Improved Wear Resistance of Nitro-Chromized Carbon Steel Using an Additional Carburizing
Abstract
:1. Introduction
2. Materials and Methods
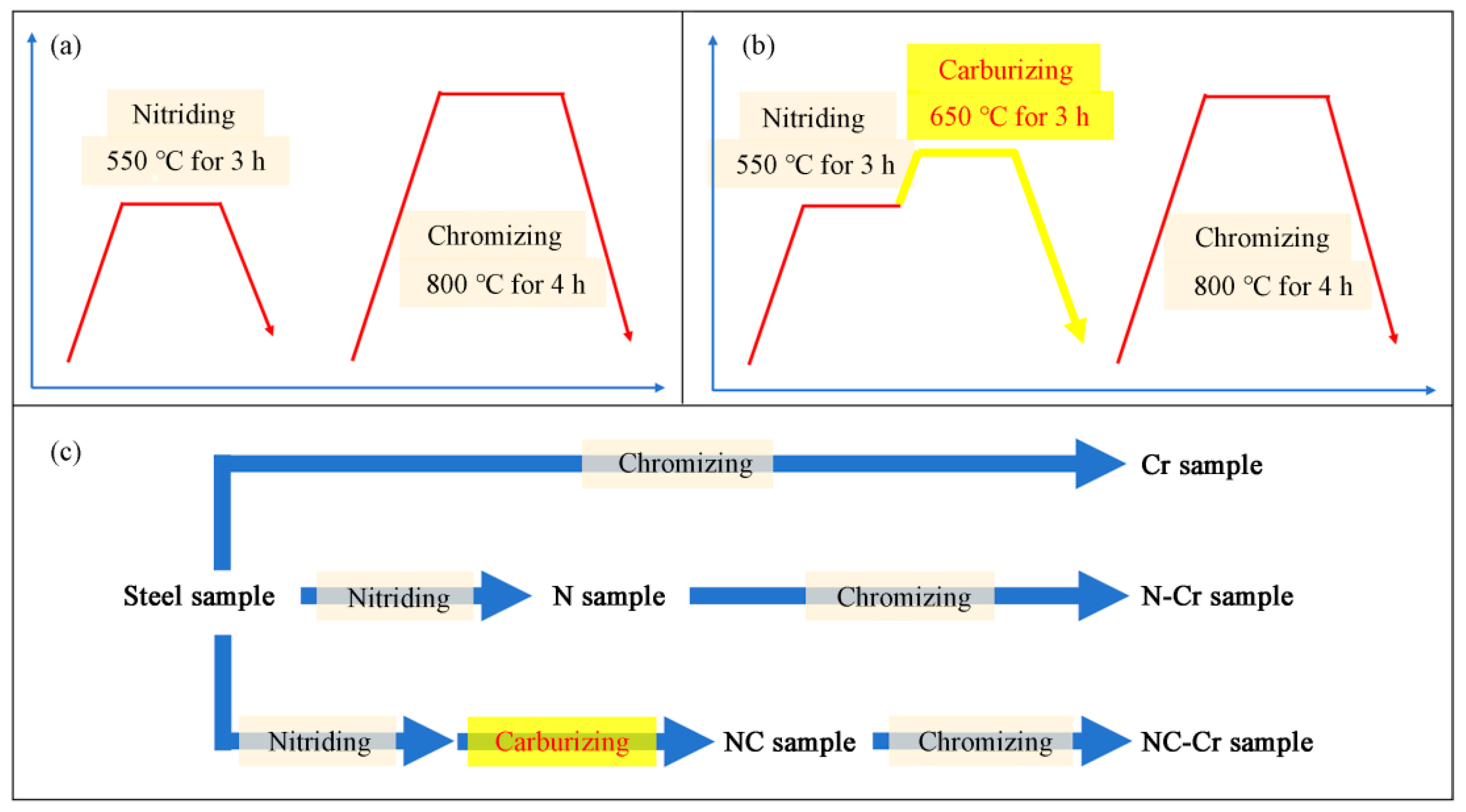
2.1. Sample Preparation
2.2. Characterization and Performance Test Methods
3. Result and Discussion
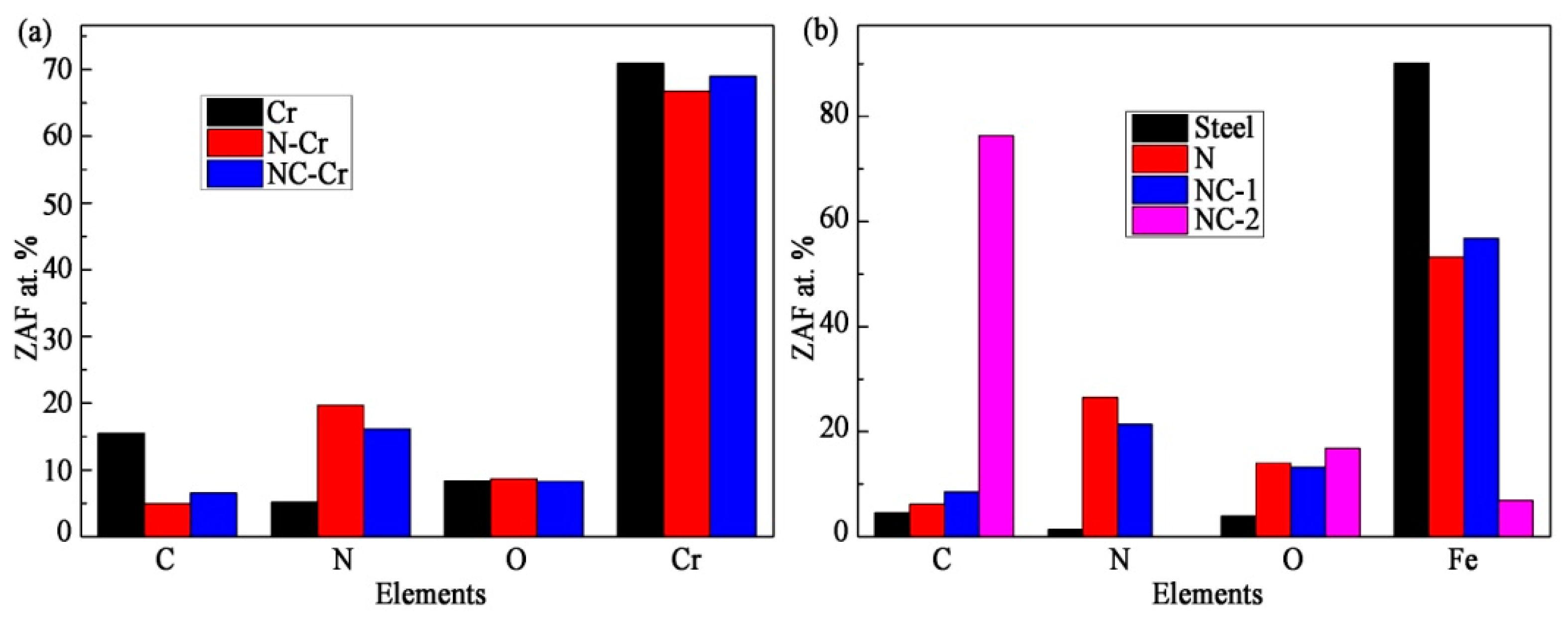
3.1. Phase Analysis and Microstructure Characteristics
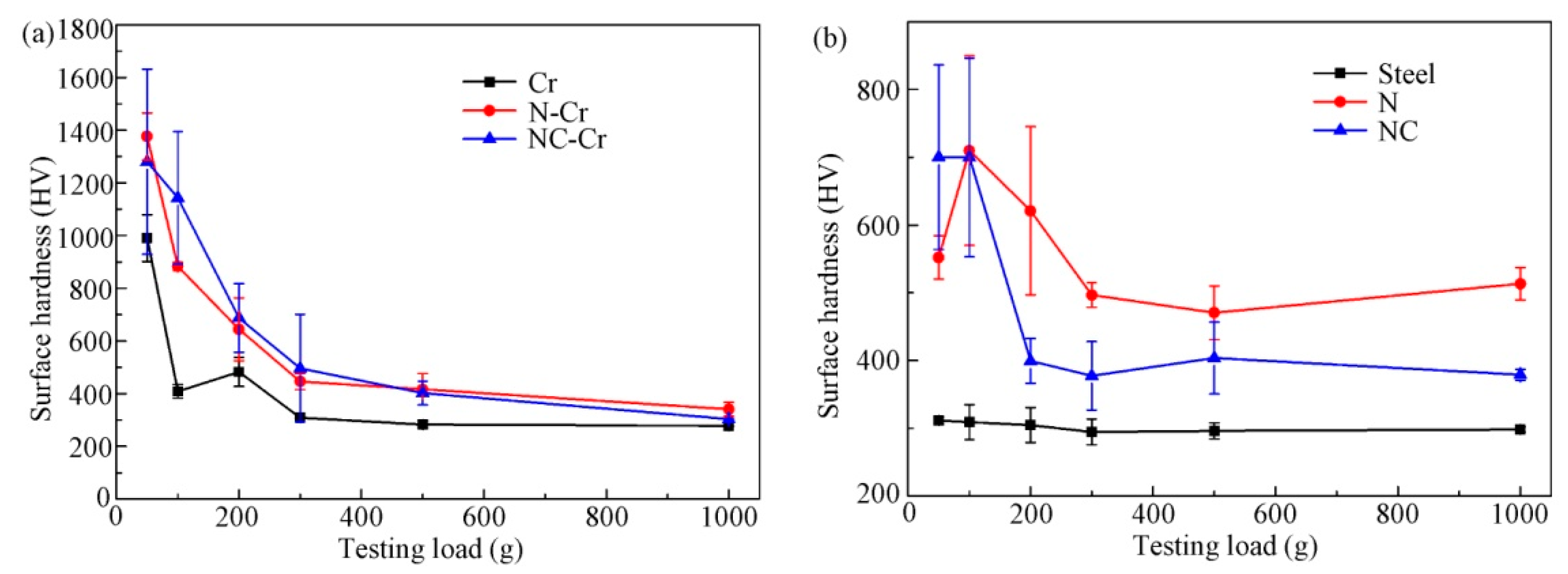
3.2. Surface Hardness and Adhesion Strength
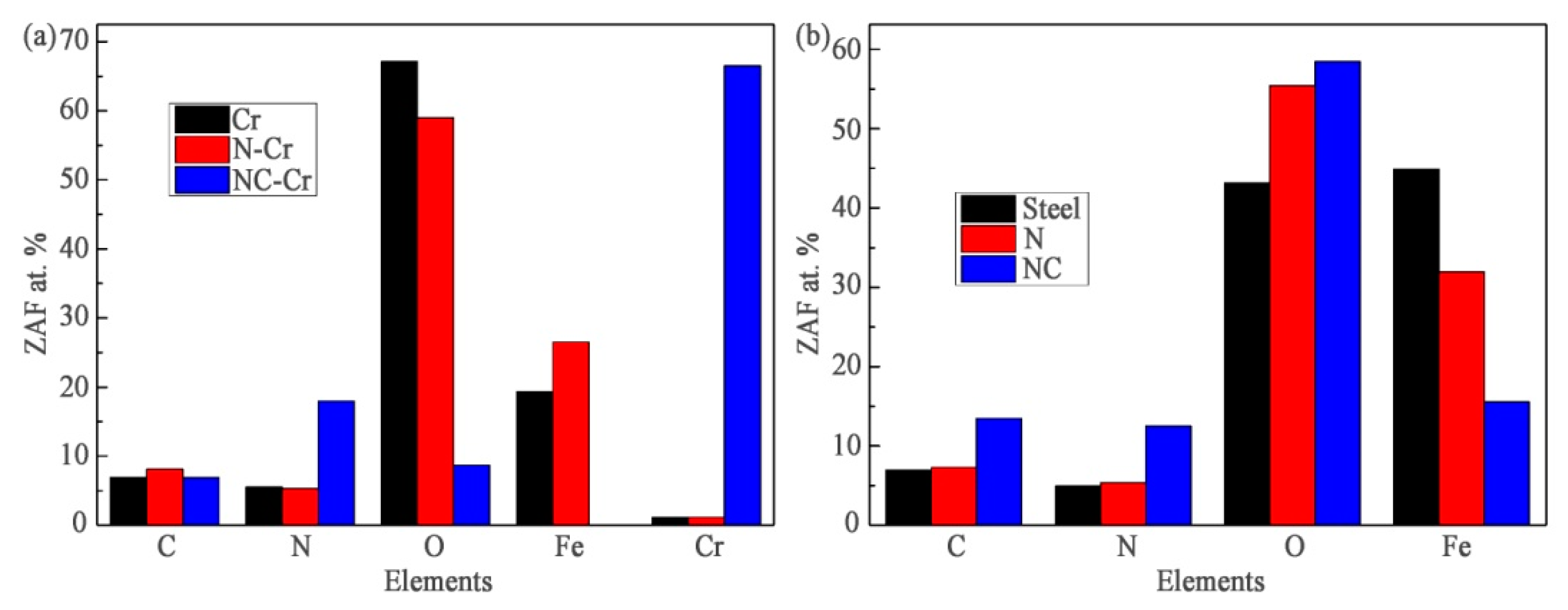
3.3. Wear Resistance
3.4. The Effect of the Additional Carburizing Process on the Wear Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tabrizi, A.T.; Aghajani, H. Study through diverse synthesis methods of chromium nitride thin layers: Areview. J. Surf. Investig. 2021, 15, 1217–1224. [Google Scholar] [CrossRef]
- Tabrizi, A.T.; Aghajani, H.; Laleh, F.F. Tribological characterization of hybrid chromium nitride thin layer synthesized on titanium. Surf. Coat. Technol. 2021, 419, 127317. [Google Scholar] [CrossRef]
- Tabrizi, A.T.; Aghajani, H.; Laleh, F.F. Tribological study of thin-electroplated chromium: Evaluation of wear rate as a function of surface roughness. Exp. Tech. 2023, 47, 369–379. [Google Scholar] [CrossRef]
- Chang, D.Y.; Lee, S.Y.; Kang, S.G. Effect of plasma nitriding on the surface properties of the chromium diffusion coating layer in iron-base alloys. Surf. Coat. Technol. 1999, 116, 391–397. [Google Scholar] [CrossRef]
- Wei, Z.; Zhu, C.; Zhou, L.; Wang, L. The enhancement effect of salt bath chromizing for P20 steel. Coatings 2020, 11, 27. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Wang, Y. Effect of Y2O3 on microstructure and oxidation of chromizing coating. Trans. Nonferrous Met. Soc. China 2008, 18, 1122–1127. [Google Scholar] [CrossRef]
