Identification and Analysis of Corrosion Mechanisms for Ground Pipelines with Hanging Rings
Abstract
:1. Introduction
2. Field Background
3. Experimental
3.1. Materials
3.2. Water Quality Analysis of Produced Liquids
3.3. Characterization of Corrosion Products
3.4. Evaluation of Corrosion Inhibitors
4. Results
4.1. Water Ion Analysis
4.2. Macro-Morphology Analysis of the Hanging Rings
4.3. Micro-Morphology Analysis of the Hanging Rings
4.3.1. Type I
4.3.2. Type II
4.3.3. Type III
4.4. Compositional Analysis of the Corrosion Product Layer
4.5. Evaluation of Corrosion Inhibitors
4.5.1. Open Circuit Potential Tests
4.5.2. Polarization Curve Tests
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biezma, M.V.; Andrés, M.A.; Agudo, D.; Briz, E. Most fatal oil and gas pipeline accidents through history: A lessons learned approach. Eng. Fail. Anal. 2020, 110, 104446. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Shi, B.; Zhu, C.; Wang, Z. Optimization and intelligent control for operation parameters of multiphase mixture transportation pipeline in oilfield: A case study. J. Pipeline Sci. Eng. 2021, 1, 367–378. [Google Scholar] [CrossRef]
- Aleksakhin, A.; Sala, D.; Golovin, K.; Kovalev, R. Reducing energy costs for pipeline transportation. Transp. Res. Procedia 2021, 57, 24–32. [Google Scholar] [CrossRef]
- Kazemi, A.; Shariat, M.H.; Hashemi, B. Investigation on corrosion behaviors of different Zones of dissimilarly welded A312TP316L and A106Gr.B in the brine solution containing H2S and CO2. J. Bio-Tribo-Corros. 2021, 7, 132. [Google Scholar] [CrossRef]
