef]
- Li, X.M.; Rui, M.C.; Song, J.Z.; Shen, Z.H.; Zeng, H.B. Carbon and graphene quantumdots for optoelectronic and energy devices: A review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
- Huang, H.; Yang, S.; Liu, Y.; Yang, Y.C.; Kang, Z.H. Photocatalytic polymerization from amino acid to protein by carbon dots at room temperature. ACS Appl. Bio Mater. 2019, 2, 5144–5153. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, C.; Zheng, X.; Gao, L.L.; Cui, Z.; Yang, H.B.; Guo, C.X.; Chi, Y.W.; Chang, M. One-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black. J. Mater. Chem. 2012, 22, 8764–8766. [Google Scholar] [CrossRef]
- Gupta, V.; Chaudhary, N.; Srivastava, R.; Sharma, G.D.; Bhardwaj, R.; Chand, S. Luminscent graphene quantum dots for organic photovoltaic devices. J. Am. Chem. Soc. 2011, 133, 9960–9963. [Google Scholar] [CrossRef]
- He, J.L.; He, Y.L.; Chen, Y.H.; Lei, B.F.; Zhuang, J.L.; Xiao, Y.; Liang, Y.; Zheng, M.T.; Zhang, H.R.; Liu, Y.L. Solid-state carbon dots with red fluorescence and efficient construction of dual-fluorescence morphologies. Small 2017, 13, 1700075. [Google Scholar] [CrossRef]
- Li, M.X.; Chen, T.; Gooding, J.J.; Liu, J.Q. A review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 10226–10333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.L.; Li, J.H.; Lu, D.S.; Zhang, K.X.; Wang, X.J.; Ma, L. Graphene quantum dots fluorescence enhancement and pH response characteristics. Chin. Opt. 2023, 16, 523–534. [Google Scholar]
- Wang, B.; Lu, S. The light of carbon dots: From mechanism to applications. Matter 2022, 5, 110–149. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Chen, N.; Qu, L. Graphene quantum dots: An emerging material for energy-related applications and beyond. Energy Environ. Sci. 2012, 5, 8869–8890. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X. One-pot hydrothermal synthesis of graphene quantum dots surface-passivated by polyethylene glycol and their photoelectric conversion under near-infrared light. R. Soc. Chem. 2012, 36, 97–101. [Google Scholar]
- Xu, A.L.; Wang, G.; Li, Y.Q.; Dong, H.; Yang, S.W.; He, P.; Ding, G.Q. Carbon-based quantum dots with solid-state photoluminescent: Mechanism, implementation, and application. Small 2020, 16, 2004621. [Google Scholar] [CrossRef]
- Ozhukil Valappil, M.K.; Pillai, V.; Alwarappan, S. Spotlighting graphene quantum dots and beyond: Synthesis, properties and sensing applications. Appl. Mater. Today 2017, 9, 350–371. [Google Scholar] [CrossRef]
- Li, K.; Zhang, G.J. Research progress of carbon quantum dots and graphene quantum dots in sensing field. Lab. Med. 2022, 37, 787–792. [Google Scholar]
- Wang, S.J.; Lenzini, F.; Chen, D.; Tanner, P.; Han, J.S.; Thiel, D.; Lobino, M.; Li, Q. Chemically derived graphene quantum dots for high-strain sensing. J. Mater. Technol. 2023, 141, 110–115. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.H.; Song, L.; Alemany, L.B.; Zhan, X.B.; Gao, G.H. Graphene quantum dots derived from carbon filers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Adsetts, J.R.; He, S.J.; Zhan, Z.Y.; Yang, L.; Wong, J.M.; Love, D.A.; Ding, Z. Electrogenerated chemiluminescence and electroluminescence of N-doped graphene quantum dots fabricated from an electrochemical exfoliation process in nitrogen-containing electrolytes. Chem. A Eur. J. 2020, 26, 15892–15900. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Feng, X.; Mueelen, K. Bottom-Up Fabrication of Photoluminescent Graphene Quantum Dots with Uniform Morphology. J. Am. Chem. Soc. 2011, 133, 15221–15223. [Google Scholar] [CrossRef] [PubMed]
