3.1. Pre-Formed Ferrite
Based on current research, the introduction of ferrite in bainitic steel can increase the bainitic nucleation interface, accelerating subsequent bainitic transformation. However, the formation of partial ferrite will cause an increase in the carbon content in the remaining austenite at the same time, which may cause an extension of the transformation time. The literature also reports the contradictory influence of pre-formed ferrite on the subsequent kinetics of bainitic transformation. Zhu et al. [
13] believed that the existence of ferrite/austenite interfaces hindered the kinetics of subsequent bainite formation. This is because the driving force of bainitic nucleation at these interfaces was lower than that at the original austenite grain boundary. This slowing effect is mainly due to the distribution of carbon into austenite during the growth process of ferrite. Zhu et al. believed that the distribution of alloying elements led to an increase in the concentration of elements (mainly C and Mn) near the ferrite/austenite interface. It is obvious that the distribution of carbon in austenite during ferrite formation increases the chemical stability of austenite, thus reducing the driving force of bainitic transformation after ferrite formation. While the results of Quidart and Brechet [
14] found that when the proportion of ferrite is small, the influence of carbon distribution on the reduction of the driving force for bainite formation is not significant, about 10% ferrite can promote the subsequent formation of bainite.
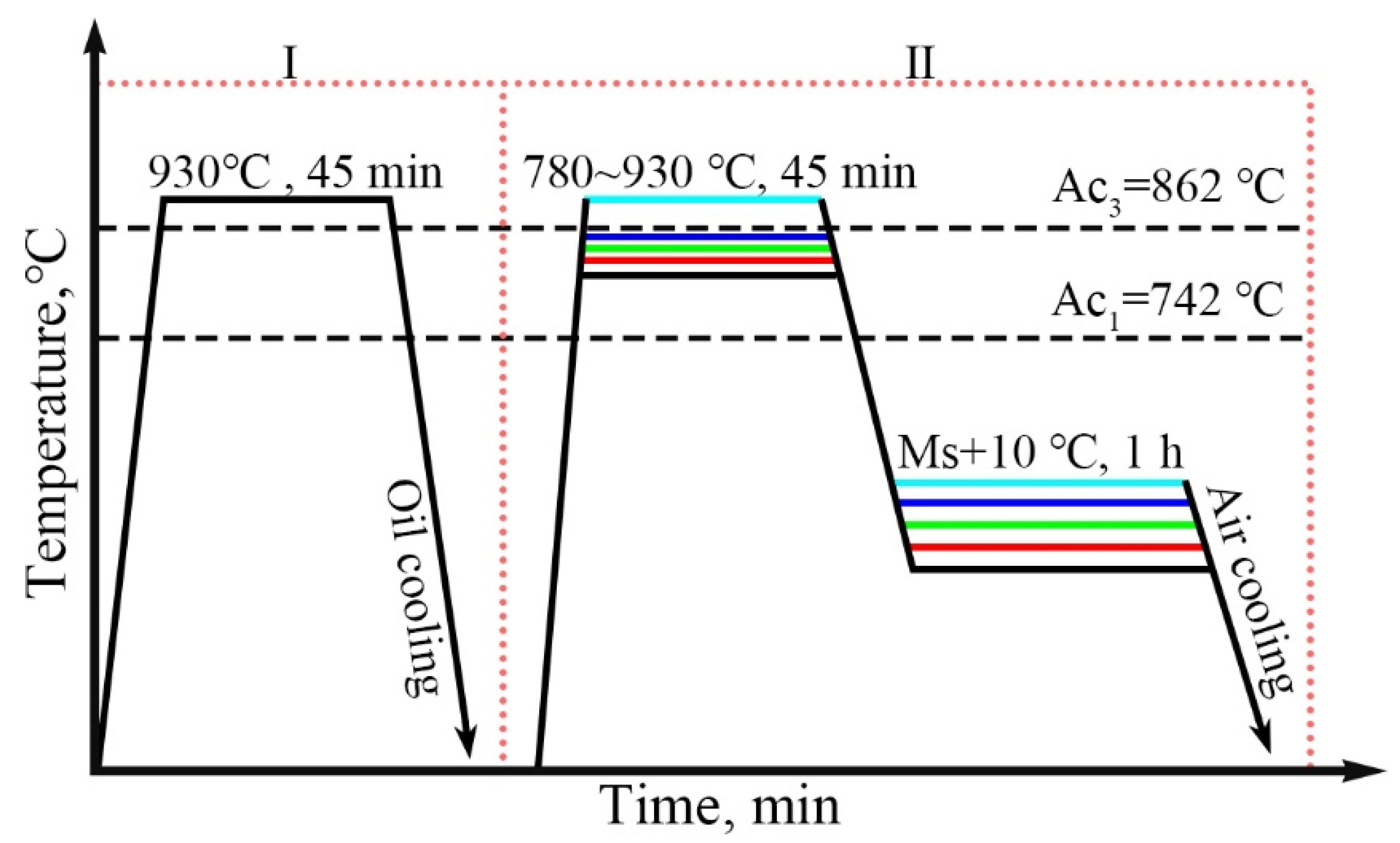
The author’s team designed a medium-carbon steel with the specific composition as follows: Fe-0.39C-1.25Mn-1.55Si-1.11Cr-0.34Mo (wt.%). The corresponding heat treatment process was developed, as shown in
Figure 1. The sample was austenitized at 930 °C in Step I and directly oil quenched after holding for 30 min to obtain martensite. Step II: Heat up to 930 °C, 840 °C, 805 °C, 795 °C, and 780 °C for 45 min and then isothermal in a salt bath furnace at Ms+10 °C, respectively.
Figure 2 shows the bainite transformation rate curves, where the insets show the isothermal curves of the tested steel under different heat-treatment processes. For all heat-treatment processes, the bainitic transformation rate is close to 0 in the 2000s, and the incubation period is almost undetected. In addition, with the increase in isothermal time, the bainite transformation rate of the five processes first increases and reaches peak value, then decreases. Autocatalytic nucleation makes bainite have a high nucleation rate [
15]. When the nucleation rate and growth rate match the best, the bainitic transformation rate reaches the peak value. After that, the original austenite grain is continuously divided by the formed bainite, increasing the probability of collision between bainite and bainite and reducing the growth rate and transformation rate. When bainite continues to grow and touches the austenite grain boundaries or collides with each other, bainitic transformation stops.