- Elgaddafi, R.; Ahmed, R.; Shah, S. Corrosion of carbon steel in CO2 saturated brine at elevated temperatures. J. Pet. Sci. Eng. 2020, 196, 107638. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, H.; Luo, J.L. Unraveling the effects of CO2 and H2S on the corrosion behavior of electroless Ni-P coating in CO2/H2S/Cl− environments at high temperature and high pressure. Corros. Sci. 2019, 148, 317–330. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Z.M.; Zheng, D.; Song, G.-L. The localized corrosion of mild steel in carbonated cement pore solution under supercritical carbon-dioxide in a simulated geothermal environment. Constr. Build. Mater. 2021, 274, 122035. [Google Scholar] [CrossRef]
- Abdar, P.S.; Hariri, M.B.; Kahyarian, A.; Nesic, S. A Revision of Mechanistic Modeling of Mild Steel Corrosion in H2S Environments. Electrochim. Acta 2021, 382, 138231. [Google Scholar] [CrossRef]
- Ren, C.; Liu, D.; Bai, Z.; Li, T. Corrosion behavior of oil tube steel in simulant solution with hydrogen sulfide and carbon dioxide. Mater. Chem. and Phys. 2005, 93, 305–309. [Google Scholar] [CrossRef]
- Dong, B.; Liu, W.; Zhang, Y.; Banthukul, W.; Zhao, Y.; Zhang, T.; Fan, Y.; Li, X. Comparison of the characteristics of corrosion scales covering 3Cr steel and X60 steel in CO2-H2S coexistence environment. J. Nat. Gas Sci. Eng. 2020, 80, 103371. [Google Scholar] [CrossRef]
- Zeng, D.; Dong, B.; Zeng, F.; Yu, Z.; Zeng, W.; Guo, Y.; Peng, Z.; Tao, Y. Analysis of corrosion failure and materials selection for CO2-H2S gas well. J. Nat. Gas Sci. Eng. 2021, 86, 103734. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Ming, N.; He, C. Effects of temperature on the corrosion behaviour of X70 steel in CO2-containing formation water. J. Nat. Gas Sci. Eng. 2021, 88, 103815. [Google Scholar] [CrossRef]
