Effects of the Second Anodization Parameters on the Hydrophobicity and Anti-Icing Properties of Al Surface with Composite Nanopore Structure
Abstract
:1. Introduction
2. Experiment Setup
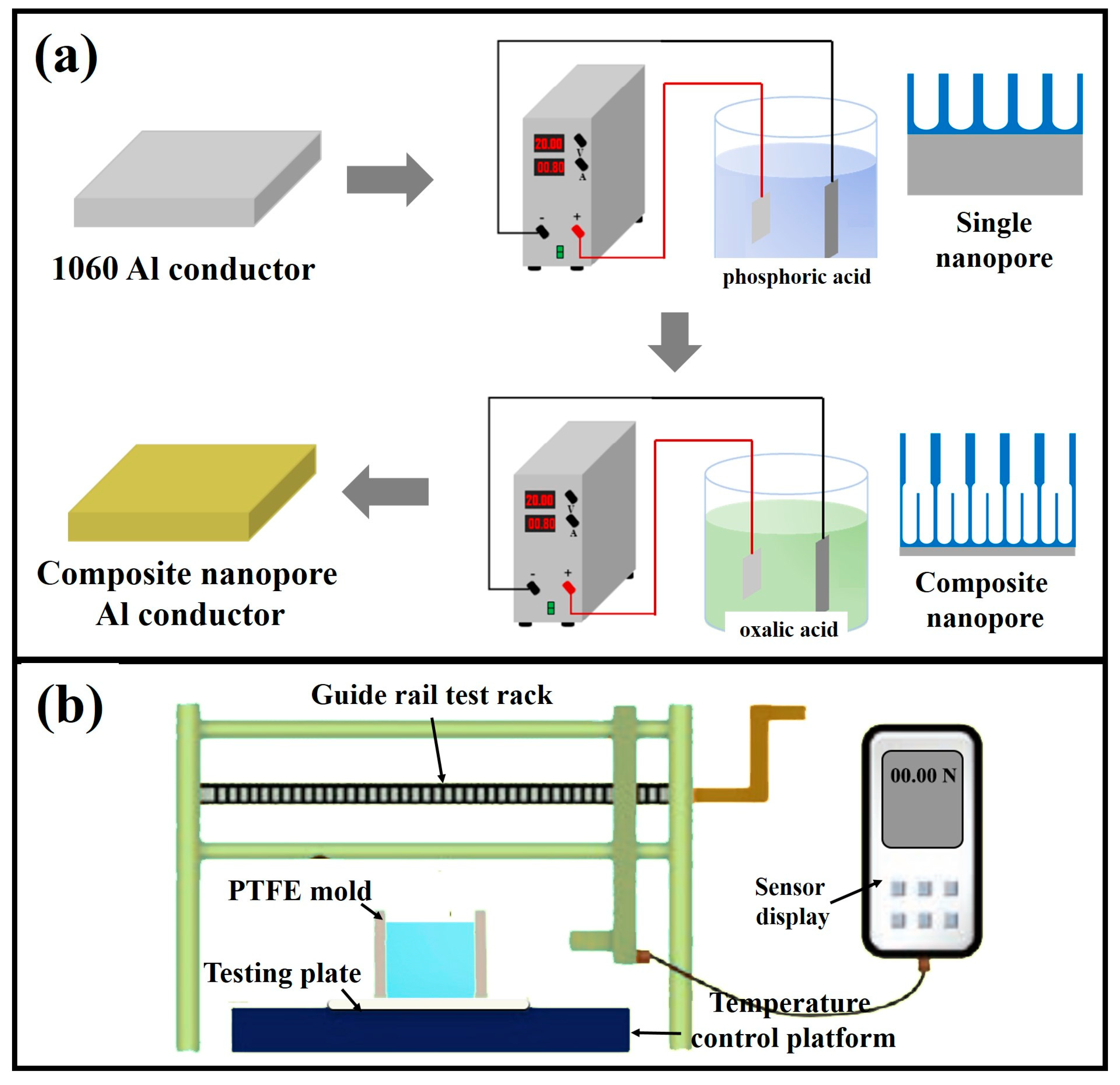
2.1. Preparation of Anti-Icing Composite Nanopore Structure
2.2. Experimental Setup
3. Results and Discussion
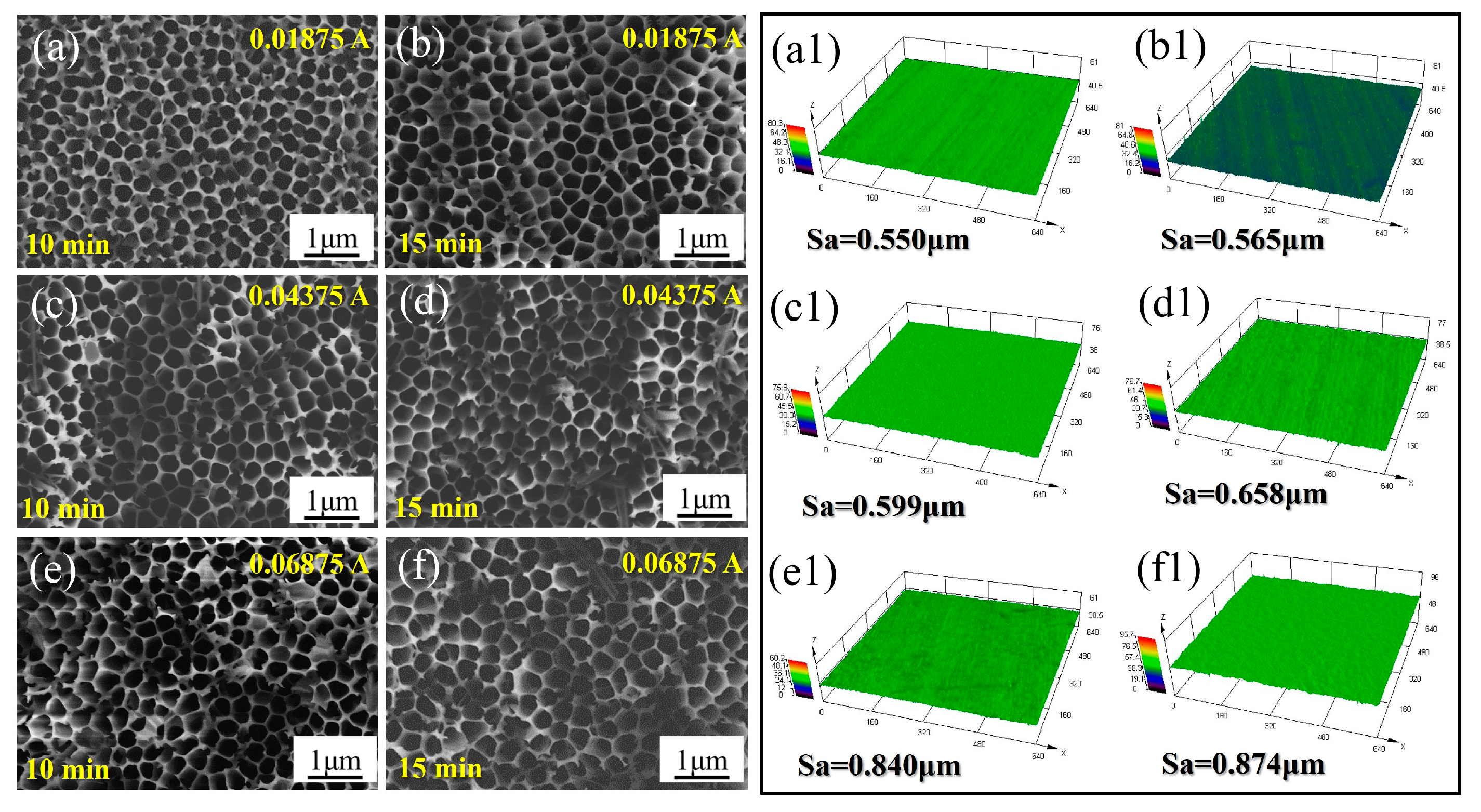
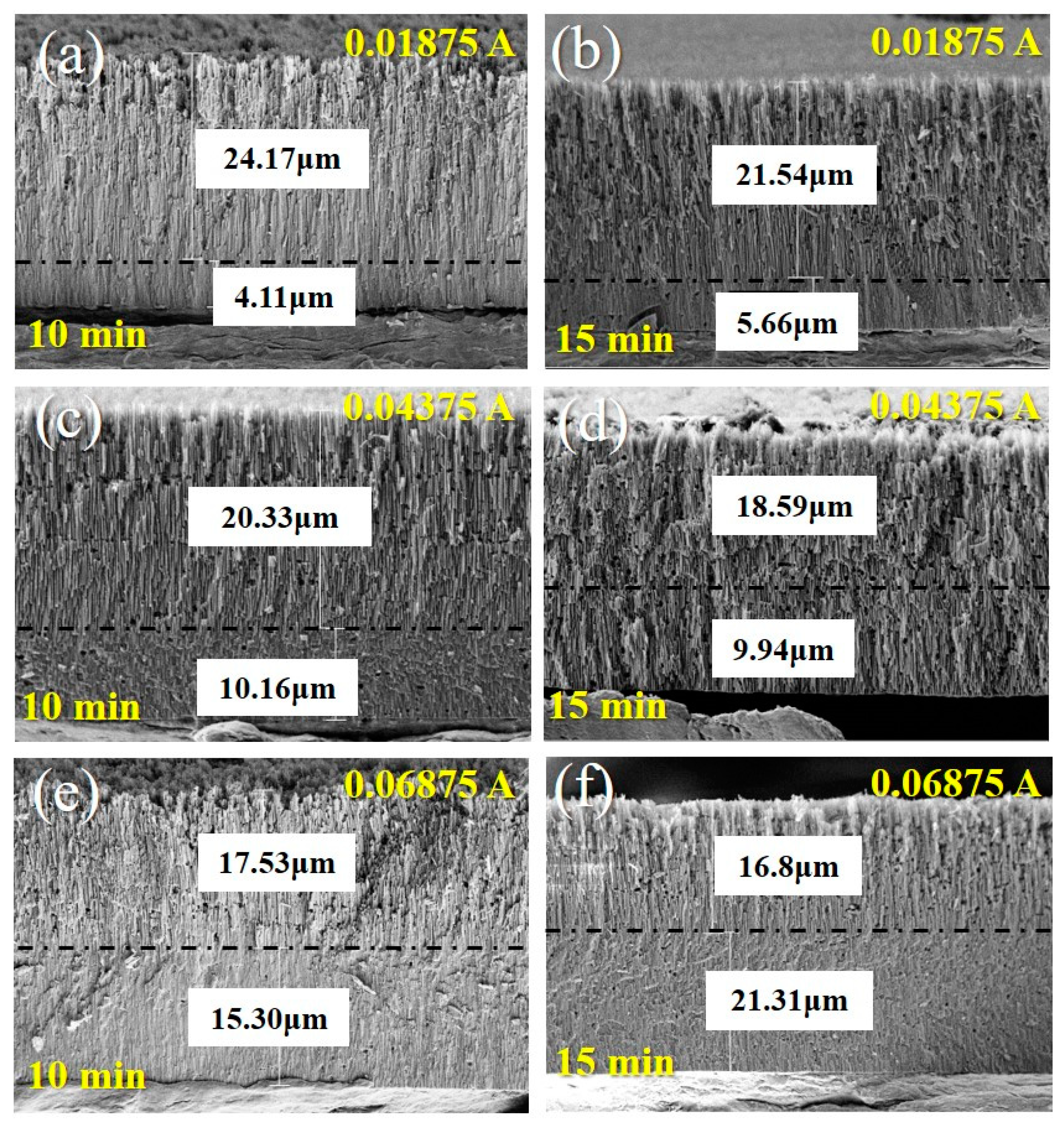
3.1. Morphologies and Micro-Structures
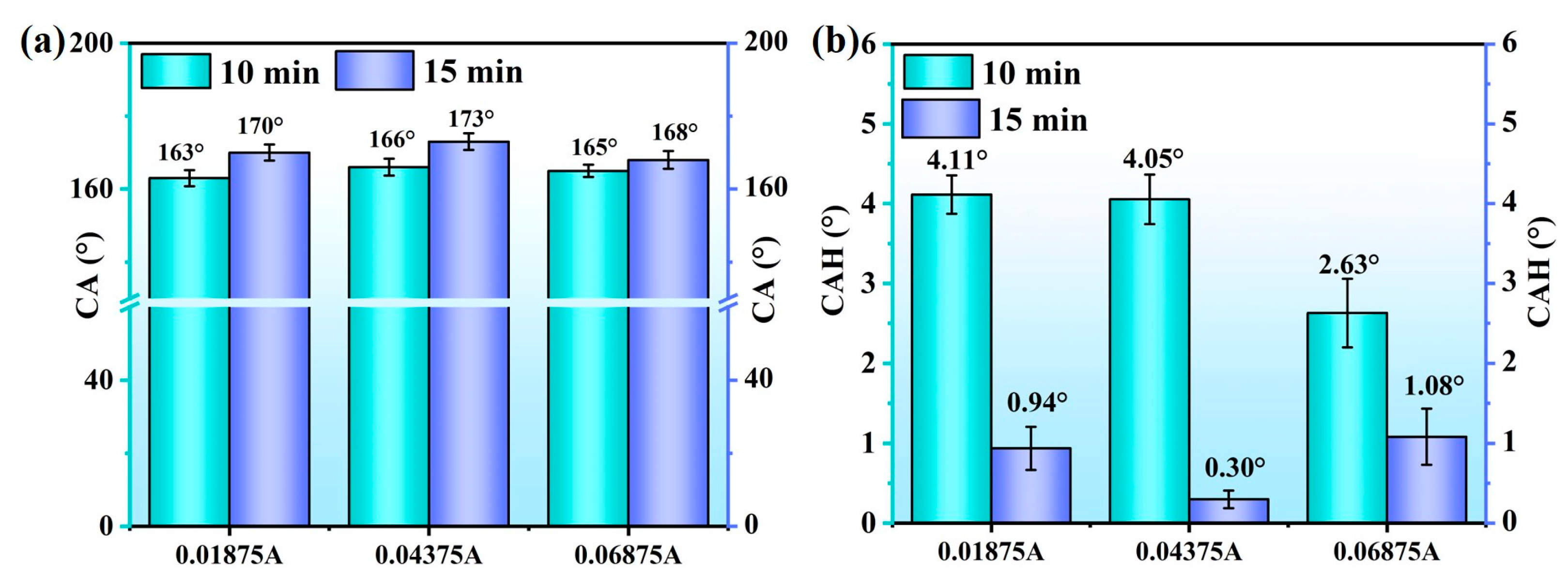
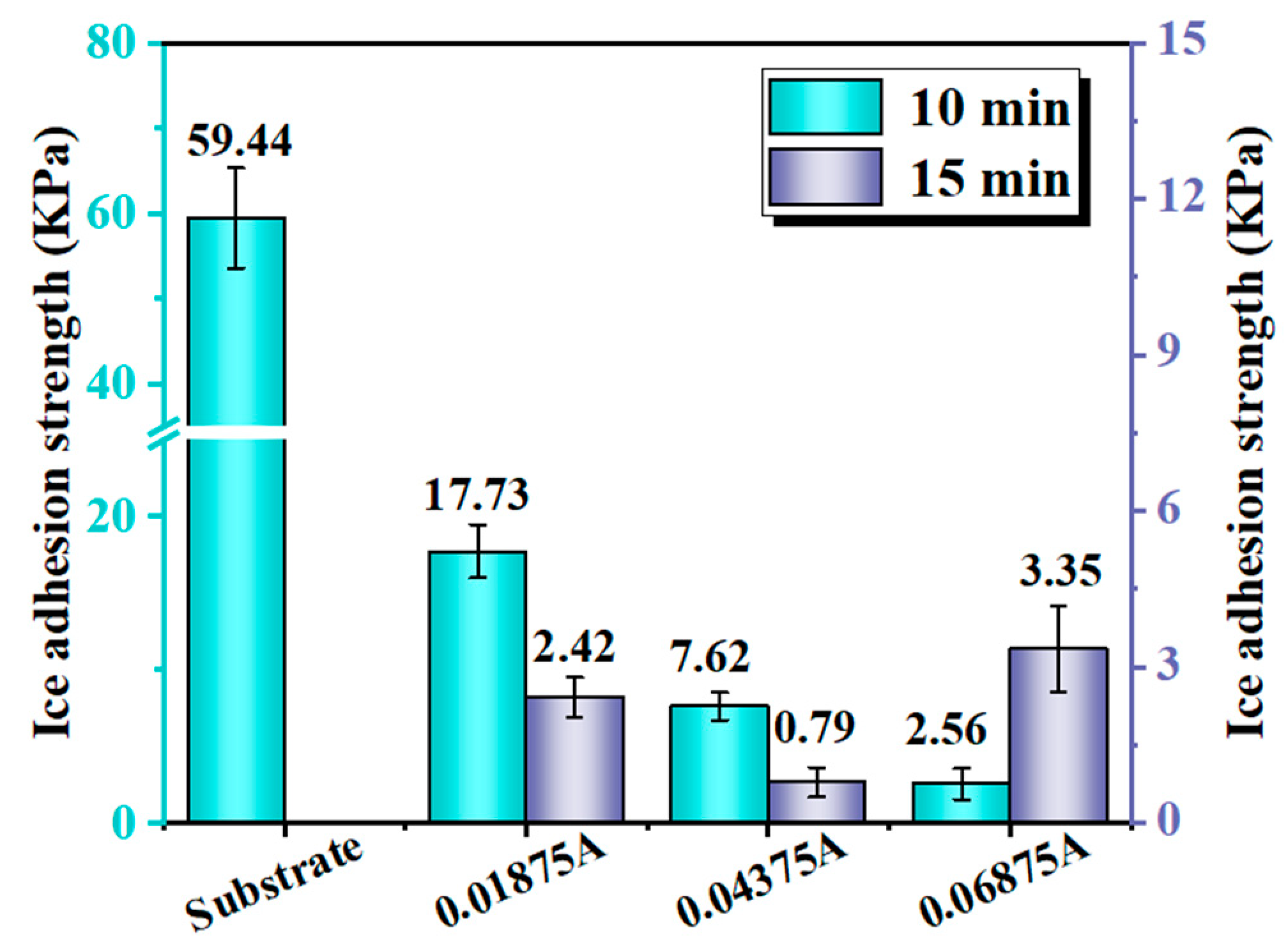
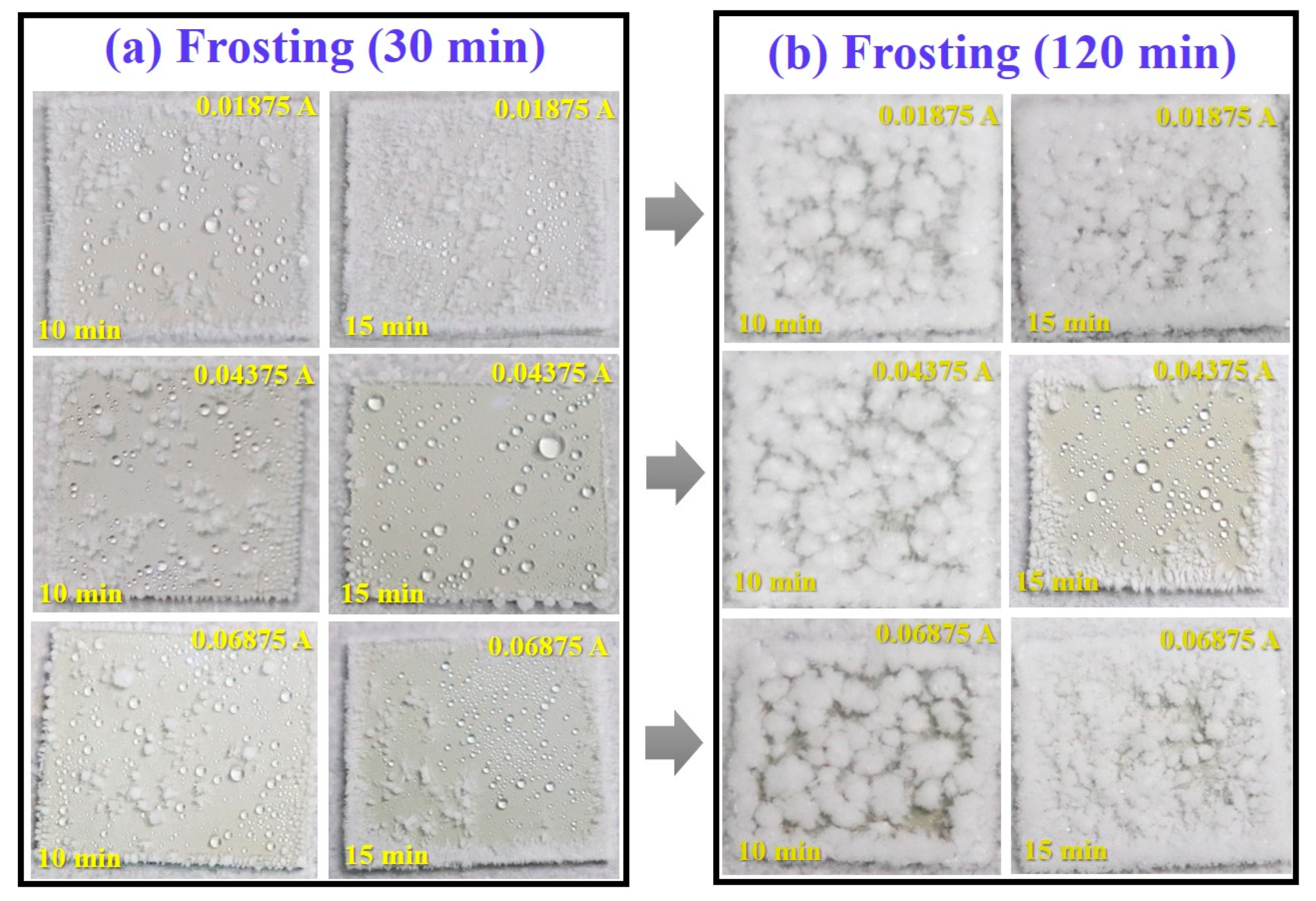
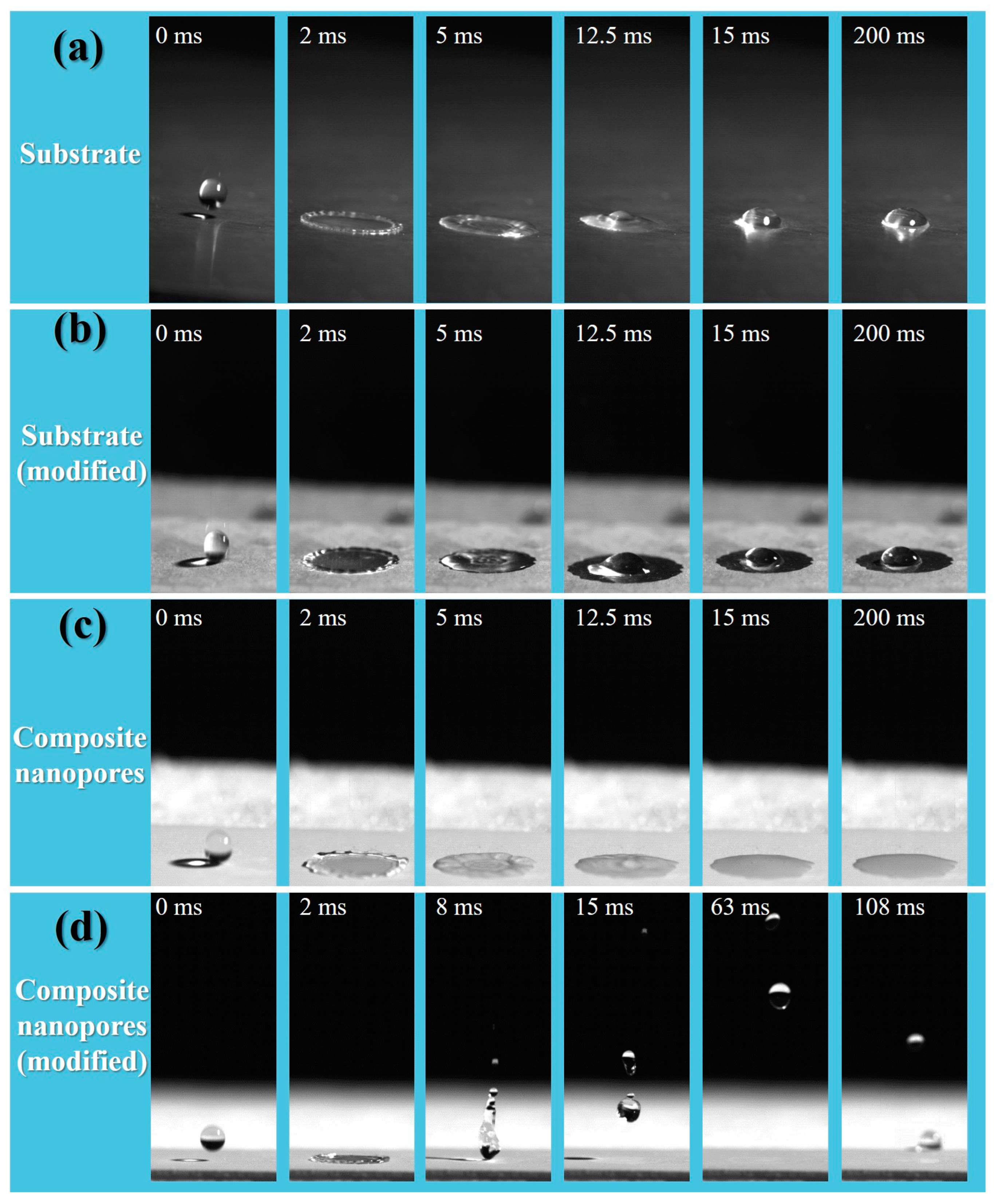
3.2. Wettability and Anti-Icing Properties
4. Conclusions
- (1)
- The optimal preparation process is to conduct secondary oxidation in oxalic acid electrolyte with an anodization current density of 0.04375 A/cm2 and anodization time of 15 min. As the current density and time increase, the surface roughness and fluctuation of anodized samples increases with the thicker upper layer.
- (2)