In a comparison of the process of two-phase zone transformation (840–780 °C) and the complete austenite temperature (930 °C) process, the peak position of the former shifts to the left, the transformation time decreases, and bainite transformation accelerates. The main reason for this phenomenon is that part of the ferrite is formed during the isothermal process in the two-phase zone of the test steel, as shown in
Figure 3. The microstructure obtained at 795 °C is the ferrite phase. The ferrite interface formed first in this part is conducive to the nucleation of bainitic ferrite, thus increasing the density of the bainitic nucleation points. Bainitic ferrite can not only nucleate at the initial nucleation point of the austenite/austenite interface, but it can also form at the austenite/ferrite interface, accelerating the bainitic transformation kinetics.
The heat treatment temperature decreases from 840 °C to 780 °C, the peak position shifts to the left, the transformation time decreases, and bainitic transformation accelerates. In fact, the main reason is related to the pre-formed ferrite content. In the two-phase zone isothermal process, the lowering temperature is beneficial for the formation of ferrites. As the temperature decreases, the ferrite content increases, resulting in an increase in the density of the bainite nucleation sites, thereby accelerating bainite transformation.
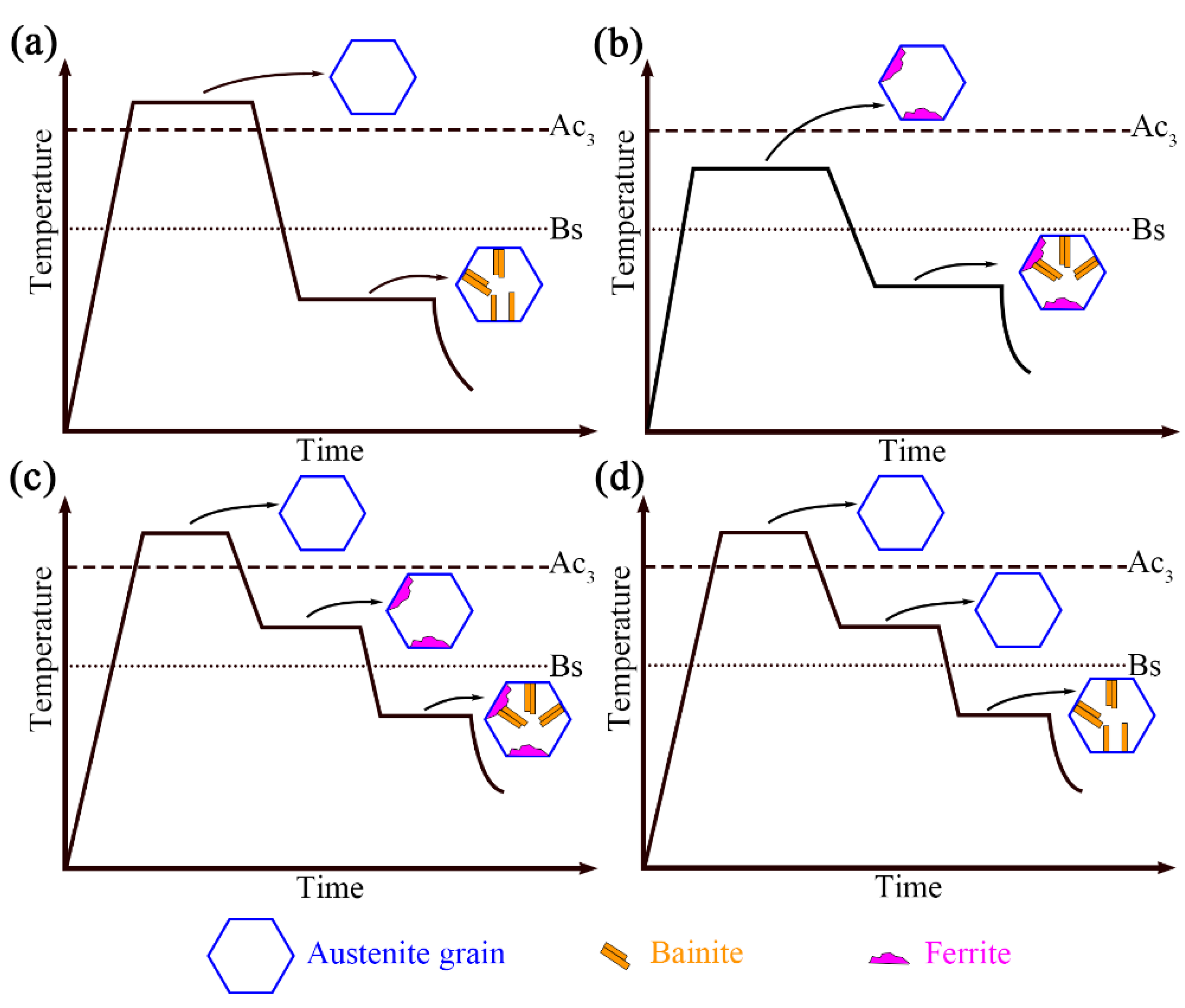
A schematic diagram of the effect of pre-formed ferrite on bainite transformation in this study and present research is shown in
Figure 4. Adding an intermediate heat treatment process between austenitizing and bainite isothermal can also be conducive to bainitic transformation, as shown in
Figure 4c. Research indicates that after complete austenitizing, adding intermediate heat treatment steps (holding below Ac3 temperature) will form ferrite at the austenite grain boundary [
16]. This step accelerates the bainitic formation kinetics, which is mainly due to an increase in the density of the bainitic nucleation sites. Bainitic ferrite can nucleate at the initial nucleation point of the austenite/austenite interface and can also form at the secondary nucleation point at the austenite/ferrite interface [
17]. The ferrite growth before bainitic transformation directly affects the available interface density for bainitic formation. The growth of ferrite leads to the formation of a ferrite/austenite interface, which means that additional interfaces are generated in the original austenite grains, and the total density of the available interfaces for bainite nucleation increases. In addition, even if no ferrite formed during the two-phase region, there is still an acceleration effect, which is related to carbon atom segregation, as shown in
Figure 4d [
18].
