Corrosion and Wear Properties of Cr Coating and ZrO2/Cr Bilayer Coating on Zr-4 Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Electrochemical Measurements and Wear Property Test
2.3. Characterizations
3. Results and Discussion
3.1. Morphology
3.2. Electrochemical Corrosion Measurement
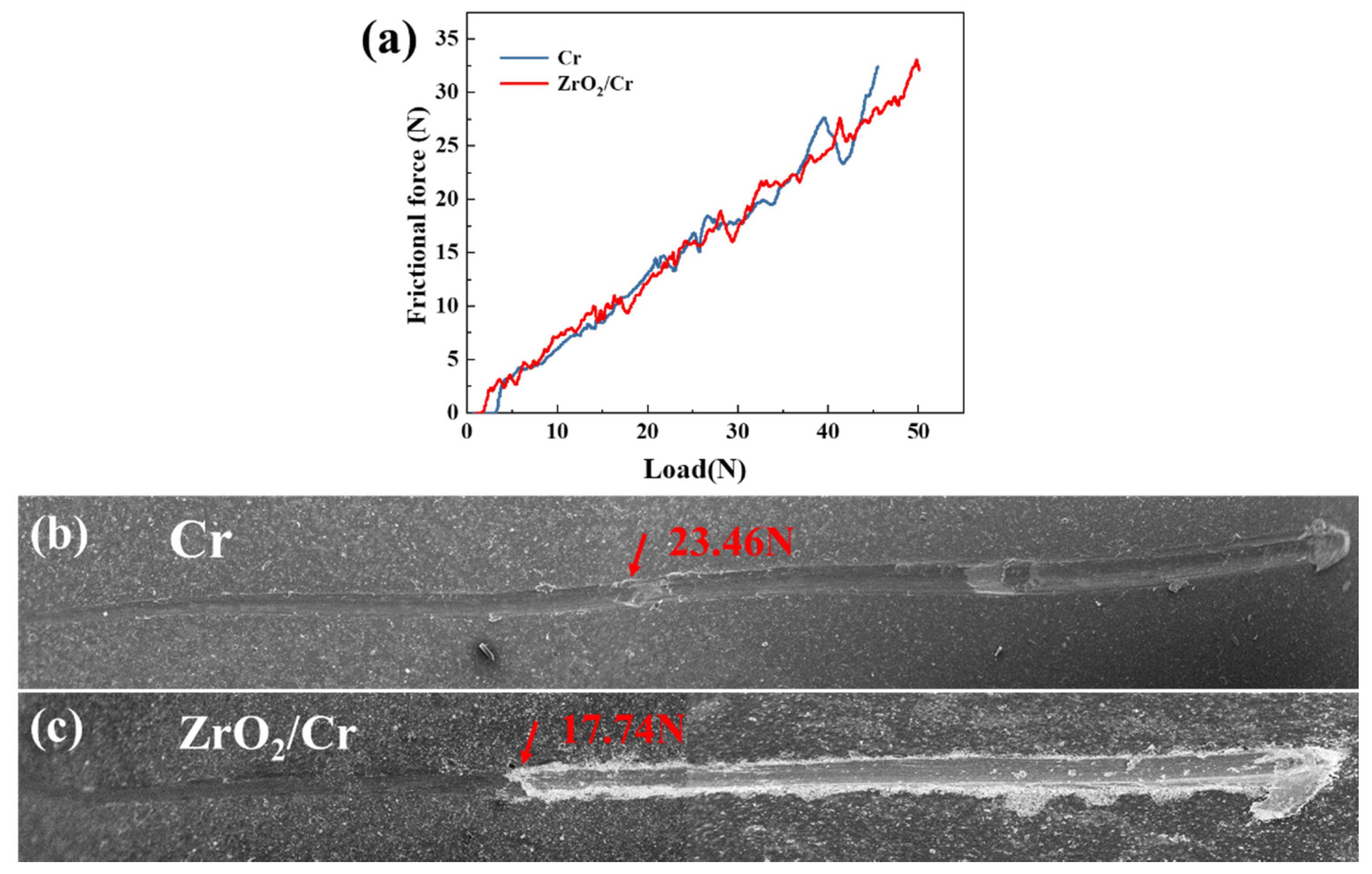
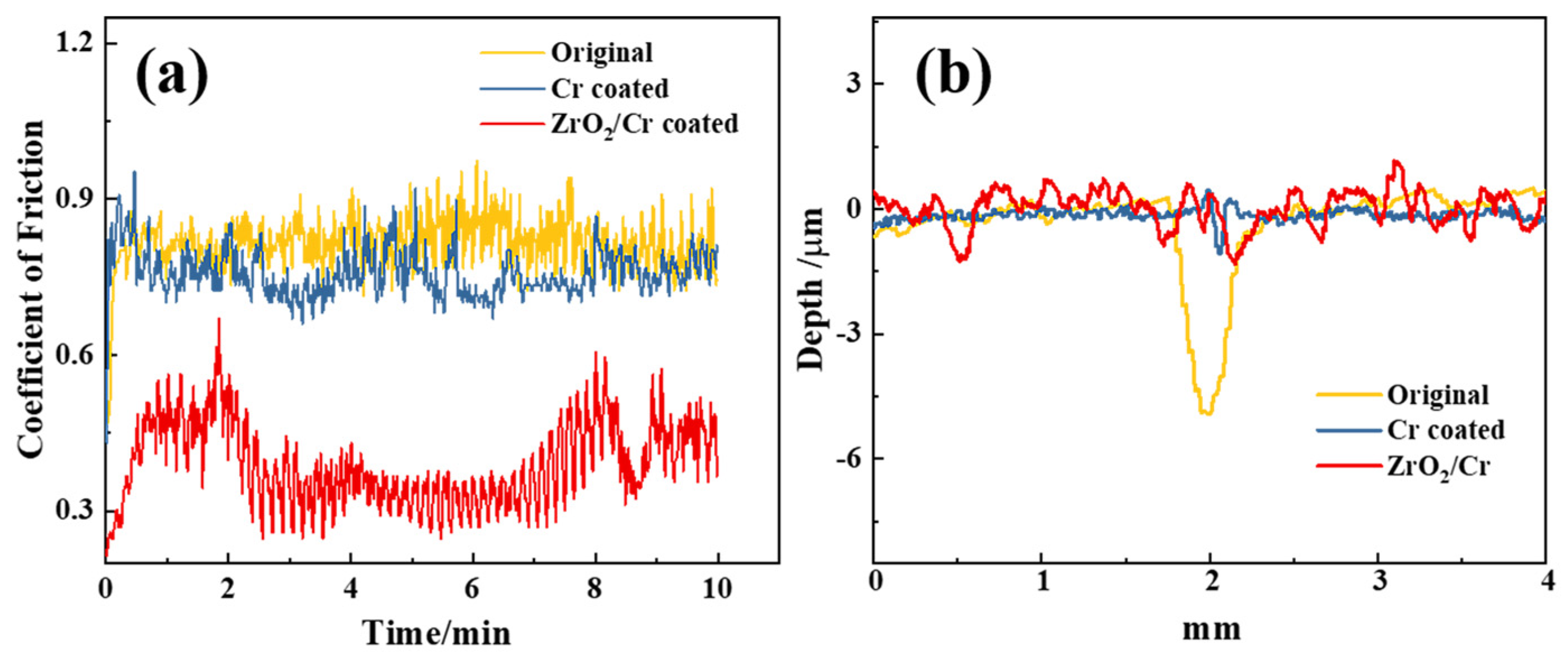
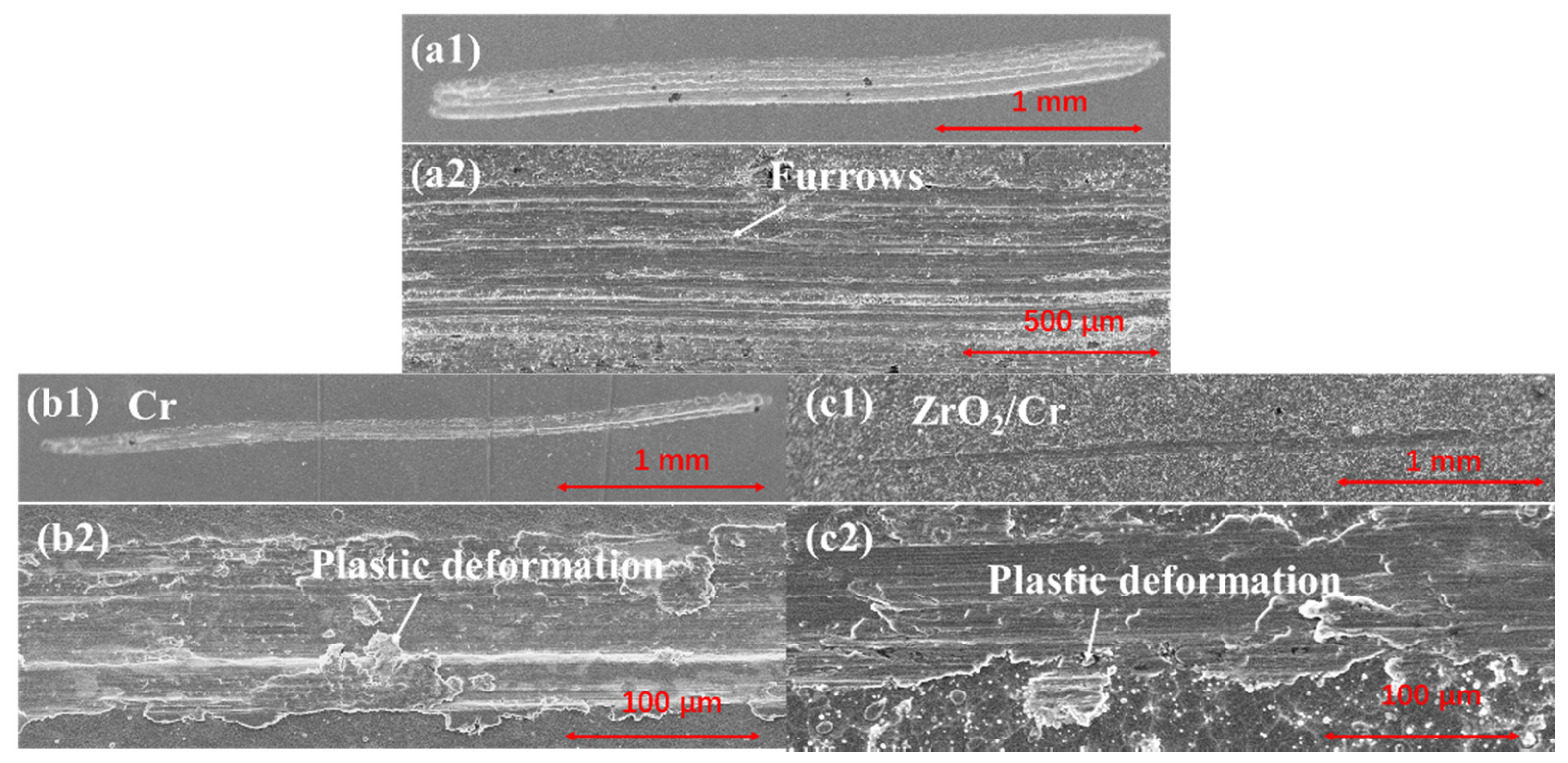
3.3. Tribological Performance
4. Conclusions
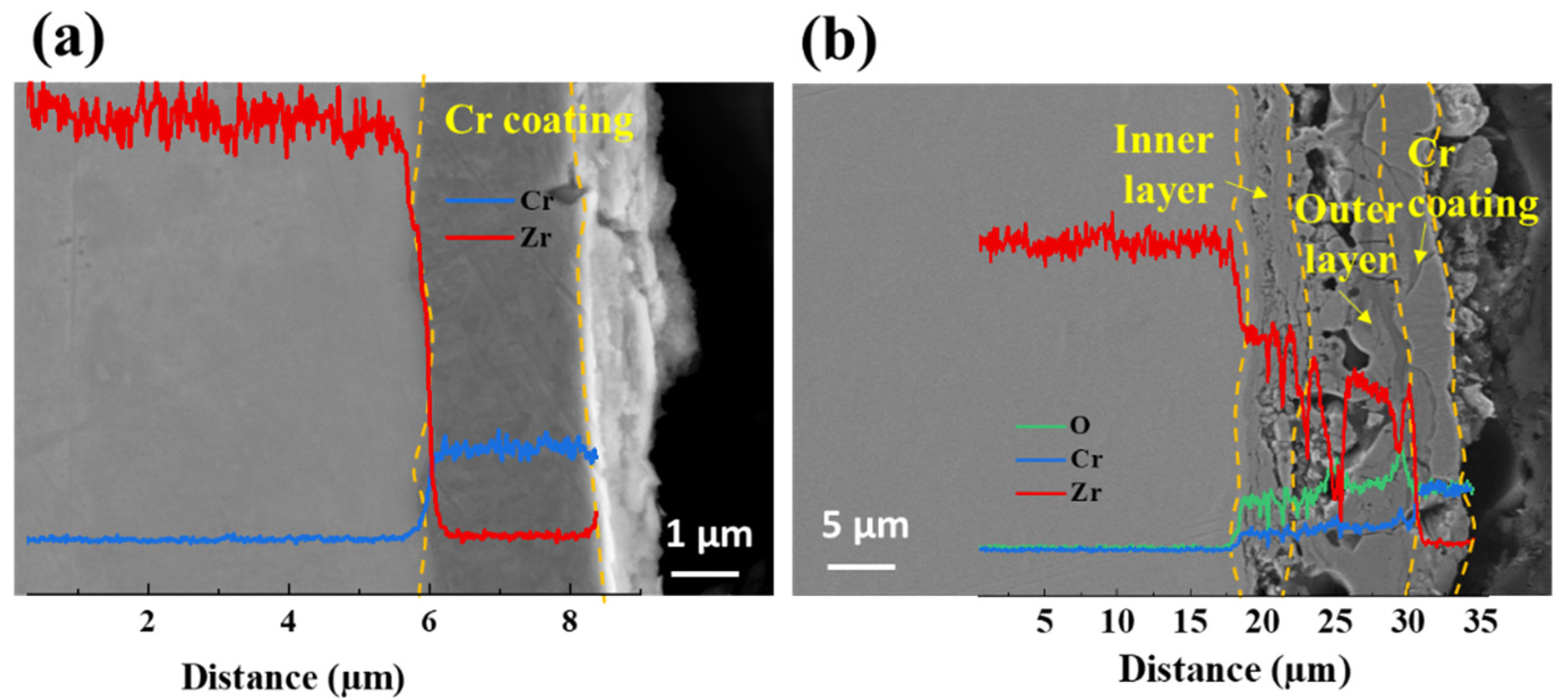
- Cr and ZrO2/Cr coatings have similar surface morphology. The more obvious surface undulates on the ZrO2/Cr coating were due to the rough surface of the PEO coating. Cross-section morphology showed that the thickness of the Cr coating was about 2.6 μm. The thickness of the ZrO2/Cr coating was about 15 ± 1.2 μm, and it was observed that Cr partly filled in the PEO coating due to the existence of micropores.