- The composite nanopore Al surface of the optimal preparation parameter has excellent hydrophobic and anti-icing properties. Among these properties, the contact angle is 173°, the contact angle hysteresis is 0.122°, the ice adhesion strength is 0.71 kPa, and the icing weight after the 8 h glaze icing reaches 0.1 g.
- (3)
- In this study, a two-step anodizing method was used to prepare superhydrophobic Al surfaces with a composite nanopore structure which effectively improves the anti-icing performance of traditional anodized single nanopore structures, especially for glaze icing protection. This study can be helpful for the further preparation of this Aluminum Conductor Steel Reinforced (ACSR) cable. Moreover, the durability of anti-icing properties still needs further exploration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, B.; Bai, J.; He, J.; Ding, C.; Dai, X.; Ci, W.; Zhu, T.; Liao, R.; Yuan, Y. A Review on Superhydrophobic Surface with Anti-Icing Properties in Overhead Transmission Lines. Coatings 2023, 13, 301. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Guo, X.; Dong, L.; Chen, J.; Zhu, M. High flashover strength and superhydrophobic coatings by constructing porous surface structure. High Volt. 2023, 8, 728–738. [Google Scholar] [CrossRef]
- Chengrong, L.; Yuzhen, L.; Xiang, C. Research issues for safe operation of power grid in China under ice-snow disasters. Power Syst. Technol. 2008, 32, 14. [Google Scholar]
- Li, T.; Li, J. Analysis of icing accident in South China power grids in 2008 and it’s countermeasures. In Proceedings of the CIRED 2009—20th International Conference and Exhibition on Electricity Distribution—Part 1, Prague, Czech Republic, 8–11 June 2009; pp. 1–4. [Google Scholar]
- Wang, J.; Fu, C.; Chen, Y.; Rao, H.; Xu, S.; Yu, T.; Li, L. Research and application of DC de–icing technology in china southern power grid. IEEE Trans. Power Deliv. 2012, 27, 1234–1242. [Google Scholar] [CrossRef]
- Ji, K.; Rui, X.; Li, L.; Leblond, A.; McClure, G. A novel ice-shedding model for overhead power line conductors with the consideration of adhesive/cohesive forces. Comput. Struct. 2015, 157, 153–164. [Google Scholar] [CrossRef]