3.2. Pre-Formed Martensite
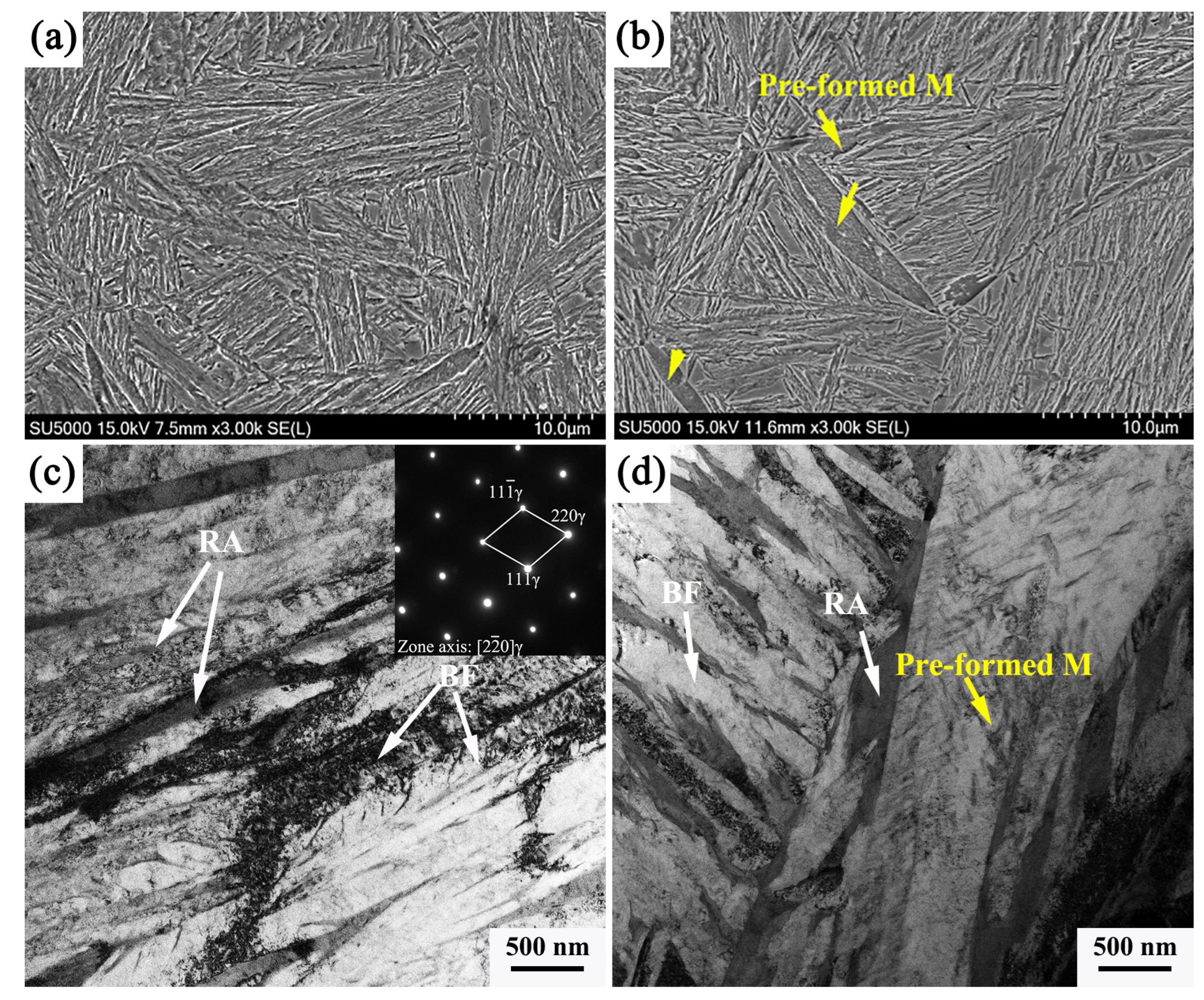
One strategy to increase the rate of bainitic transformation is to form partial martensite before bainitic formation. The heat treatment is that the sample is quenched below the Ms temperature to form a limited amount of martensite, and then isothermal above the Ms temperature or quenching below the Ms temperature and holding enough time to form bainite. Kawata et al. [
19] found that pre-formed martensite in Fe-0.2C-8Ni alloys results in faster bainitic transformation of the austenite/martensite dual phase than that in single-phase austenite. It is suggested that the acceleration of bainitic transformation is caused by an increase in the nucleation sites at the martensite/austenite interface, and it is supposed that dislocations released into the austenite during martensitic transformation contribute to nucleation of the bainite. Gong et al. [
7] conducted a comparative study of the phase transformation behavior of nano-bainite for two processes: quenching and bainite transformation (QBT) and direct isothermal bainite transformation (DIT). For the QBT process, after quenching the sample between Ms and Mf temperature, the sample heats up to the bainitic isothermal transformation temperature. During quenching, a small amount of martensite is introduced, and the volume fraction of martensite (VM) is 6%. Compared to the DIT process, bainitic transformation occurs faster in the QBT process. This indicates that the pre-formed martensite accelerates nano-bainite transformation. The analysis showed that bainitic lath presented almost the same orientation as the neighboring pre-formed martensite plate. In addition, the dislocation introduced in austenite during martensitic transformation is the accompanying variant selection of auxiliary bainitic transformation, which is a favorable bainitic nucleation point. Furthermore, Smanio et al. [
20] suggested that the kinetics of bainite transformation after introducing a certain amount of martensite first is consistent with that after the formation of the same amount of bainite.