- Lazareva, A.; Owen, J.; Vargas, S.; Barker, R.; Neville, A. Investigation of the evolution of an iron carbonate layer and its effect on localized corrosion of X65 carbon steel in CO2 corrosion environments. Corros. Sci. 2021, 192, 109849. [Google Scholar] [CrossRef]
- Peng, Z.; Lv, F.; Feng, Q.; Zheng, Y. Enhancing the CO2-H2S corrosion resistance of oil well cement with a modified epoxy resin. Constr. Build. Mater. 2022, 326, 126854. [Google Scholar] [CrossRef]
- Anijdan, S.H.M.; Arab, G.; Sabzi, M.; Sadeghi, M.; Eivani, A.R.; Jafarian, H.R. Sensitivity to hydrogen induced cracking, and corrosion performance of an APIX65 pipeline steel in H2S containing environment: Influence of heat treatment and its subsequent microstructural changes. J. Mater. Res. Technol. 2021, 15, 1–16. [Google Scholar] [CrossRef]
- Avelino, A.F.; Araújo Walney, S.; Dias, D.F.; Santos, L.P.M.; Correia, A.N.; Lima-Neto, P. Corrosion investigation of the 18Ni 300 grade maraging steel in aqueous chloride medium containing H2S and CO2. Electrochim. Acta 2018, 286, 339–349. [Google Scholar] [CrossRef]
- Asadian, M.; Sabzi, M.; Anijdan, S.H. The effect of temperature, CO2, H2S gases and the resultant iron carbonate and iron sulfide compounds on the sour corrosion behaviour of ASTM A-106 steel for pipeline transportation. Int. J. Press. Vessel. Pip. 2019, 171, 184–193. [Google Scholar] [CrossRef]
- Silva, S.C.; Silva, A.B.; Gomes, J.A.C.P. Hydrogen embrittlement of API 5L X65 pipeline steel in CO2 containing low H2S concentration environment. Eng. Fail. Anal. 2021, 120, 105081. [Google Scholar] [CrossRef]