- Dai, X.; Wu, L.; Ci, W.; Yao, W.; Yuan, Y.; Xie, Z.; Jiang, B.; Wang, J.; Andrej, A.; Pan, F. Dual self-healing effects of salicylate intercalated MgAlY-LDHs film in-situ grown on the micro-arc oxidation coating on AZ31 alloys. Corros. Sci. 2023, 220, 111285. [Google Scholar] [CrossRef]
- Ci, W.; Chen, X.; Sun, Y.; Dai, X.; Zhu, G.; Zhao, D.; Pan, F. Effect of Zn on mechanical and corrosion properties of Mg-Sc-Zn alloys. J. Mater. Sci. Technol. 2023, 158, 31–42. [Google Scholar] [CrossRef]
- Golovin, K.; Kobaku, S.P.R.; Lee, D.H.; DiLoreto, E.T.; Mabry, J.M.; Tuteja, A. Designing durable icephobic surfaces. Sci. Adv. 2016, 2, e1501496. [Google Scholar] [CrossRef]
- Xiang, H.; Yuan, Y.; Zhu, T.; Dai, X.; Zhang, C.; Gai, Y.; Liao, R. A novel durable anti-icing slippery surfaces with dendritic porous structure. Mater. Today Phys. 2023, 35, 101137. [Google Scholar] [CrossRef]
- Irajizad, P.; Nazifi, S.; Ghasemi, H. Icephobic surfaces: Definition and figures of merit. Adv. Colloid Interface Sci. 2019, 269, 203–218. [Google Scholar] [CrossRef]
- Xiang, H.; Yuan, Y.; Zhu, T.; Dai, X.; Zhang, C.; Gai, Y.; Liao, R. Anti-Icing Mechanism for a Novel Slippery Aluminum Stranded Conductor. ACS Appl. Mater. Interfaces 2023, 15, 34215–34229. [Google Scholar] [CrossRef]