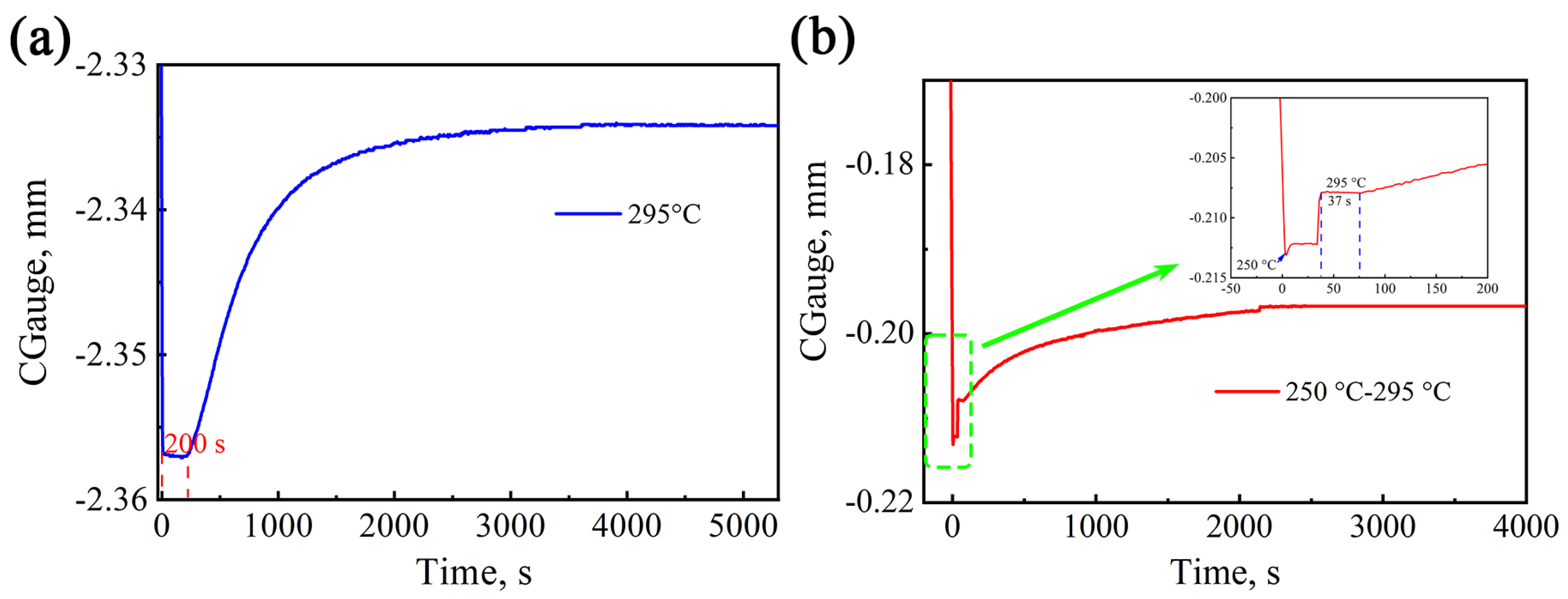
In this paper, the team investigated the effect of pre-formed martensite on bainitic transformation in medium-carbon steel with the following composition: 0.41C-1.53Mn-1.55Si-1.23Cr-0.37Mo-0.54Al-0.34Ni. The Ms temperature was tested to be 285 °C using DIL805A dilatometry. The isothermal transformation curve of the test steel at 295 °C and the curve at 250 °C isothermal for 10 s and then at 295 °C is shown in
Figure 5.
According to the expansion curves, the incubation period of bainite transformation at 295 °C is 200 s, and the total transformation time is 4200 s. Martensitic transformation occurs instantaneously at a temperature of 250 °C below Ms, and no obvious incubation period was observed. Moreover, the martensitic phase transformation occurs within a limited time of 10 s at a homogeneous temperature. There is a direct relationship between martensite transformation and temperature; that is, the lower the temperature, the larger the amount of martensite. The formula for calculating the volume fraction of martensite (
) is
. Thus, the volume fraction of pre-formed martensite at this temperature is 31.9%. Subsequently, the temperature rises to 295°C, and bainitic transformation occurs after a short incubation period of 37 s. The shorter incubation period is due to the fact that the pre-formed martensite provides many favorable interfaces for bainitic ferrite nucleation, which is more favorable than nucleation at the grain boundaries. At this stage, bainite nucleation can occur at both the parent austenite grain boundaries and the martensitic interfaces. The heat treatment process was observed using in situ high-temperature confocal microscopy, as shown in
Figure 6, where it can be clearly seen that the bainitic ferrite grows on the pre-formed martensite at a temperature of 295 °C.
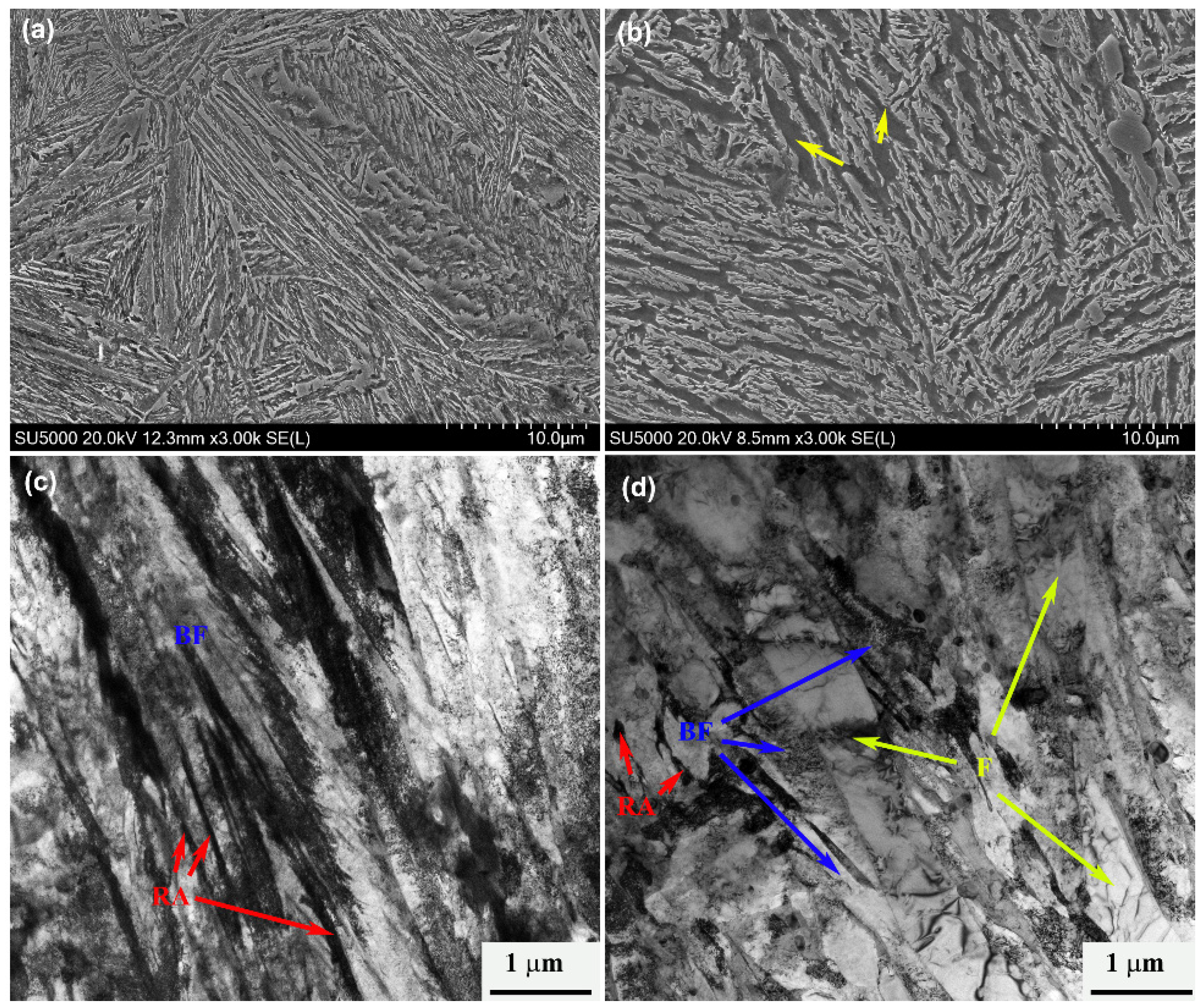
Figure 7 shows the SEM and TEM images of the two heat treatment processes; after the traditional one-stage bainitic transformation process, the microstructure consists of bainitic ferrite and retained austenite, and the width of the bainitic ferrite plate is more uniform, as shown in
Figure 7a,c. Compared with the microstructure after the pre-formed martensite process, the wide and long plate in the microstructure can be clearly seen, which is more obvious under TEM observation. Nano-scale carbides distribute on the lath, and this morphology is a typical pre-formed martensite. This part of the pre-formed martensite undergoes self-tempering during the second-stage isothermal process, and thus, precipitation of carbides occurs. It is also observed that the size of the bainitic ferrite plate obtained in this process is thin in the width direction.
From a crystallographic point of view, previous studies have indicated that pre-formed bainite and pre-formed martensite have the same orientation relationship with the parent austenite. And, both had identical crystallographic features, including the same habit plane, displacement directions, orientation relationships, and shape deformation matrices. In addition, austenite undergoes plastic deformation caused by martensite transformation; the bainitic poles are displaced, and bainitic transformation is determined by the displacement mechanism [