- Zeng, D.; Zeng, F.; Peng, Z.; Yu, Z.; Tao, Y.; Zeng, W.; Guo, Y. Fracture failure analysis of the sacrificial anode protector in a water injection well. Eng. Fail. Anal. 2020, 112, 104479. [Google Scholar] [CrossRef]
- Li, L.; Yan, J.; Xiao, J.; Sun, L.; Fan, H.; Wang, J. A comparative study of corrosion behavior of S-phase with AISI 304 austenitic stainless steel in H2S/CO2/Cl− media. Corros. Sci. 2021, 187, 109472. [Google Scholar] [CrossRef]
- Hua, Y.; Xu, S.; Wang, Y.; Taleb, W.; Sun, J.; Zhang, L.; Barker, R.; Neville, A. The formation of FeCO3 and Fe3O4 on carbon steel and their protective capabilities against CO2 corrosion at elevated temperature and pressure. Corros. Sci. 2019, 157, 392–405. [Google Scholar] [CrossRef]
- Abadeh, H.K.; Javidi, M. Assessment and influence of temperature, NaCl and H2S on CO2 corrosion behavior of different microstructures of API 5L X52 carbon steel in aqueous environments. J. Nat. Gas Sci. Eng. 2019, 67, 93–107. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, X.; Du, L.; Li, J.; Li, P.; Yu, C.; Misra, R.D.K.; Wang, Y. Comparison of corrosion behaviour of low-alloy pipeline steel exposed to H2S/CO2-saturated brine and vapour-saturated H2S/CO2 environments. Electrochim. Acta 2017, 232, 528–541. [Google Scholar] [CrossRef]
- Chen, C.; Li, C.; Reniers, G.; Yang, F. Safety and security of oil and gas pipeline transportation: A systematic analysis of research trends and future needs using WoS. J. Clean. Prod. 2021, 279, 123583. [Google Scholar] [CrossRef]