- Lee, J.W.; Duh, J.G. Evaluation of microstructures and mechanical properties of chromized steels with different carbon contents. Surf. Coat. Technol. 2004, 177, 525–531. [Google Scholar] [CrossRef]
- Baggio-Scheid, V.H.; DeVasconcelos, G.; Oliveira, M.A.S.; Ferreira, B.C. Duplex surface treatment of chromium pack diffusion and plasma nitriding of mild steel. Surf. Coat. Technol. 2003, 163, 313–317. [Google Scholar] [CrossRef]
- Lin, N.; Xie, F.; Yang, H.; Tian, W.; Wang, H.; Tang, B. Assessments on friction and wear behaviors of P110 steel and chromizing coating sliding against two counterparts under dry and wet conditions. Appl. Surf. Sci. 2012, 258, 4960–4970. [Google Scholar] [CrossRef]
- Dong, Z.; Zhou, T.; Liu, J.; Zhang, X.; Shen, B.; Hu, W.; Liu, L. Cavitation erosion behaviors of surface chromizing layer on 316L stainless steel. Ultrason Sonochem. 2019, 58, 104668. [Google Scholar] [CrossRef]
- Lin, N.; Guo, J.; Xie, F.; Zou, J.; Tian, W.; Yao, X.; Zhang, H.; Tang, B. Comparison of surface fractal dimensions of chromizing coating and P110 steel for corrosion resistance estimation. Appl. Surf. Sci. 2014, 311, 330–338. [Google Scholar] [CrossRef]
- Hakami, F.; Heydarzadeh Sohi, M.; Rasizadeh Ghani, J. Duplex surface treatment of AISI 1045 steel via plasma nitriding of chromized layer. Thin Solid Film. 2011, 519, 6792–6796. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, G.S.; Kim, B.S. Mechanical properties of duplex layer formed on AISI403 stainless steel by chromizing and boronizing treatment. Surf. Coat. Technol. 2004, 177, 178–184. [Google Scholar] [CrossRef]