21]. In summary, in the presence of pre-formed martensite, bainitic transformation starts both at the austenite grain boundaries and at the martensite/austenite interfaces. The rate of bainite formation at the martensite/austenite interface does not affect bainitic transformation at the austenite grain boundaries. However, the bainitic transformation at the martensite/austenite interface leads to the formation of a bainite/austenite interface that favors autocatalytic nucleation. The effect of the martensite/austenite interface on the rate of bainite nucleation is closely related to the content of pre-formed martensite, which affects both the density of the austenite nucleation sites and austenite carbon enrichment.
3.3. Precipitated Particles
Microalloying technology has gradually matured in the field of materials and has been widely applied in a variety of steels. By adding trace carbon and nitride-forming elements (generally no more than 0.2%), trace inclusions or second-phase precipitation precipitates appear in the steel, which would affect nucleation and grain boundary migration. At the same time, it interacts with carbon and nitrogen elements to play the role of precipitation strengthening, fine-grain strengthening, and then control recrystallization, so as to improve the microstructure of the steel and enhance its comprehensive properties [
22,
23,
24].
As an example, element V is a strong carbon-nitride-forming element. Fine VC or V(C, N) precipitation phases are formed mainly during the transformation of austenite to ferrite or during the austenite/ferrite isothermal transformation, and the content of such particles is closely temperature dependent. The lattice mismatch between V(C, N) and ferrite is low. Therefore, V(C, N) can act as a preferential nucleation site for acicular ferrite, promote intercrystalline ferrite transformation, and can effectively refine ferrite grains. Therefore, the V-containing precipitated phase can play a dual role in precipitation strengthening and fine grain strengthening in steel. Present studies have concluded that the steel microalloying with V could improve of the mechanical properties, including strength, ductility and impact properties [
25]. The presence of fine VC particles in steel with the addition of 0.06 wt.% V can increase the yield strength and tensile strength of the steel by 60 MPa and 95 MPa, respectively. Interestingly, it is without loss of toughness and ductility [
26].
The tested steel used in this study was V micro-alloyed medium-carbon bainitic steel with the chemical composition (wt.%) of Fe-0.40C-1.53Mn-1.53Si-1.09Cr-0.31Mo-0.15V. The Ac1 and Ac3 temperatures were 731 °C and 864 °C, respectively. The high-temperature double-stage isothermal heat treatment process was designed based on the phase transformation temperature. All samples with preheated treatment were homogenized and annealed at 1050 °C for 3 h and quenched in oil to room temperature. Double-stage high-temperature + bainitic phase transformation heat treatment process: The samples were heated up to 950 °C and held for 30 min. Then, they were rapidly cooled down to 890 °C, 870 °C, 860 °C, and 848 °C and held for 30 min in order to precipitate different amounts of VC particles. Subsequently, bainite isothermal transformation was carried out at 320 °C for 2 h.