- Alamri, A.H. Localized corrosion and mitigation approach of steel materials used in oil and gas pipelines—An overview. Eng. Fail. Anal. 2020, 116, 104735. [Google Scholar] [CrossRef]
- Tang, S.; Zhu, C.; Cui, G.; Xing, X.; Mu, J.; Li, Z. Analysis of internal corrosion of supercritical CO2 pipeline. Corros. Rev. 2021, 39, 1–3. [Google Scholar] [CrossRef]
- Li, K.; Zeng, Y.; Luo, J.-L. Influence of H2S on the general corrosion and sulfide stress cracking of pipelines steels for supercritical CO2 transportation. Corros. Sci. 2021, 190, 109639. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, L.; Li, D.; Lu, M.; Xue, J.; Zhong, W. Corrosion and stress corrosion cracking behavior of 316 L austenitic stainless steel in high H2S-CO2-Cl− environment. J. Mater. Sci. 2013, 48, 3708–3715. [Google Scholar] [CrossRef]
- Li, J.; Zhong, X.; Wang, T.; Shang, T.; Hu, J.; Zhang, Z.; Zeng, D.; Hou, D.; Shi, T. Synergistic effect of erosion and hydrogen on properties of passive film on 2205 duplex stainless steel. J. Mater. Sci. Technol. 2021, 67, 1–10. [Google Scholar] [CrossRef]
- Keitelman, A.; Alvarez, M. 40 Years of J.R. Galvele’s Localized Acidification Pitting Model: Past, Present, and Future. Corrosion 2017, 73, 8–17. [Google Scholar] [CrossRef]
- BinMerdhah, A.B. Inhibition of barium sulfate scale at high-barium formation water. J. Pet. Sci. Eng. 2012, 90–91, 124–130. [Google Scholar] [CrossRef]
- Wang, H.; Hu, J.; Yang, Z.; Yin, Z.; Xiong, Q.; Zhong, X. The study of a highly efficient and environment-friendly scale inhibitor for calcium carbonate scale in oil fields. Petroleum 2021, 7, 325–334. [Google Scholar] [CrossRef]
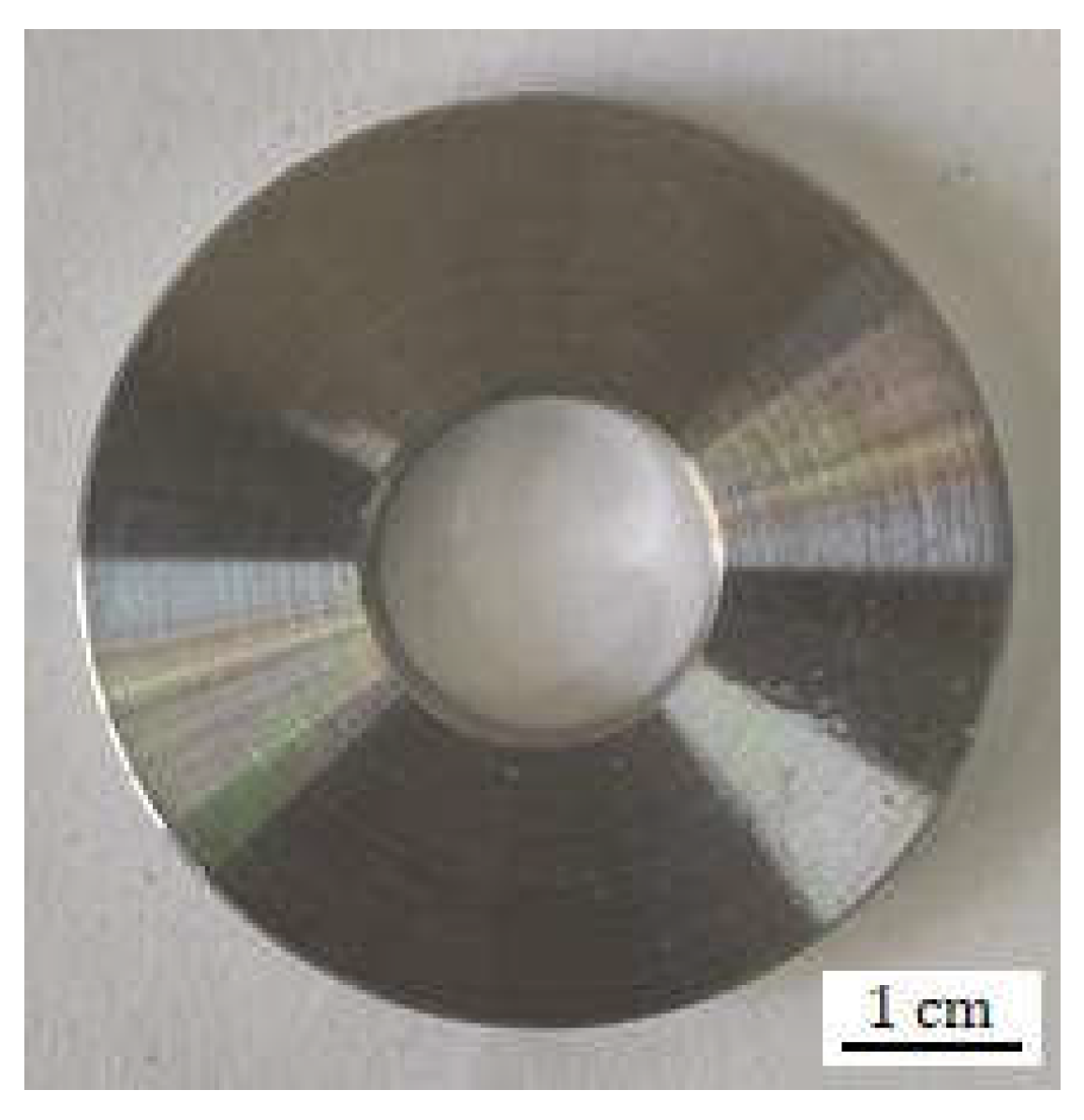