- Lee, J.; Lee, M.-H.; Choi, C.-H. Design of Robust Lubricant-Infused Surfaces for Anti-Corrosion. ACS Appl. Mater. Interfaces 2022, 14, 2411–2423. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, Y.; Zhang, D.; Xu, N.; Zhu, X. Wettability, durability and corrosion properties of slippery laser-textured aluminum alloy surface under water impact. Surf. Coat. Technol. 2020, 394, 125856. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Biring, S. Nanoplatform based on ideally ordered arrays of short straight and long beer bottle-shaped nanochannels. Microporous Mesoporous Mater. 2019, 287, 71–76. [Google Scholar] [CrossRef]
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Modin, E.B. Modus Operandi of Protective and Anti-icing Mechanisms Underlying the Design of Longstanding Outdoor Icephobic Coatings. ACS Nano 2019, 13, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Parin, R.; Martucci, A.; Sturaro, M.; Bortolin, S.; Bersani, M.; Carraro, F.; Del Col, D. Nano-structured aluminum surfaces for dropwise condensation. Surf. Coat. Technol. 2018, 348, 1–12. [Google Scholar] [CrossRef]
- Sastry, S. Ins and outs of ice nucleation. Nature 2005, 438, 746–747. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Rakitin, A.; Li, J.; Vedeneev, A.S.; Xu, J.M. Electronic Transport in Y-Junction Carbon Nanotubes. Phys. Rev. Lett. 2000, 85, 3476–3479. [Google Scholar] [CrossRef]
- Dai, X.; Yuan, Y.; Liao, R.; Liu, G.; Zhang, C.; Huang, H. Experimental Studies of a Novel Anti-Icing Aluminum Conductor With Excellent Durability and Improved Electrical Performance. IEEE Trans. Power Deliv. 2023, 1–12. [Google Scholar] [CrossRef]
- Wang, F.; Lv, F.; Liu, Y.; Li, C.; Lv, Y. Ice adhesion on different microstructure superhydrophobic aluminum surfaces. J. Adhes. Sci. Technol. 2013, 27, 58–67. [Google Scholar] [CrossRef]

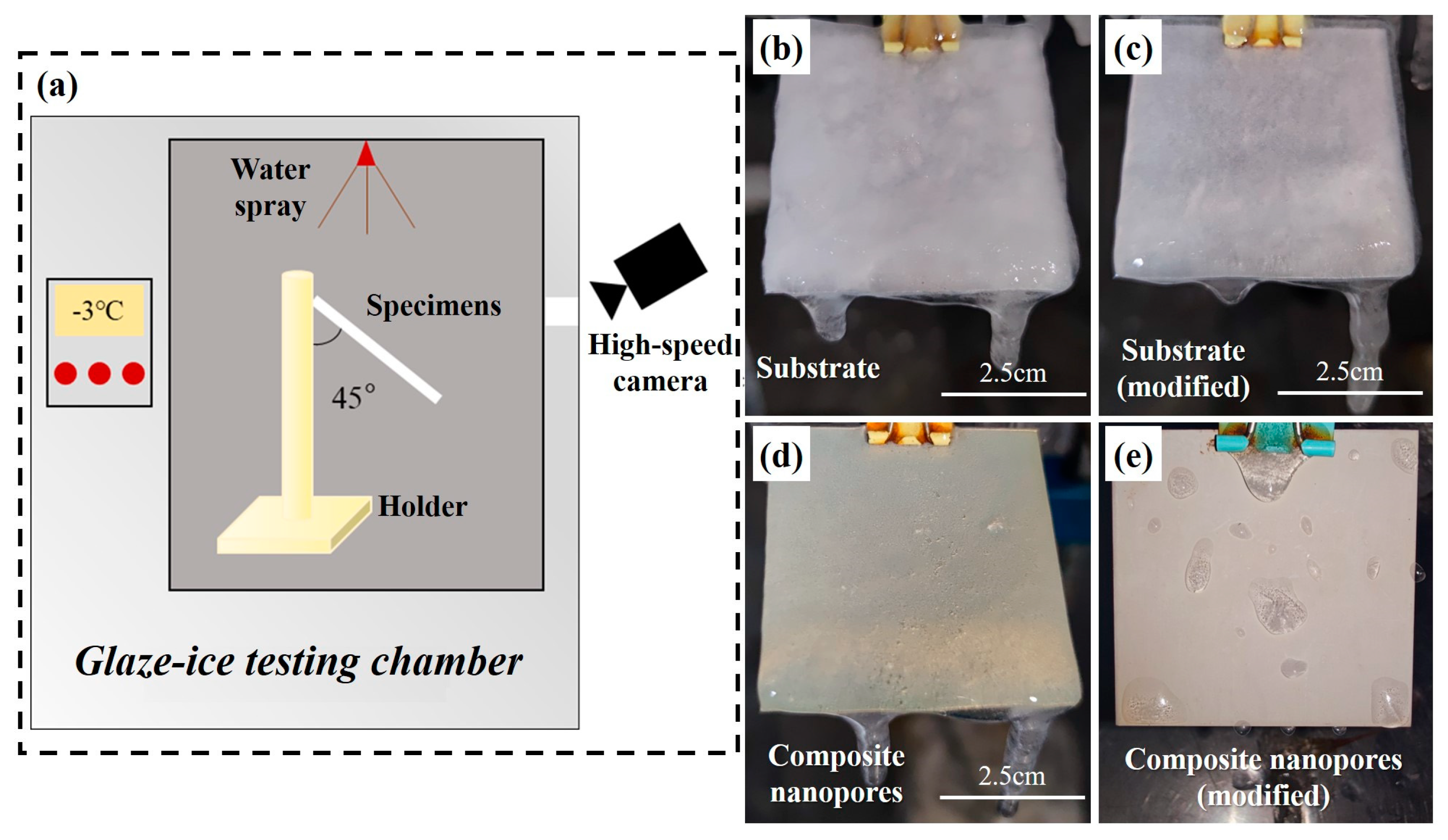
| Specimens | Substrate | Substrate (Modified) | Composite Nanopores | Composite Nanopores (Modified) |
|---|---|---|---|---|
| Icing weight (g) | 17.33 | 17.68 | 14.83 | 0.10 |