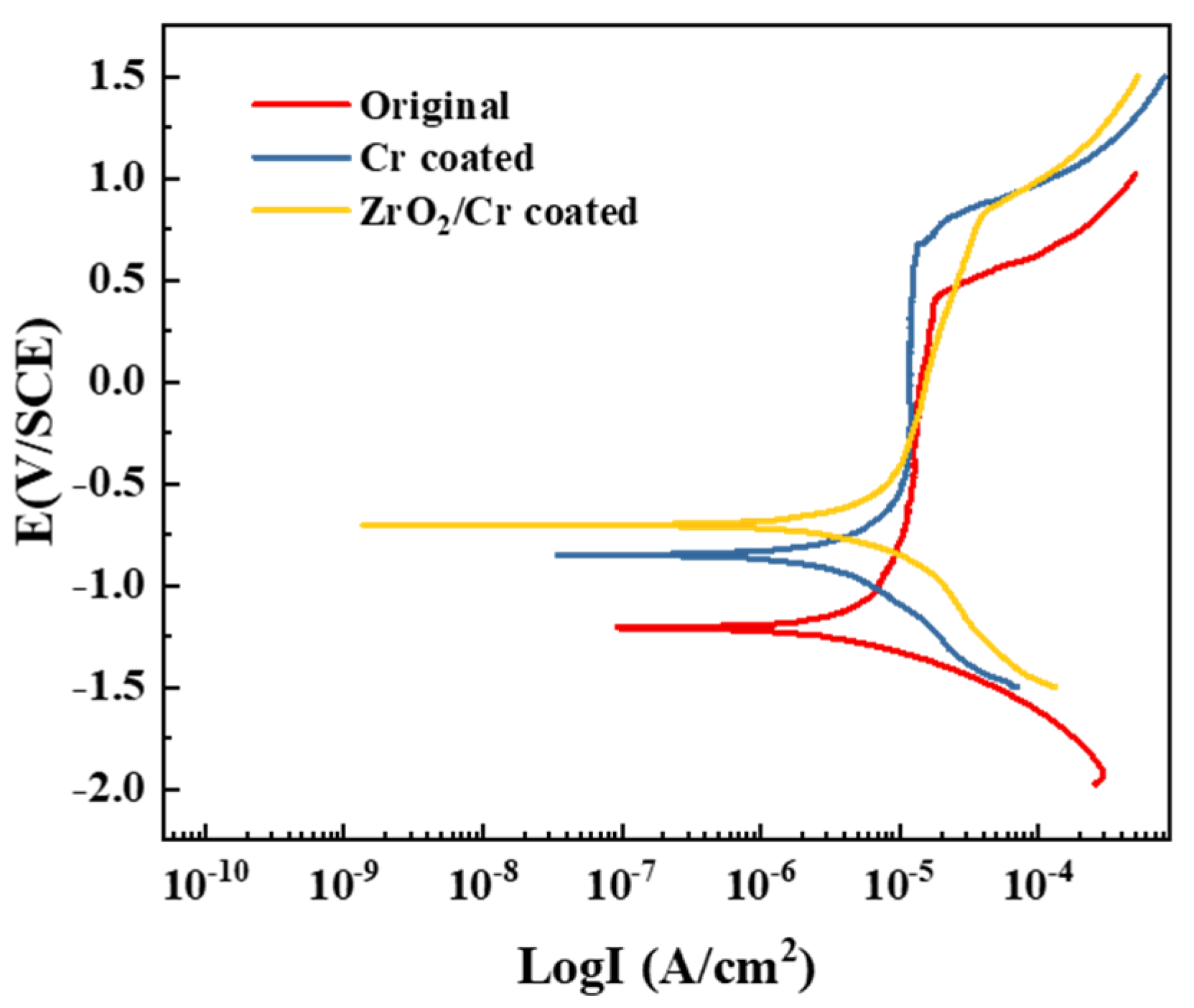
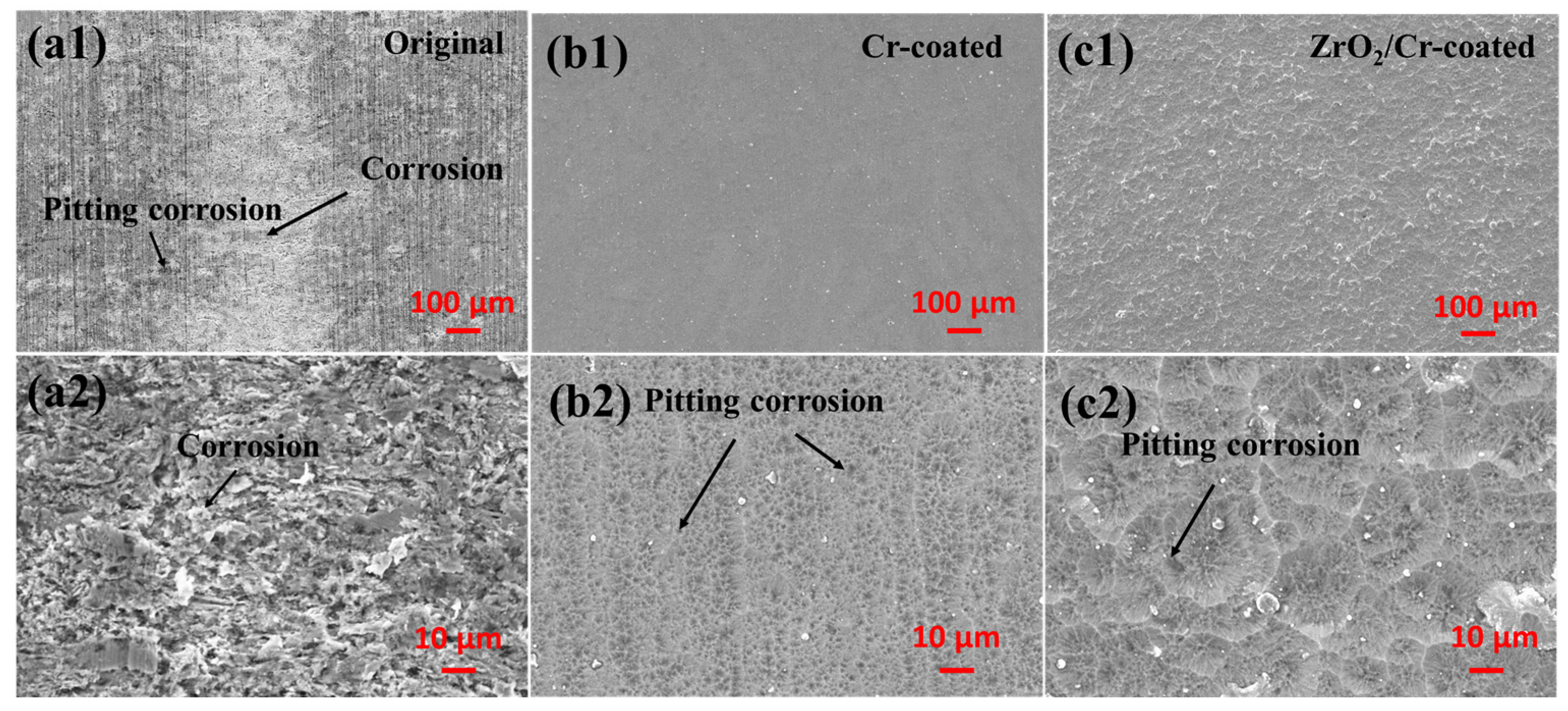
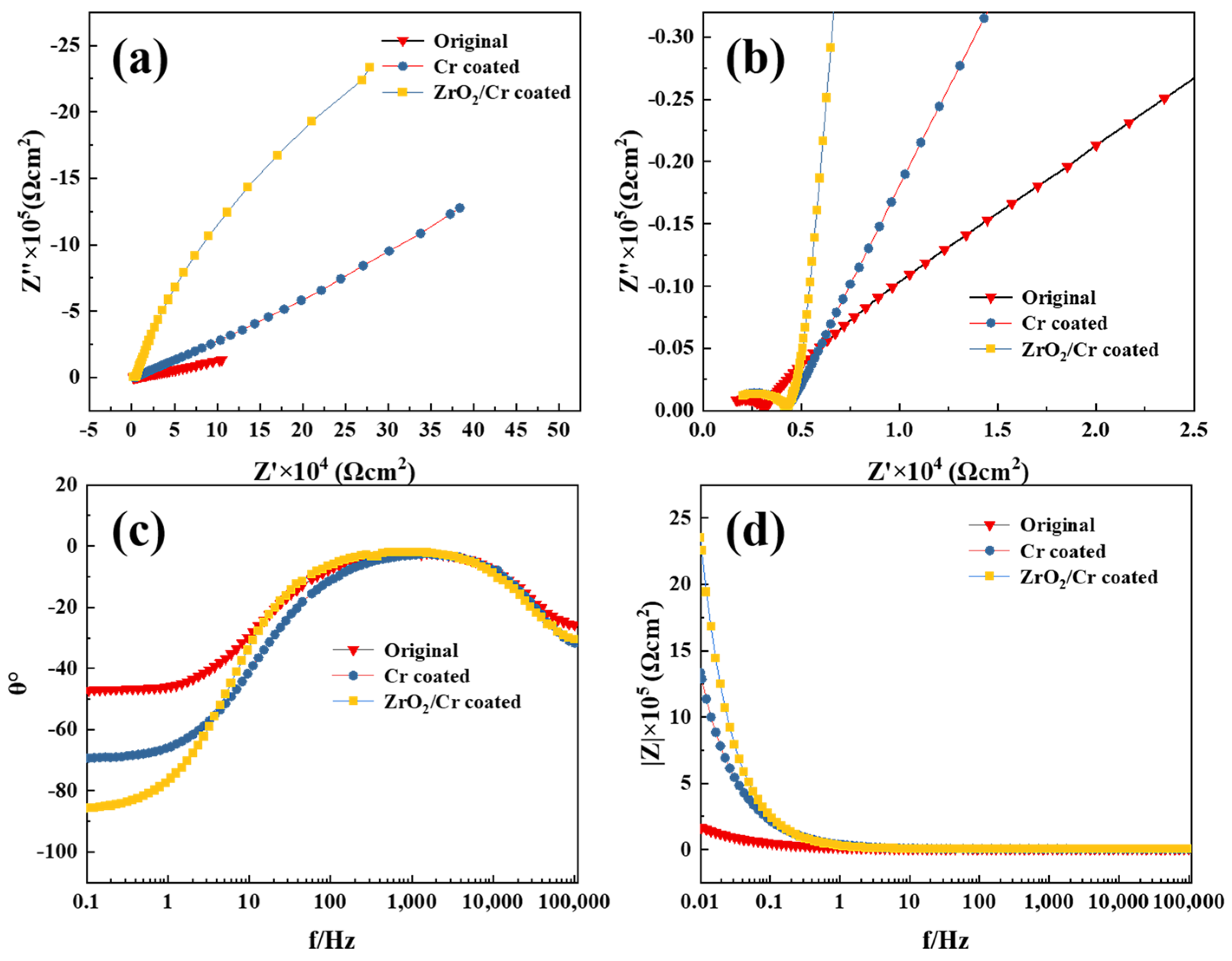
- Pitting corrosion occurred on the surface of the original and coated Zr-4 alloys during the electrochemical corrosion. The ZrO2/Cr-coated Zr-4 alloy exhibited superior corrosion resistance with an iorr value of 1.86 × 10−6 A/cm2, which is lesser than that of the Cr-coated Zr-4 alloy with an iorr value of 6.42 × 10−6 A/cm2 owing to its double-barrier construction and high thickness (15 ± 1.2 μm). The coating on the Zr-4 alloy had better corrosion resistance than that of the original Zr-4 alloy with an iorr value of 1.02 × 10−5 A/cm2.
- Critical loads of Cr and ZrO2/Cr coatings were 23.46 and 17.74 N, respectively, and the lower critical load of ZrO2/Cr coatings is ascribed to the high coating porosity of the PEO coating. The wear resistance of the coated Zr-4 alloy was enhanced due to the increase in hardness. The wear depth of the coating on the Zr-4 alloy was much lower than that of the original Zr-4 alloy, which is attributed to the change of the wear mechanism from abrasive wear to fatigue wear.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Animasaun, I.L.; Shah, N.A.; Wakif, A.; Mahanthesh, B.; Sivaraj, R.; Koriko, O.K. Ratio of Momentum Diffusivity to Thermal Diffusivity: Introduction, Meta-Analysis, and Scrutinization; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Wang, X.; Guan, H.; Liao, Y.; Zhu, M.; Xu, C.; Jin, X.; Liao, B.; Xue, W.; Zhang, Y.; Bai, G. Enhancement of high temperature steam oxidation resistance of Zr–1Nb alloy with ZrO2/Cr bilayer coating. Corros. Sci. 2021, 187, 109494. [Google Scholar] [CrossRef]
- Bischoff, J.; Delafoy, C.; Chaari, N.; Vauglin, C.; Buchanan, K.; Barberis, P.; Monsifrot, E.; Schuster, F.; Brachet, J.; Nimishakavi, K. Cr-coated cladding development at Framatome. Top Fuel 2018, 2018, A0152. [Google Scholar]
- Park, J.-H.; Kim, H.-G.; Park, J.-y.; Jung, Y.-I.; Park, D.-J.; Koo, Y.-H. High temperature steam-oxidation behavior of arc ion plated Cr coatings for accident tolerant fuel claddings. Surf. Coat. Technol. 2015, 280, 256–259. [Google Scholar] [CrossRef]