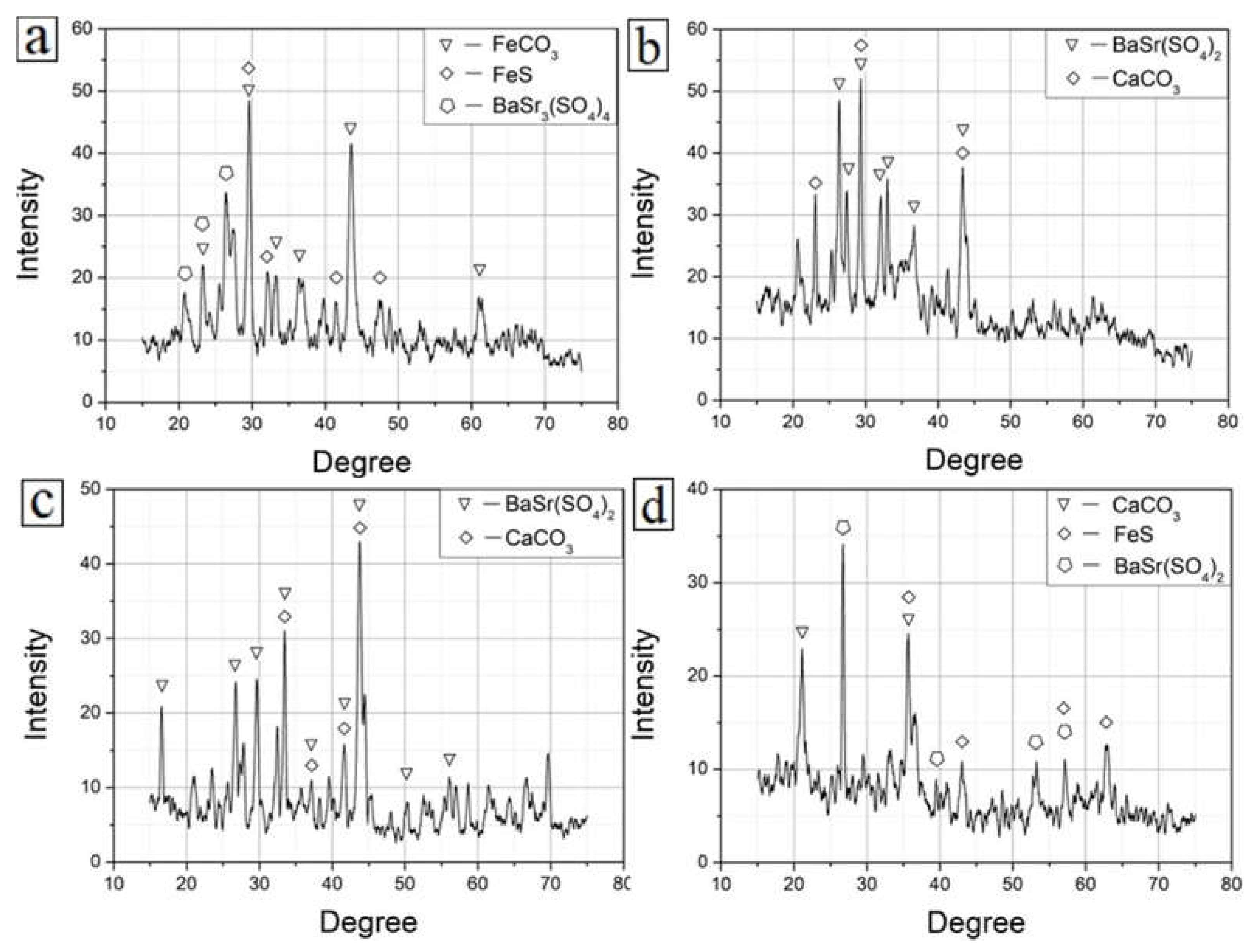
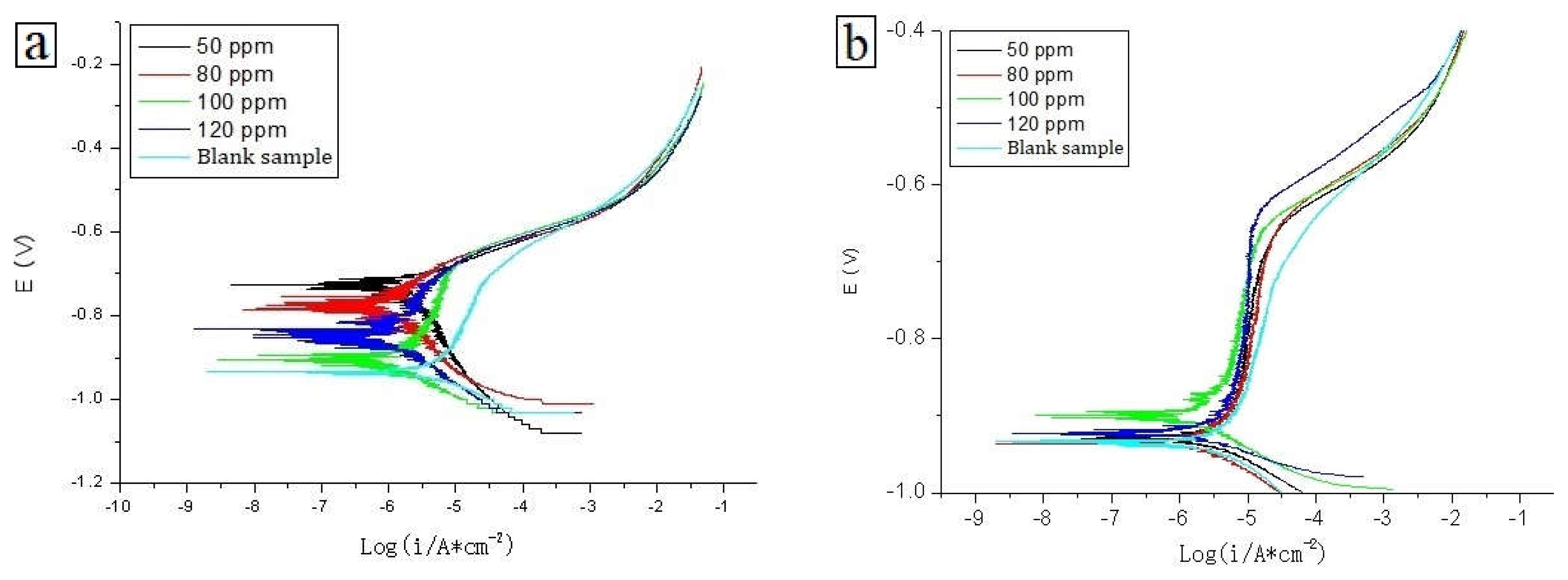
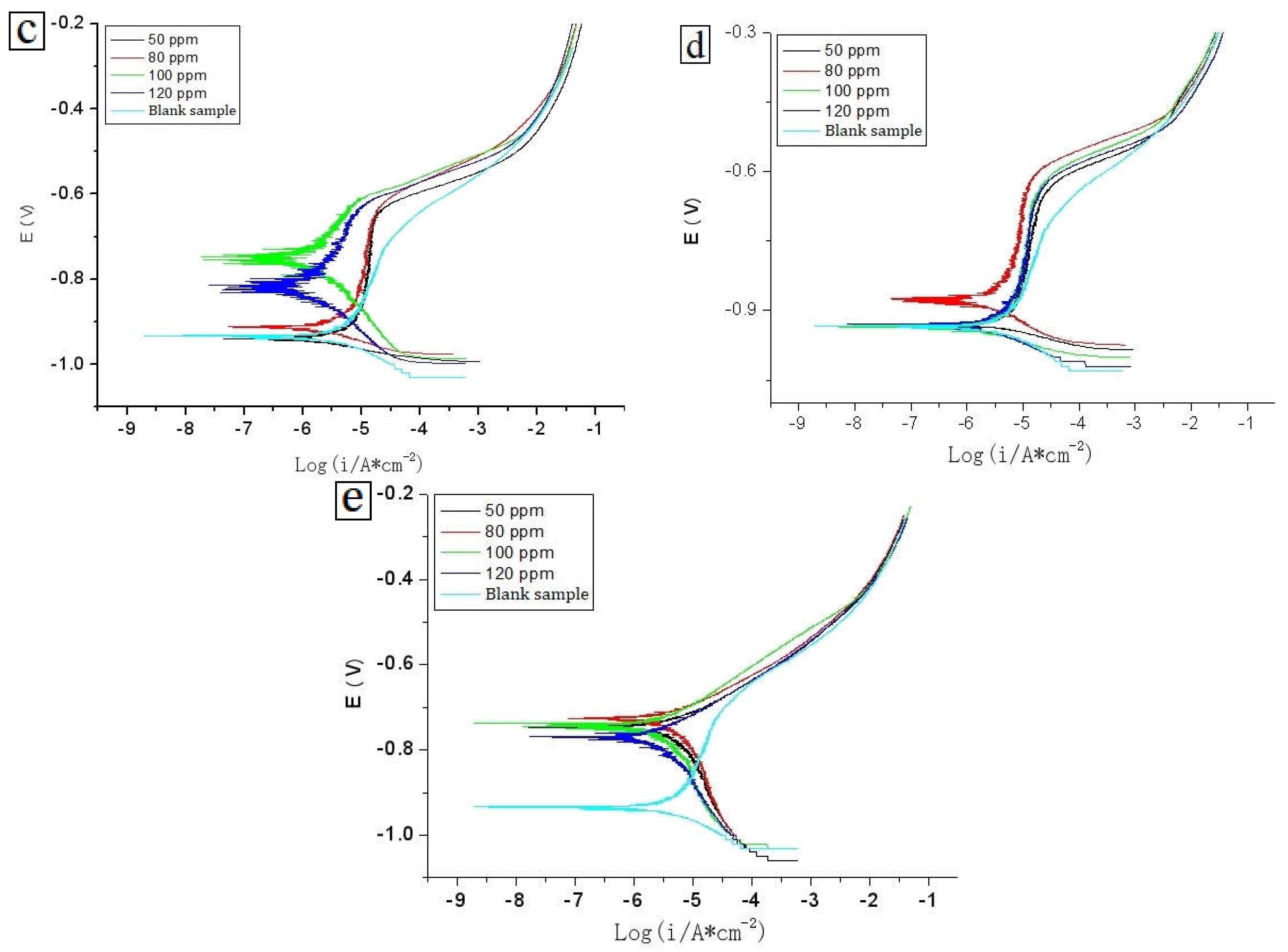
| C | Si | Mn | P | S | Cr | Mo | Ni | Cu | B | Co | Nb | Ti | V | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.20 | 0.22 | 0.49 | 0.02 | 0.02 | 0.02 | <0.01 | 0.03 | 0.04 | <0.001 | 0.006 | <0.001 | <0.001 | <0.005 | 98.94 |
| Sample Position | Ba2+ | Ca2+ | Na+ + K+ | SO42− | HCO3− | Cl− | Water Type |
|---|---|---|---|---|---|---|---|
| 1 | 707.36 | 64.12 | 21,612.92 | 31.28 | 617.43 | 34,093.68 | Sodium bicarbonate |
| 2 | 1019.06 | 268.50 | 30,669.44 | 285.30 | 791.37 | 47,050.94 | Sodium bicarbonate |
| 3 | 278.35 | 40.79 | 20,021.06 | 862.16 | 1064.8 | 29,829.90 | Sodium bicarbonate |
| 4 | 170.79 | 78.11 | 4900.42 | 599.25 | 942.74 | 7465.77 | Sodium sulphate |
| 5 | 932.95 | 238.04 | 17,400.86 | 426.39 | 661.24 | 27,075.86 | Calcium chloride |
| 6 | 906.18 | 275.24 | 16,042.01 | 1807.12 | 539.09 | 24,056.37 | Sodium sulphate |
| 7 | 912.55 | 286.40 | 18,114.96 | 199.20 | 462.07 | 27,971.75 | Calcium chloride |
| 8 | 274.86 | 122.36 | 8987.88 | 436.92 | 647.96 | 15,130.63 | Calcium chloride |
| 9 | 1164.91 | 409.14 | 30,638.20 | 177.30 | 374.44 | 48,411.37 | Calcium chloride |
| Position | Element | Weight Percentage | Atomic Percentage |
|---|---|---|---|
| #1 | C | 37.02 | 45.93 |
| O | 56.08 | 52.24 | |
| S | 2.37 | 1.10 | |
| Ca | 0.30 | 0.11 | |
| Sr | 2.61 | 0.44 | |
| Ba | 1.62 | 0.18 | |
| #4 | C | 7.99 | 11.97 |
| O | 73.09 | 82.17 | |
| S | 5.60 | 3.14 | |
| Cl | 1.19 | 0.61 | |
| Sr | 7.06 | 1.45 | |
| Ba | 5.06 | 0.66 | |
| #5 | C | 21.73 | 27.39 |
| O | 76.12 | 72.02 | |