- Grejtak, T.; Qu, J. Improving mechanical properties of carbon and tool steels via chromizing. Adv. Appl. Ceram. 2023, 1, 1–11. [Google Scholar] [CrossRef]
- Meng, T.; Guo, Q.; Xi, W.; Ding, W.; Liu, X.; Lin, N.; Yu, S.; Liu, X. Effect of surface etching on the oxidation behavior of plasma chromizing-treated AISI 440B stainless steel. Appl. Surf. Sci. 2018, 433, 855–861. [Google Scholar] [CrossRef]
- Bai, C.Y.; Wen, T.-M.; Hou, K.H.; Pu, N.W.; Ger, M.D. The characteristics and performance of AISI 1045 steel bipolar plates with chromized coatings for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2011, 36, 3975–3983. [Google Scholar] [CrossRef]
- Li, S.; Yang, Z.; Wan, Q.; Hou, J.; Xiao, Y.; Zhang, X.; Gao, R.; Meng, L. Increase in wear resistance of traction wheel via chromizing: A study combining experiments and simulations. Coatings 2022, 12, 1275. [Google Scholar] [CrossRef]
- Taktak, S.; Ulker, S.; Gunes, I. High temperature wear and friction properties of duplex surface treated bearing steels. Surf. Coat. Technol. 2008, 202, 3367–3377. [Google Scholar] [CrossRef]
- Cho, K.H.; Lee, W.G.; Lee, S.B.; Jang, H. Corrosion resistance of chromized 316L stainless steel for PEMFC bipolar plates. J. Power Sources 2008, 178, 671–676. [Google Scholar] [CrossRef]
- Chi, C.; He, Z.; Gao, Y.; Xu, Z. Thermodynamic analysis of carbon migration in W1-1.0C steel in plasma surface chromizing. J. Univ. Sci. Technol. Beijing 2006, 13, 131–134. [Google Scholar] [CrossRef]
- Lin, N.; Xie, F.; Zhong, T.; Wu, X.; Tian, W. Influence of adding various rare earths on microstructures and corrosion resistance of chromizing coatings prepared via pack cementation on P110 steel. J. Rare Earths 2010, 28, 301–304. [Google Scholar] [CrossRef]