- Massey, C.P.; Terrani, K.A.; Dryepondt, S.N.; Pint, B.A. Cladding burst behavior of Fe-based alloys under LOCA. J. Nucl. Mater. 2016, 470, 128–138. [Google Scholar] [CrossRef]
- Hu, X.; Terrani, K.A.; Wirth, B.D.; Snead, L.L. Hydrogen permeation in FeCrAl alloys for LWR cladding application. J. Nucl. Mater. 2015, 461, 282–291. [Google Scholar] [CrossRef]
- Ott, L.J.; Robb, K.R.; Wang, D. Preliminary assessment of accident-tolerant fuels on LWR performance during normal operation and under DB and BDB accident conditions. J. Nucl. Mater. 2014, 448, 520–533. [Google Scholar] [CrossRef]
- Kashkarov, E.; Sidelev, D.; Syrtanov, M.; Tang, C.; Steinbrück, M. Oxidation kinetics of Cr-coated zirconium alloy: Effect of coating thickness and microstructure. Corros. Sci. 2020, 175, 108883. [Google Scholar] [CrossRef]
- Han, X.; Chen, C.; Tan, Y.; Feng, W.; Peng, S.; Zhang, H. A systematic study of the oxidation behavior of Cr coatings on Zry4 substrates in high temperature steam environment. Corros. Sci. 2020, 174, 108826. [Google Scholar] [CrossRef]
- Guo, Z.; Dailey, R.; Zhou, Y.; Sun, Z.; Wang, J.; Corradini, M.L. Effect of ATF Cr-coated-Zircaloy on BWR in-vessel accident progression during a station blackout. Nucl. Eng. Des. 2021, 372, 110979. [Google Scholar] [CrossRef]
- Yeom, H.; Maier, B.; Johnson, G.; Dabney, T.; Lenling, M.; Sridharan, K. High temperature oxidation and microstructural evolution of cold spray chromium coatings on Zircaloy-4 in steam environments. J. Nucl. Mater. 2019, 526, 151737. [Google Scholar] [CrossRef]
- McCafferty, E. Standard electrode potentials of the elements as a fundamental periodic property of atomic number. Electrochim. Acta 2007, 52, 5884–5890. [Google Scholar] [CrossRef]
- Jin, D.; Ni, N.; Guo, Y.; Zou, Z.; Wang, X.; Guo, F.; Zhao, X.; Xiao, P. Corrosion of the bonding at FeCrAl/Zr alloy interfaces in steam. J. Nucl. Mater. 2018, 508, 411–422. [Google Scholar] [CrossRef]