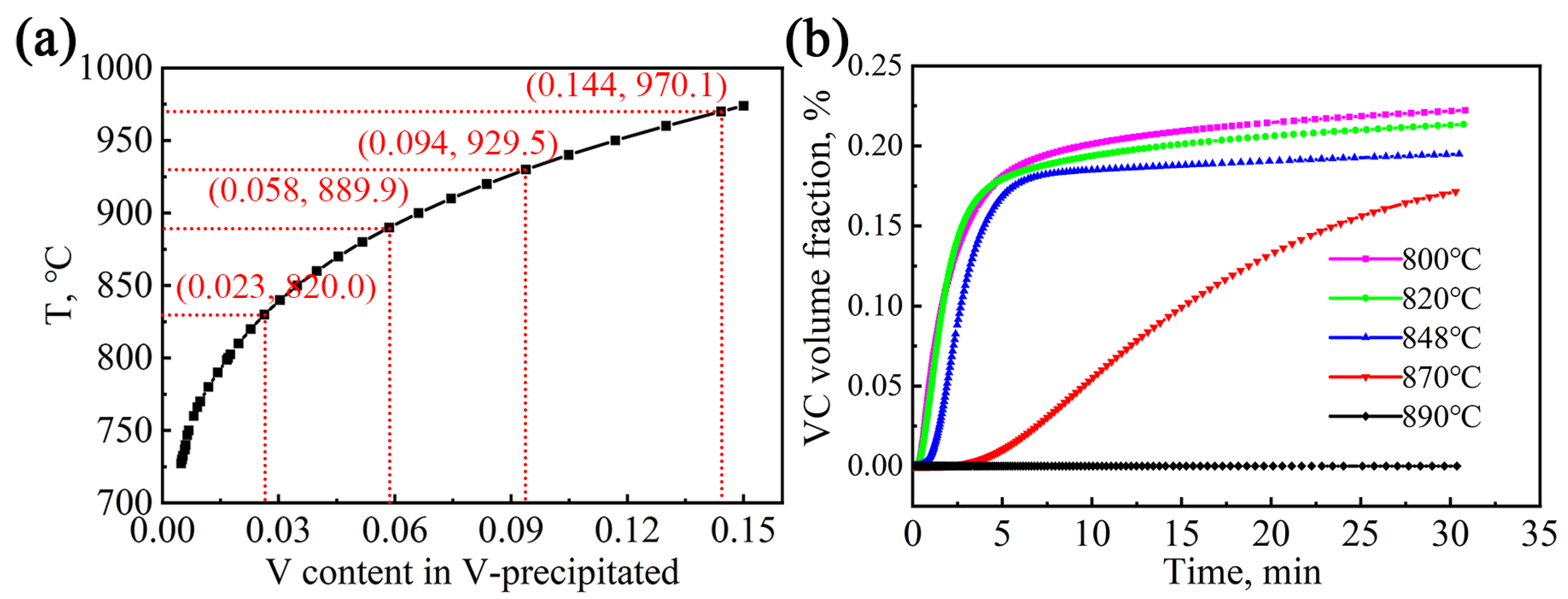
Using the precipitation module of Thermal-Calc software (2020b, Stockholm, Sweden using the TCFE11 and MOBFE6 databases), the evolution of the precipitation and solid solution content of the V element in the test steel during the second-stage high-temperature isothermal process was simulated and calculated, as shown in
Figure 8. With the increase in austenitizing temperature, more V is in solution into the matrix. When the temperature reaches near 970 °C, the V element completely dissolves into the austenitic matrix, as shown in
Figure 8a. In addition, with the increase in isothermal time, the volume fraction of VC particulates in the austenite matrix increases rapidly and then gradually levels off. After 30 min of holding time for all processes, the change in the volume fraction of the VC particles tends to be stable. The isothermal temperature increases from 800 °C to 890 °C, and the volume fraction of VC precipitation decreases. At 890 °C for 30 min, the volume fraction of VC precipitation is extremely small compared with other processes.
Figure 9 shows the kinetics curves of bainitic transformation measured at 320 °C after austenitizing at different temperatures.
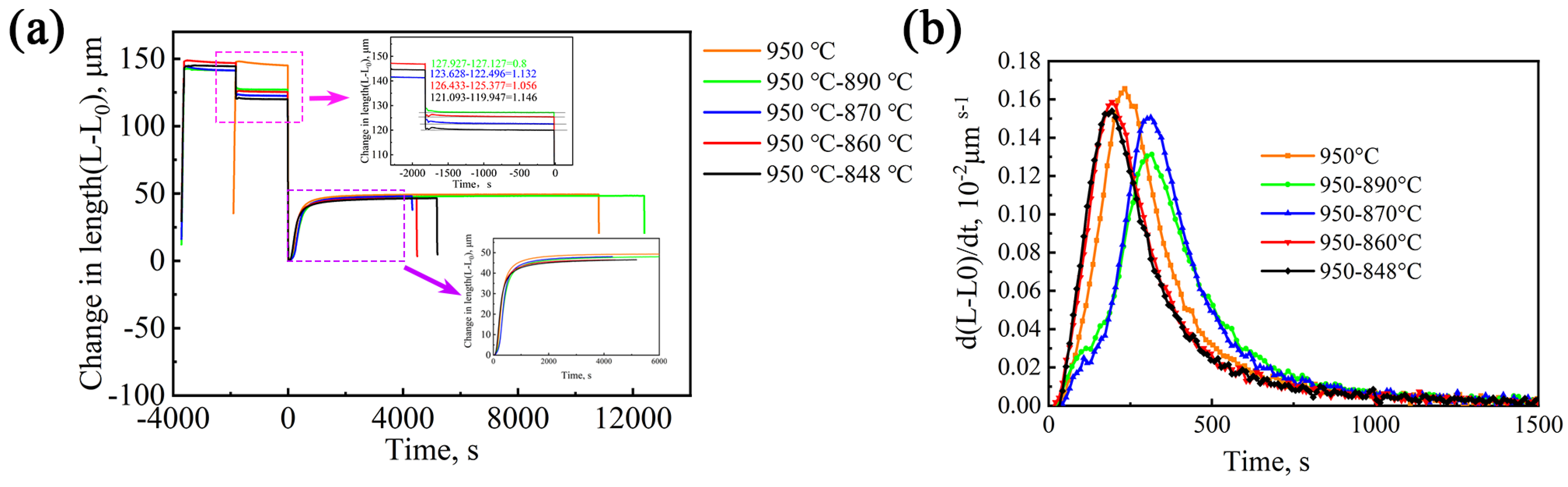
Figure 9a shows the thermal expansion curves of the overall heat treatment process. Zooming in on the pink rectangular area in the figure, one can observe that the isothermal temperature decreased from 890 °C to 848 °C, and the expansion amount of the sample gradually increased from 0.800 μm to 1.146 μm, which is due to the increase in VC precipitation. The large contraction of the expansion curve represents more precipitation. At the beginning of the second stage of the isothermal curve, the curve has some ups and downs, which are caused by temperature instability during the cooling process of the dilatometer. Zooming in on the purple rectangular area in the figure, one can observe the isothermal transformation curves of bainitic transformation under different processes. The incubation period is short (approximately 30 s), and the transformation can be completed in approximately 2 h.