| Specimens with Structures | Technique | Contact Angle (°) | Anti-Icing Behavior | Ref. |
|---|---|---|---|---|
| Anodized Al plate with composite structure | Anodization | 172 | 0.79 kPa; delay frosting to 2 h; 0.1 g ice formed after 8 h of the glaze icing | This study |
| Acid-etched surface with FAS | Acid etching | 165 | 0.58 kPa | [22] |
| Polyamide mesh structure with SiO2 nanoparticles | Depositing | 153 | 1.9 kPa; delay frosting to ~18 min | [1] |
| Nano-texture by laser processing | Laser ablation | 153 | Little snow accumulated on the surface after outdoor time of 3 years | [17] |
| Anodized Al plate with single structure | Anodization | 156 | Some big glaze ice at the top edge after 80 min | [1] |
| Slippery surface with dendritic structure | Anodization and SLIPS | ~105 | ~5 kPa; 1.9 g ice formed after 3 h of the glaze icing | [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Bai, J.; Yang, L.; Zhang, L.; Dai, X.; Zhang, C.; Hua, X.; Zhu, T.; Xiang, H.; Liao, R.; et al. Effects of the Second Anodization Parameters on the Hydrophobicity and Anti-Icing Properties of Al Surface with Composite Nanopore Structure. Coatings 2023, 13, 1859. https://doi.org/10.3390/coatings13111859
Li B, Bai J, Yang L, Zhang L, Dai X, Zhang C, Hua X, Zhu T, Xiang H, Liao R, et al. Effects of the Second Anodization Parameters on the Hydrophobicity and Anti-Icing Properties of Al Surface with Composite Nanopore Structure. Coatings. 2023; 13(11):1859. https://doi.org/10.3390/coatings13111859
Chicago/Turabian StyleLi, Bo, Jie Bai, Liuqing Yang, Lusong Zhang, Xu Dai, Cheng Zhang, Xujiang Hua, Tao Zhu, Huiying Xiang, Ruijin Liao, and et al. 2023. "Effects of the Second Anodization Parameters on the Hydrophobicity and Anti-Icing Properties of Al Surface with Composite Nanopore Structure" Coatings 13, no. 11: 1859. https://doi.org/10.3390/coatings13111859
APA StyleLi, B., Bai, J., Yang, L., Zhang, L., Dai, X., Zhang, C., Hua, X., Zhu, T., Xiang, H., Liao, R., & Yuan, Y. (2023). Effects of the Second Anodization Parameters on the Hydrophobicity and Anti-Icing Properties of Al Surface with Composite Nanopore Structure. Coatings, 13(11), 1859. https://doi.org/10.3390/coatings13111859