- Djemmah, S.; Madi, Y.; Voué, M.; Haddad, A.; Allou, D.; Oualllam, S.; Bouchafaa, H.; Rezzoug, A. Effect of Mg addition on morphology, roughness and adhesion of crchromized layer produced by pack cementation. IJE Trans. A Basics 2023, 36, 1773–1782. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, J.; Lu, K. Wear and corrosion properties of a low carbon steel processed by means of SMAT followed by lower temperature chromizing treatment. Surf. Coat. Technol. 2006, 201, 2796–2801. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Z.; Lu, K. Enhanced chromizing kinetics of tool steel by means of surface mechanical attrition treatment. Mater. Sci. Eng. A 2010, 527, 995–1002. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, J.; Lu, K. Chromizing behaviors of a low carbon steel processed by means of surface mechanical attrition treatment. Acta Mater. 2005, 53, 2081–2089. [Google Scholar] [CrossRef]
- Zeng, J.; Hu, J.; Yang, X.; Xu, H.; Li, H.; Guo, N. Evolution of the microstructure and properties of pre-boronized coatings during pack-cementation chromizing. Coatings 2020, 10, 159. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, J.; Yang, Y.; Yang, X.; Li, H.; Guo, N. Microstructures and wear resistance of boron-chromium duplex-alloyed coatings prepared by a two-step pack cementation process. Coatings 2019, 9, 529. [Google Scholar] [CrossRef]
- Cao, H.; Luo, C.; Liu, J.; Zou, G. Phase transformations in low-temperature chromized 0.45 wt.% C plain carbon steel. Surf. Coat. Technol. 2007, 201, 7970–7977. [Google Scholar] [CrossRef]