- Surviliene, S.; Lisowska-Oleksiak, A.; Češuniene, A. Effect of ZrO2 on corrosion behaviour of chromium coatings. Corros. Sci. 2008, 50, 338–344. [Google Scholar] [CrossRef]
- Skinner, G.B.; Johnston, H.L. Thermal expansion of zirconium between 298 K and 1600 K. J. Chem. Phys. 1953, 21, 1383–1384. [Google Scholar] [CrossRef]
- Patil, R.; Subbarao, E. Axial thermal expansion of ZrO2 and HfO2 in the range room temperature to 1400 °C. J. Appl. Crystallogr. 1969, 2, 281–288. [Google Scholar] [CrossRef]
- Dubrovinskaia, N.; Dubrovinsky, L.; Saxena, S.; Sundman, B. Thermal expansion of chromium (Cr) to melting temperature. Calphad 1997, 21, 497–508. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Han, X.; Feng, W.; Zhou, X.; Peng, S.; Zhang, H. Oxidation resistance improvement of Zr-4 alloy in 1000 °C steam environment using ZrO2/FeCrAl bilayer coating. Surf. Coat. Technol. 2018, 349, 807–815. [Google Scholar] [CrossRef]
- Wang, X.; Liao, Y.; Xu, C.; Guan, H.; Zhu, M.; Gao, C.; Jin, X.; Pang, P.; Du, J.; Liao, B. Steam oxidation behavior of ZrO2/Cr-coated pure zirconium prepared by plasma electrolytic oxidation followed by filtered cathodic vacuum arc deposition. J. Alloy. Compd. 2021, 883, 160798. [Google Scholar] [CrossRef]
- Blau, P.J. A multi-stage wear model for grid-to-rod fretting of nuclear fuel rods. Wear 2014, 313, 89–96. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Cai, Z.-B.; Ding, Y.; Cui, X.-J.; Yang, Z.-B.; Zhu, M.-H. Characterization of graphene oxide/ZrO2 composite coatings deposited on zirconium alloy by micro-arc oxidation. Appl. Surf. Sci. 2020, 506, 144928. [Google Scholar] [CrossRef]
- Li, Z.-W.; Di, S.-C. Effect of anode pulse-width on the microstructure and wear resistance of microarc oxidation coatings. Metals 2017, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Lv, K.; Du, Z.; Chen, W.; Ji, P.; Wang, M. Effects of graphene on the wear and corrosion resistance of micro-arc oxidation coating on a titanium alloy. Metals 2021, 12, 70. [Google Scholar] [CrossRef]
- Arunnellaiappan, T.; Arun, S.; Hariprasad, S.; Gowtham, S.; Ravisankar, B.; Rameshbabu, N. Fabrication of corrosion resistant hydrophobic ceramic nanocomposite coatings on PEO treated AA7075. Ceram. Int. 2018, 44, 874–884. [Google Scholar]