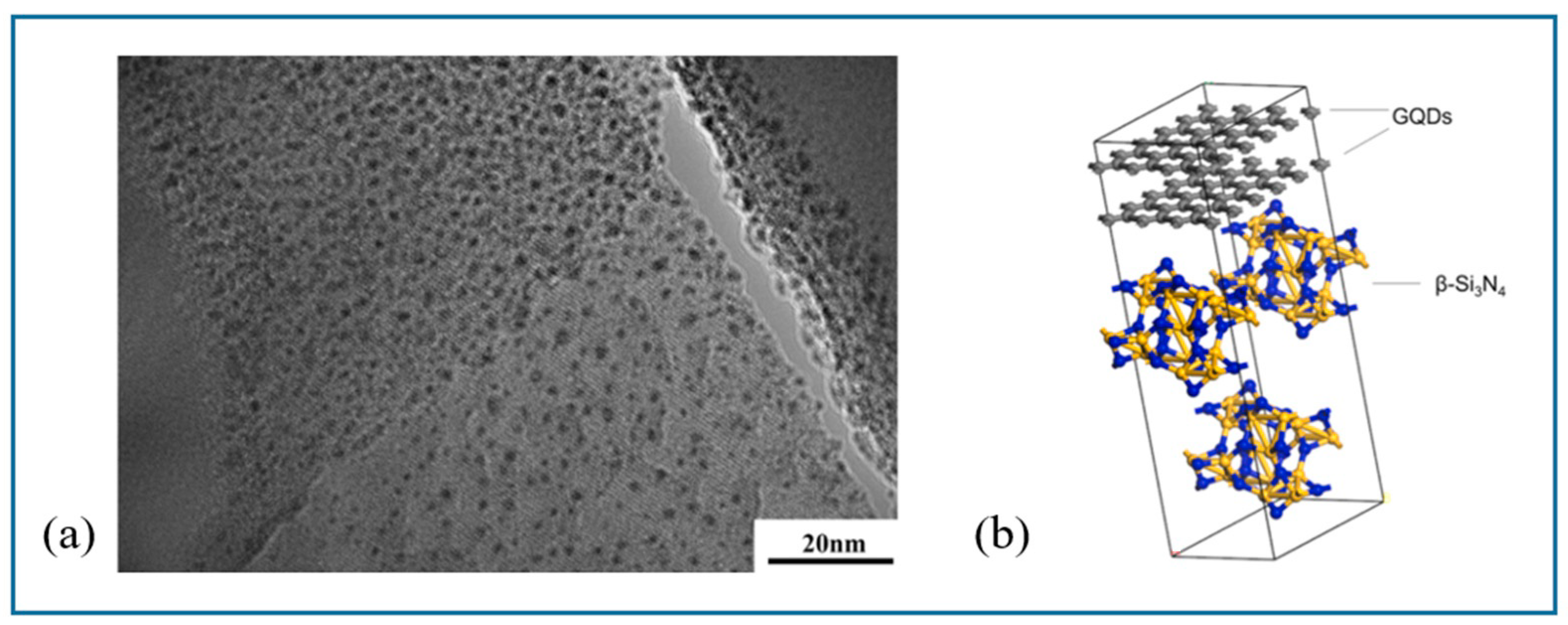
- Chen, W.; Zhao, Z.Q.; Lou, R. Study on self-derived products of nanometer lignin in silicon nitride ceramics during sintering process. Results Mater. 2021, 12, 100228. [Google Scholar] [CrossRef]
- Lin, L.X.; Zhang, S.W. Creating high yield water soluble luminescent graphene quantum dots via exfoliating and disintegrating carbon nanotubes and graphite flakes. Chem. Commun. 2012, 48, 10177–10179. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-Doped Graphene Quantum Dots with Oxygen-Rich Functional Groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef]
- Teymourinia, H.; Salavati-Niasari, M.; Amiri, O.; Safardoust-Hojaghan, H. Synthesis of graphene quantum dots from corn powder and their application in reduce charge recombination and increase free charge carriers. J. Mol. Liq. 2017, 242, 447–455. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, D.; Han, S.; Hu, P.F.; Liu, R.L. Bottom-up fabrication of photoluminescent carbon dots with uniform morphology via a soft–hard template approach. Chem. Commun. 2013, 49, 4920–4922. [Google Scholar] [CrossRef]
- Minati, L.; Del Piano, A. Facile Synthesis of Water-Soluble, Highly-Fluorescent Graphene Quantum Dots from Graphene Oxide Reduction for Efficient Cell Labelling. C J. Carbon Res. 2019, 5, 77. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.; Yang, Y.; Cui, J.H.; Rong, J.H. One-step preparation of nitrogen-doped graphene quantum dots from oxidized debris of graphene oxide. J. Mater. Chem. B 2012, 1, 39–42. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, L.; Shang, W.; Xie, W.J.; Ma, H.; Fu, Y.Y.; Fang, D.C.; Sun, H.; Fan, L.Z.; Han, M.; et al. Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells. J. Mater. Chem. 2012, 22, 7461–7467. [Google Scholar] [CrossRef]
- Tetsuka, H.; Asahi, R.; Nagoya, A.; Okamoto, K.; Tajima, I. Optically tunable amino-functionalized graphene quantum dots. Adv. Mater. 2012, 24, 5333–5338. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.B.; Ji, R.B.; Li, X.M.; Bai, G.X.; Liu, C.P.; Hao, J.H.; Lin, J.Y.; Jiang, H.X. Deep ultraviolet to near-infrared emission and photoresponse in layered N-doped graphene quantum dots. ACS Nano 2014, 8, 6312–6320. [Google Scholar] [CrossRef]
- Yang, S.W.; Zhu, C.; Sun, J.; Peng, H.; Yuan, N.Y.; Ding, J.N.; Ding, G.Q.; Xie, X.M. Triphenylphosphine modified graphene quantum dots: Spectral modulation for full spectrum of visible light with high quantum yield. RSC Adv. 2015, 5, 33347–33350. [Google Scholar] [CrossRef]