Figure 9b shows the rate curves of bainite transformation of the test steel under different processes. The rate curves of bainite transformation of the five processes all show a trend of first increasing and then decreasing, and their peak transformation rates are different. Compared with the direct isothermal process at 950 °C, the highest peaks of the 950–890 °C and 950–870 °C processes move to the right, which indicates that the bainitic transformation time increases. The highest peaks of the 950–860 °C and 950–848 °C processes move to the left, and the transformation time decreases. This indicates that the isothermal temperature of the second stage affects bainite transformation. The lower temperature in the second stage is beneficial to accelerate transformation, while the higher temperature in the second stage is not conducive to bainitic transformation.
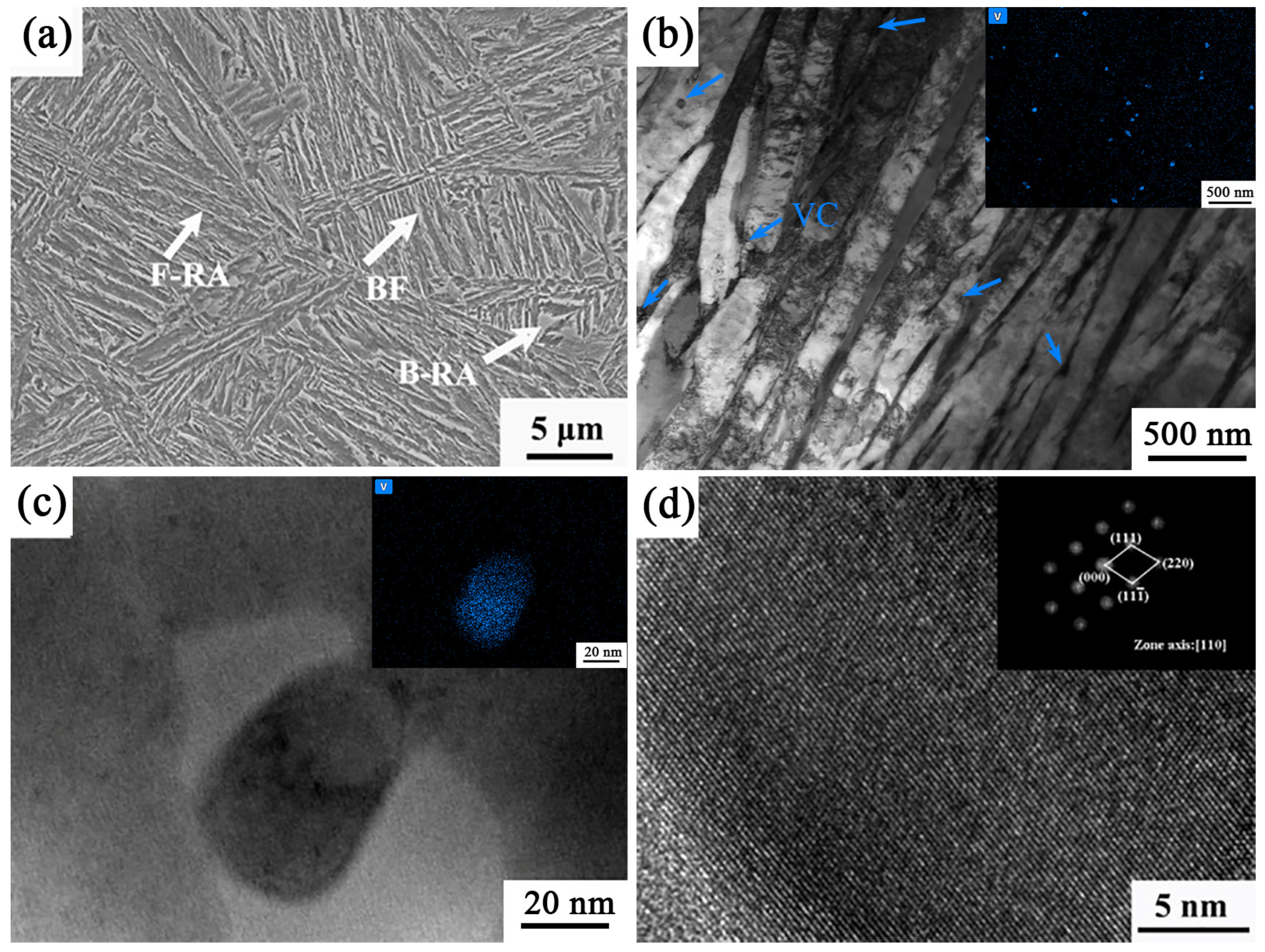
Figure 10 shows the microstructure of the sample under the process of 950–848 °C. The microstructure is mainly composed of bainitic ferrite and retained austenite. Although the temperature range of 848 °C is a two-phase zone temperature, there is no ferrite formation under this process condition, which is a typical carbide-free bainite microstructure. From
Figure 10a, it can be seen that the direction of the bainite laths is more complex, and no bainite ferrite laths are observed throughout the grain, suggesting an increase in the nucleation charge of bainite. A large number of dislocations on the bainitic ferrite laths can be seen in
Figure 10b. During the growth of the lath-like bainitic ferrite, C atoms diffuse around, and the carbon content of the retained austenite rises to form a thin film of retained austenite. The presence of VC particles between microstructures can be observed from the corresponding EDS patterns. V atom segregation is more obvious, and the size distribution of particles is between 36 and 76 nm. Zooming in to observe the TEM images and the corresponding EDS maps, it is not difficult to find the presence of second-phase particles.