- Cao, H.; Luo, C.; Liu, J.; Wu, C.; Zou, G. Formation of a nanostructured CrN layer on nitrided tool steel by low-temperature chromizing. Scripta Mater. 2008, 58, 786–789. [Google Scholar] [CrossRef]
- Wu, C.; Hong, Y.; Chen, W.; Chen, J.; Yuan, M.; Liao, X. A double strengthened surface layer fabricated by nitro-chromizing on carbon steel. Surf. Coat. Technol. 2016, 298, 83–92. [Google Scholar] [CrossRef]
- Hakami, F.; HeydarzadehSohi, M.; RasizadehGhani, J.; Ebrahimi, M. Chromizing of plasma nitrided AISI 1045 steel. Thin Solid Film. 2011, 519, 6783–6786. [Google Scholar] [CrossRef]
- Ozdemir, O.; Sen, S.; Sen, U. Formation of chromium nitride layers on AISI 1010 steel by nitro-chromizing treatment. Vacuum 2007, 81, 567–570. [Google Scholar] [CrossRef]
- Durmaz, M.; Kilinc, B.; Abakay, E.; Sen, U.; Sen, S. Tribological properties of CrN coatings deposited by nitro-chromizing treatment on AISI D2 steel. AIP Conf. Proc. 2015, 1653, 020034. [Google Scholar] [CrossRef]
- Chen, W.; Wu, C.; Liu, Z.; Ni, S.; Hong, Y.; Zhang, Y.; Chen, J. Phase transformations in the nitrocarburizing surface of carbon steels revisited by microstructure and property characterizations. Acta Mater. 2013, 61, 3963–3972. [Google Scholar] [CrossRef]
- Woehrle, T.; Leineweber, A.; Mittemeijer, E.J. Microstructural and phase evolution of compound layers growing on α–iron during gaseous nitrocarburizing. Metall. Mater. Trans. A 2012, 43, 2401–2413. [Google Scholar] [CrossRef]
- Nikolussi, M.; Leineweber, A.; Mittemeijer, E.J. Microstructure and crystallography of massive cementite layers on ferrite substrates. Acta Mater. 2008, 56, 5837–5844. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, M.; Zhang, Y.; Zhang, C.; Wang, X. Self-lubricating and anti-corrosion amorphous carbon/Fe3C composite coating on M50NiL steel by low temperature plasma carburizing. Surf. Coat. Technol. 