- Ge, J.C.; Lan, M.H.; Zhou, B.J.; Liu, M.M.; Guo, L.; Wang, H.; Jia, Q.Y.; Niu, G.; Huang, X.; Zhou, H.Y.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, Z.L.; Tian, Z.Q.; Zhang, L.; Liu, C.; Lin, Y.; Qi, B.; Pang, D.W. Electrochemical Tuning of Luminescent Carbon Nanodots: From Preparation to Luminescence Mechanism. Adv. Mater. 2011, 23, 5801–5806. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Chen, C.; Yang, X.L.; Li, C.Z. Facile Preparation and Upconversion Luminescence of Graphene Quantum Dots. Chem. Commun. 2011, 47, 2580–2582. [Google Scholar] [CrossRef]
- Mandal, D.; Khatun, S.; Gupta, A.N.; Chandra, A. DNA supported graphene quantum dots for Ag ion sensing. Nanotechnology 2019, 30, 255501. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Biswal, M.; Mhamane, D.; Gokhale, R.; Ogale, S. Large scale synthesis of graphene quantum dots (GQDs) from waste biomass and their use as an efficient and selective photoluminescence on-off-on probe for Ag(+) ions. Nanoscale 2014, 6, 11664–11670. [Google Scholar] [CrossRef]
- Sharma, E.; Vashisht, D.; Vashisht, A.; Vats, V.; Singh, K. Facile synthesis of sulfur and nitrogen codoped graphene quantum dots for optical sensing of Hg and Ag ions. Chem. Phys. Lett. 2019, 730, 436–444. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, L.; Lan, C.; Zhao, J.J.; Su, Y.B.; Zhao, S.L. One-pot green synthesis of oxygen-rich nitrogen-doped graphene quantum dots and their potential application in pH-sensitive photoluminescence and detection of mercury(II) ions. Talanta 2015, 142, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Wu, B.; Li, Z.; Wang, S.; Liu, Y.; Pan, D.; Wu, M. Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing. Chem. Eng. J. 2016, 300, 75–82. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Q.; Chen, D.; Liu, Z.D.; Zheng, X.H. Facile and Highly Effective Synthesis of Controllable Lattice Sulfur-Doped Graphene Quantum Dots via Hydrothermal Treatment of Durian. ACS Appl. Mater. Interfaces 2018, 10, 5750–5759. [Google Scholar] [CrossRef]
- Qian, Z.S.; Shan, X.Y.; Chai, L.J.; Chen, J.R.; Feng, H. A fluorescent nanosensor based on graphene quantum dots-aptamer probe and graphene oxide platform for detection of lead (II) ion. Biosens. Bioelectron. 2015, 68, 225–231. [Google Scholar] [CrossRef]
- Yu, J.; Yao, Z.H.; He, K.Y.; Xing, B.C.; Wang, Q.; Cheng, K.J.; Wang, L.; Xu, X.H. Nanomaterials-based optical biosensor for detection of mycotoxins in traditional Chinese medicine. Chin. J. Anal. Chem. 2023, 51, 472–483. [Google Scholar]
- Lu, J.; Yan, M.; Ge, L.; Wang, S.W.; Yan, J.X.; Yu, J.H. Electrochemiluminescence of blue-luminescent graphene quantum dots and its application in ultrasensitive aptasensor for adenosine triphosphate detection. Biosens. Bioelectron. 2013, 47, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.P.; Qiu, W.B.; Zhao, H.F.; Xiang, C.Y.; Qiu, J.D. Electrochemiluminescence resonance energy transfer between graphene quantum dots and graphene oxide for sensitive protein kinase activity and inhibitor sensing. Anal. Chim. Acta 2016, 904, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.F.; Qiu, H.Z.; Lin, X.H.; Dong, L.; Liu, A.L. A unique turn-off fluorescent strategy for sensing dopamine based on formed polydopamine (pDA) using graphene quantum dots (GQDs) as fluorescent probe. Sens. Actuators B Chem. 2015, 221, 7–14. [Google Scholar]
- Lei, X.Y.; Zuo, G.F.; Wang, P.; Li, Z.F. Research progress and analytical application of electrochemical biosensor based on N-GQDs. J. Funct. Mater. Devices 2020, 26, 184–190. [Google Scholar]