- Wu, J.; Lu, P.; Dong, L.; Zhao, M.; Li, D.; Xue, W. Combination of plasma electrolytic oxidation and pulsed laser deposition for preparation of corrosion-resisting composite film on zirconium alloys. Mater. Lett. 2020, 262, 127080. [Google Scholar] [CrossRef]
- Zou, Z.; Xue, W.; Jia, X.; Du, J.; Wang, R.; Weng, L. Effect of voltage on properties of microarc oxidation films prepared in phosphate electrolyte on Zr–1Nb alloy. Surf. Coat. Technol. 2013, 222, 62–67. [Google Scholar] [CrossRef]
- Rosalbino, F.; Macciò, D.; Giannoni, P.; Quarto, R.; Saccone, A. Study of the in vitro corrosion behavior and biocompatibility of Zr-2.5 Nb and Zr-1.5 Nb-1Ta (at%) crystalline alloys. J. Mater. Sci. Mater. Med. 2011, 22, 1293–1302. [Google Scholar] [CrossRef]
- Sharifi, H.; Aliofkhazraei, M.; Darband, G.B.; Shrestha, S. A review on adhesion strength of peo coatings by scratch test method. Surf. Rev. Lett. 2018, 25, 1830004. [Google Scholar] [CrossRef]
- Arun, S.; Arunnellaiappan, T.; Rameshbabu, N. Fabrication of the nanoparticle incorporated PEO coating on commercially pure zirconium and its corrosion resistance. Surf. Coat. Technol. 2016, 305, 264–273. [Google Scholar] [CrossRef]
- Bischoff, J.; Delafoy, C.; Vauglin, C.; Barberis, P.; Roubeyrie, C.; Perche, D.; Duthoo, D.; Schuster, F.; Brachet, J.-C.; Schweitzer, E.W. AREVA NP’s enhanced accident-tolerant fuel developments: Focus on Cr-coated M5 cladding. Nucl. Eng. Technol. 2018, 50, 223–228. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, F.; Dong, J.; Wu, X.; Xue, Z.; Matykina, E.; Skeldon, P.; Thompson, G. Comparison of plasma electrolytic oxidation of zirconium alloy in silicate-and aluminate-based electrolytes and wear properties of the resulting coatings. Electrochim. Acta 2012, 85, 25–32. [Google Scholar] [CrossRef]
- Reed, B.; Wang, R.; Lu, R.Y.; Qu, J. Autoclave grid-to-rod fretting wear evaluation of a candidate cladding coating for accident-tolerant fuel. Wear 2021, 466, 203578. [Google Scholar] [CrossRef]