Figure 10c shows the TEM photographs of the sample under the process of 950–848 °C.
Figure 10d shows a high-resolution image of
Figure 10c with the diffraction spot obtained by Fourier transform. The precipitated carbide was identified as VC by comparing it with PDF cards of standard VC particles.
The second-stage isothermal process is in the high-temperature zone, which affects bainitic transformation. The second-stage isothermal process controls in the two-phase zone temperature are beneficial for accelerating transformation. The reasons for the analysis are as follows: (1) The difference in phase transformation may be related to carbon segregation. In this study, the second-stage temperature of the 950–860 °C process and the 950–848 °C process is at the temperature of the two-phase zone, but the microstructure of the sample does not appear ferrite (as shown in
Figure 10), so the acceleration of bainitic transformation kinetics is not due to the transformation of the ferrite/austenite interface. However, the second-stage isothermal temperature of the 950–860 °C process and the 950–848 °C process is within the temperature range of the two-phase zone, which has a driving force for the formation of ferrite. Therefore, it can be speculated that carbon segregation to the austenite grain boundary leads to a fluctuation in the carbon composition nearby, which can lead to the formation of stable ferrite cores during the second-stage isothermal process; the mechanism is shown in
Figure 4d. The increase in the number of ferrite cores BCC structure directly increases the nucleation density of bainite. (2) A large number of VC particles will precipitate in these two processes, and the phase interface with the increase in VC particles provides the preferential nucleation position. The double factors lead to the acceleration of bainitic transformation.
Adding V in the continuous cooling process can broaden the bainitic transformation zone [
27]. Bainitic ferrite can nucleate at the position with a low carbon concentration near the vanadium carbide/austenite interface. Due to the different thermal expansion coefficients of VC and austenite, strain fields and dislocation fields are generated, thus reducing the barriers to bainitic ferrite nucleation [
28]. Ravi et al. [
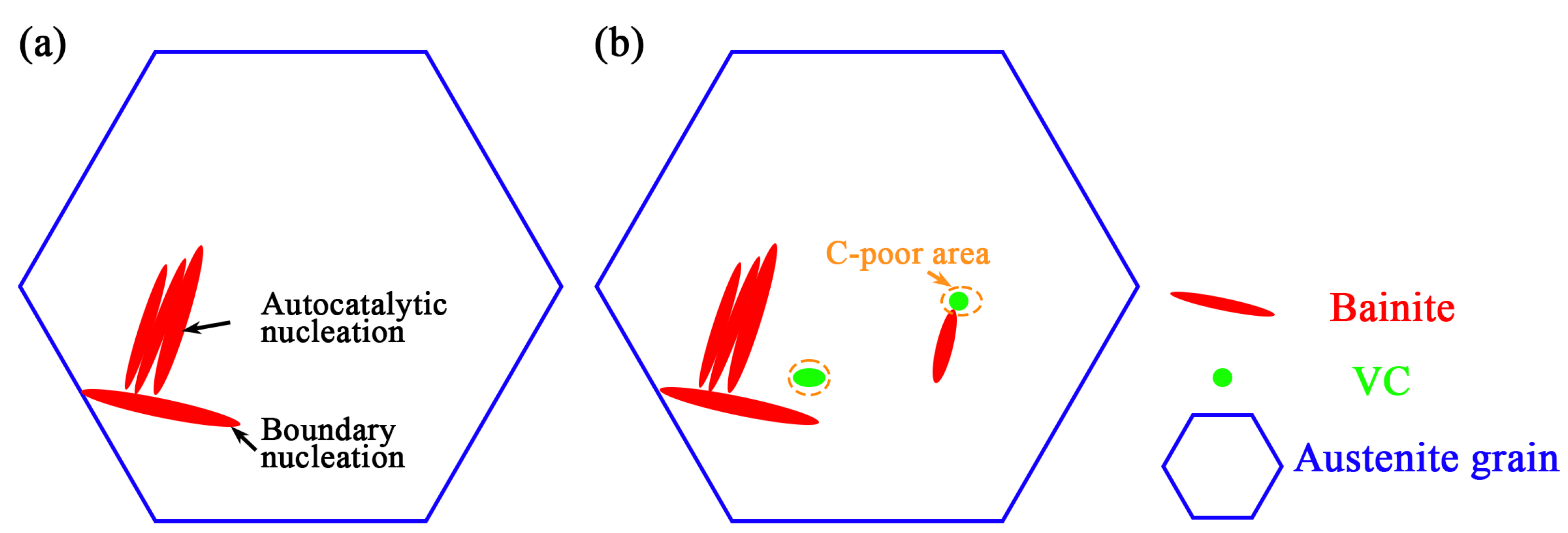
16] have shown that the nucleation ability of bainite is affected by the interface energy between the precipitates and the matrix. Before bainite isothermal treatment, the research team introduced small VC particles at the bay area to significantly accelerate the initial bainitic transformation and shorten the incubation period [
29]. VC particles consume carbon atoms, and a C-poor area forms around the VC particles. The bainitic ferrite nucleates at the VC/austenite interface, consuming the interface energy between the austenite and the VC and then producing the interface energy between the bainitic ferrite and the austenite and the interface energy between the bainitic ferrite and the VC. The precipitation of VC reduces the activation energy of bainitic ferrite and accelerates the phase transformation. The acceleration mechanism is shown in
Figure 11. In addition, the research team has studied the transformation kinetics of two kinds of medium-carbon bainite steels with different N contents by introducing AlN particles in previous reports [
30]. When the N content increases from 20 ppm to 210 ppm, bainitic transformation significantly accelerates, and the incubation time and transformation time shorten by 50% and 20%, respectively. At the same time, the transformation degree improves, and the microstructure refines. The heterogeneous nucleation sites provided by the AlN particles are the main reason for the accelerating bainite transformation.