- Kalkal, A.; Pradhan, R.; Kadian, S.; Manik, G.; Packirisamy, G. Biofunctionalized graphene quantum dots based fluorescent biosensor towards efficient detection of small cell lung cancer. ACS Appl. Bio Mater. 2020, 3, 4922–4932. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Liu, Y.; Cui, J.J.; Liu, H.; Wang, P.; Li, Y.Y.; Chen, L.; Zhao, Z.D.; Dong, Y.H. A novel label-free electrochemical immunosensor based on functionalized nitrogen-doped graphene quantum dots for carcinoembryonic antigen detection. Biosens. Bioelectron. 2017, 90, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Chen, W. In Situ Growth of Surfactant-Free Gold Nanoparticles on Nitrogen-Doped Graphene Quantum Dots for Electrochemical Detection of Hydrogen Peroxide in Biological Environments. Anal. Chem. 2014, 87, 1903. [Google Scholar] [CrossRef] [PubMed]
- Muthurasu, A.; Ganesh, V. Horseradish Peroxidase Enzyme Immobilized Graphene Quantum Dots as Electrochemical Biosensors. Appl. Biochem. Biotechnol. 2014, 174, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y. Analysis of Electronic Sensor in Daily Life. Mod. Ind. Econ. Inf. 2022, 12, 220–221. (In Chinese) [Google Scholar]
- Sreeprasad, T.S.; Rodriguez, A.A.; Colston, J.; Graham, A.; Shishkin, E.; Pallem, V.; Berry, V. Electron-Tunneling Modulation in Percolating Network of Graphene Quantum Dots: Fabrication, Phenomenological Understanding, and Humidity/Pressure Sensing Applications. Nano Lett. 2013, 13, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Dai, J.; Zhang, F.; Yang, M. Advanced Fiber-Optic Relative Humidity Sensor Based on Graphene Quantum Dots Doped Polyimide Coating. IEEE Photonics Technol. Lett. 2022, 34, 725–728. [Google Scholar] [CrossRef]
- Wang, L.J.; Cao, G.; Tu, T.; Li, H.; Zhou, C.; Hao, X.J.; Su, Z.; Guo, G.C.; Jiang, H.W.; Guo, G.P. A graphene quantum dot with a single electron transistor as an integrated charge sensor. Am. Inst. Phys. 2010, 97, 183–214. [Google Scholar] [CrossRef]
| Scholars | GQDs | Types of Metal Ions |
|---|---|---|
| Mandal [48] | DNA GQDs | Ag+ |
| Suryawanshi [49] | Am-GQDs | Ag+ |
| Sharma [50] | S, N-GQDs | Ag+ and Hg2+ |
| Shi [51] | N-OGQD | Hg2+ |
| Wang [52] | PEI-GQDs | Fe 3+ and Cu 2+ ions |
| Wang [53] | modified GQDs with sulfhydryl group | Hg+, Zn2+, Ag+ and Cd+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Zhou, F.; Li, W.; Ao, G.; Xu, X.; Yang, L. Research Progress on the Application of Graphene Quantum Dots. Coatings 2023, 13, 1956. https://doi.org/10.3390/coatings13111956
Tan X, Zhou F, Li W, Ao G, Xu X, Yang L. Research Progress on the Application of Graphene Quantum Dots. Coatings. 2023; 13(11):1956. https://doi.org/10.3390/coatings13111956
Chicago/Turabian StyleTan, Xiangyu, Fangrong Zhou, Wenyun Li, Gang Ao, Xiaowei Xu, and Le Yang. 2023. "Research Progress on the Application of Graphene Quantum Dots" Coatings 13, no. 11: 1956. https://doi.org/10.3390/coatings13111956
APA StyleTan, X., Zhou, F., Li, W., Ao, G., Xu, X., & Yang, L. (2023). Research Progress on the Application of Graphene Quantum Dots. Coatings, 13(11), 1956. https://doi.org/10.3390/coatings13111956