- Brachet, J.-C.; Idarraga-Trujillo, I.; Le Flem, M.; Le Saux, M.; Vandenberghe, V.; Urvoy, S.; Rouesne, E.; Guilbert, T.; Toffolon-Masclet, C.; Tupin, M. Early studies on Cr-Coated Zircaloy-4 as enhanced accident tolerant nuclear fuel claddings for light water reactors. J. Nucl. Mater. 2019, 517, 268–285. [Google Scholar] [CrossRef]



| Material | Zr [15] 25–870 °C | ZrO2 [16] 25–1040 °C | Cr [17] 25–1020 °C | |
|---|---|---|---|---|
| Property | ||||
| thermal expansion coefficient (×10−6/K) | 5.5 (a axis) | 10.3 (a axis) | 16.6 | |
| Sample | Ecorr (V/SCE) | icorr (A/cm2) |
|---|---|---|
| Original Zr-4 alloy | −1.216 | 1.02 × 10−5 |
| Cr-coated Zr-4 alloy | −0.847 | 6.42 × 10−6 |
| ZrO2/Cr-coated Zr-4 alloy | −0.702 | 1.86 × 10−6 |
| Sample | Rs (Ω cm2) | Cdl (Ω−1·cm2·s−n) | n | Rct (Ω cm2) | Cc (Ω−1·cm2·s−n) | N | Rc (Ω cm2) | Cp (Ω−1·cm2·s−n) | n | Rp (Ω cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Original | 1177 | 2.73 × 10−5 | 0.61 | 9.25 × 104 | - | - | - | - | - | - |
| Cr-coated | 1066 | 6.66 × 10−6 | 0.79 | 9.48 × 108 | 2.36 × 10−7 | 0.92 | 3180 | - | - | - |
| ZrO2/Cr-coated | 1109 | 6.18 × 10−6 | 0.97 | 3.39 × 107 | 3.5 × 10−9 | 0.89 | 3949 | 1.54 × 10−6 | 0.77 | 1.61 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Qiu, L.; Hu, X.; Jiang, H. Corrosion and Wear Properties of Cr Coating and ZrO2/Cr Bilayer Coating on Zr-4 Alloy. Coatings 2022, 12, 1281. https://doi.org/10.3390/coatings12091281
Pan X, Qiu L, Hu X, Jiang H. Corrosion and Wear Properties of Cr Coating and ZrO2/Cr Bilayer Coating on Zr-4 Alloy. Coatings. 2022; 12(9):1281. https://doi.org/10.3390/coatings12091281
Chicago/Turabian StylePan, Xiaolong, Longshi Qiu, Xiaogang Hu, and Haixia Jiang. 2022. "Corrosion and Wear Properties of Cr Coating and ZrO2/Cr Bilayer Coating on Zr-4 Alloy" Coatings 12, no. 9: 1281. https://doi.org/10.3390/coatings12091281
APA StylePan, X., Qiu, L., Hu, X., & Jiang, H. (2022). Corrosion and Wear Properties of Cr Coating and ZrO2/Cr Bilayer Coating on Zr-4 Alloy. Coatings, 12(9), 1281. https://doi.org/10.3390/coatings12091281