2016, 304, 142–149. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, M.; Zhang, Y.; Li, D.; Zhang, C.; Zhu, Y.; Wang, Y. Catalytic growth of diamond-like carbon on Fe3C-containing carburized layer through a single-step plasma-assisted carburizing process. Carbon 2017, 122, 1–8. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, M.; Zhang, Y. Tribological behavior of diamond-like carbon in-situ formed on Fe3C-containing carburized layer by plasma carburizing. Appl. Surf. Sci. 2019, 479, 482–488. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Zhang, Z.; Zhang, S.; Zhang, S.; Wang, Q. Characterization of microstructure and surface properties of GLC film deposited in plasma nitriding system. Diam. Relat. Mater. 2021, 119, 108570. [Google Scholar] [CrossRef]
- Li, J.; Men, S.; Zhang, Z.; Yang, Y.; Sun, Y.; Ding, J.; Wang, Q. Structural, mechanical, and tribological properties of GLC film on a nitrided layer prepared in a glow-discharge plasma nitriding system. Vacuum 2021, 193, 110543. [Google Scholar] [CrossRef]
- Dong, J.; Hoffmann, F.; Kluemper-Westkamph, H.; Zoch, H.W. Influence of CO or CO2 as carbon donator on the development of the compound layer during nitrocarburizing of alloyed steels. Mater. Perform. Charact. 2012, 1, 103926. [Google Scholar] [CrossRef]
- Li, S.; Manory, R.R. Surface morphology and compound layer pores of plasma nitrocarburized low carbon steel. Metall. Mater. Trans. A 1996, 27, 135. [Google Scholar] [CrossRef]
- Middendorf, C.; Mader, W. Growth and microstructure of iron nitride layers and pore formation in ε-Fe3N. Z. Metalldk. 2013, 94, 333–340. [Google Scholar] [CrossRef]