| Fe | 2.15 | 0.58 | |
| #8 | C | 17.97 | 23.12 |
| O | 78.65 | 75.95 | |
| Fe | 3.38 | 0.94 |
| Open Circuit Potential/mV | HHS08-1# | HHS08-3# | PD43-1# | RX-21# | YD-03# | |
|---|---|---|---|---|---|---|
| Concentration/ppm | ||||||
| 0 | −654.903 | |||||
| 50 | −683.579 | −635.609 | −591.592 | −584.503 | −676.496 | |
| 80 | −609.474 | −632.350 | −576.529 | −572.260 | −665.889 | |
| 100 | −646.365 | −611.680 | −586.034 | −618.911 | −644.755 | |
| 120 | −673.752 | −577.737 | −596.989 | −635.958 | −671.883 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, Y.; Li, Y.; Yao, Y.; Gan, Q.; Wang, Y.; Wang, L.; Wen, L.; Li, S.; Yang, D.; Ji, J.; et al. Identification and Analysis of Corrosion Mechanisms for Ground Pipelines with Hanging Rings. Coatings 2022, 12, 1257. https://doi.org/10.3390/coatings12091257
Xi Y, Li Y, Yao Y, Gan Q, Wang Y, Wang L, Wen L, Li S, Yang D, Ji J, et al. Identification and Analysis of Corrosion Mechanisms for Ground Pipelines with Hanging Rings. Coatings. 2022; 12(9):1257. https://doi.org/10.3390/coatings12091257
Chicago/Turabian StyleXi, Yuntao, Yidi Li, Yang Yao, Qingming Gan, Yixu Wang, Lei Wang, Lei Wen, Shilei Li, Daoyong Yang, Jiangtao Ji, and et al. 2022. "Identification and Analysis of Corrosion Mechanisms for Ground Pipelines with Hanging Rings" Coatings 12, no. 9: 1257. https://doi.org/10.3390/coatings12091257
APA StyleXi, Y., Li, Y., Yao, Y., Gan, Q., Wang, Y., Wang, L., Wen, L., Li, S., Yang, D., Ji, J., & Lei, S. (2022). Identification and Analysis of Corrosion Mechanisms for Ground Pipelines with Hanging Rings. Coatings, 12(9), 1257. https://doi.org/10.3390/coatings12091257