- Zamharir, M.J.; Aghajani, H.; Tabrizi, A.T. Evaluation of adhesion strength of TiN layer applied on 316L substrate by electrophoretic deposition. J. Aust. Ceram. Soc. 2021, 57, 1219–1230. [Google Scholar] [CrossRef]
- Schubert, T.; Löser, W.; Schinnerling, S.; Bächer, I. Alternative phase formation in thin strip casting of stainless steels. Mater. Sci. Technol. -Lond. 2013, 11, 181–185. [Google Scholar] [CrossRef]
- Hong, Y.; Dong, D.; Lin, S.; Wang, W.; Tang, C.; Kuang, T.; Dai, M. Improving surface mechanical properties of the selective laser melted 18Ni300 maraging steel via plasma nitriding. Surf. Coat. Technol. 2021, 406, 126675. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Huang, S.; Deng, B.; Yu, Y.; He, C.; Xu, W.; Fan, T. Improved Wear Resistance of Nitro-Chromized Carbon Steel Using an Additional Carburizing. Coatings 2023, 13, 1858. https://doi.org/10.3390/coatings13111858
Hong Y, Huang S, Deng B, Yu Y, He C, Xu W, Fan T. Improved Wear Resistance of Nitro-Chromized Carbon Steel Using an Additional Carburizing. Coatings. 2023; 13(11):1858. https://doi.org/10.3390/coatings13111858
Chicago/Turabian StyleHong, Yue, Shuqi Huang, Bin Deng, Yingmei Yu, Chupeng He, Wei Xu, and Touwen Fan. 2023. "Improved Wear Resistance of Nitro-Chromized Carbon Steel Using an Additional Carburizing" Coatings 13, no. 11: 1858. https://doi.org/10.3390/coatings13111858
APA StyleHong, Y., Huang, S., Deng, B., Yu, Y., He, C., Xu, W., & Fan, T. (2023). Improved Wear Resistance of Nitro-Chromized Carbon Steel Using an Additional Carburizing. Coatings, 13(11), 1858. https://doi.org/10.3390/coatings13111858


.jpg)

