Influence of Magnesium Content on the Physico-Chemical Properties of Hydroxyapatite Electrochemically Deposited on a Nanostructured Titanium Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Biofunctionalization of cp-Ti
2.3. Characterization
3. Results and Discussion
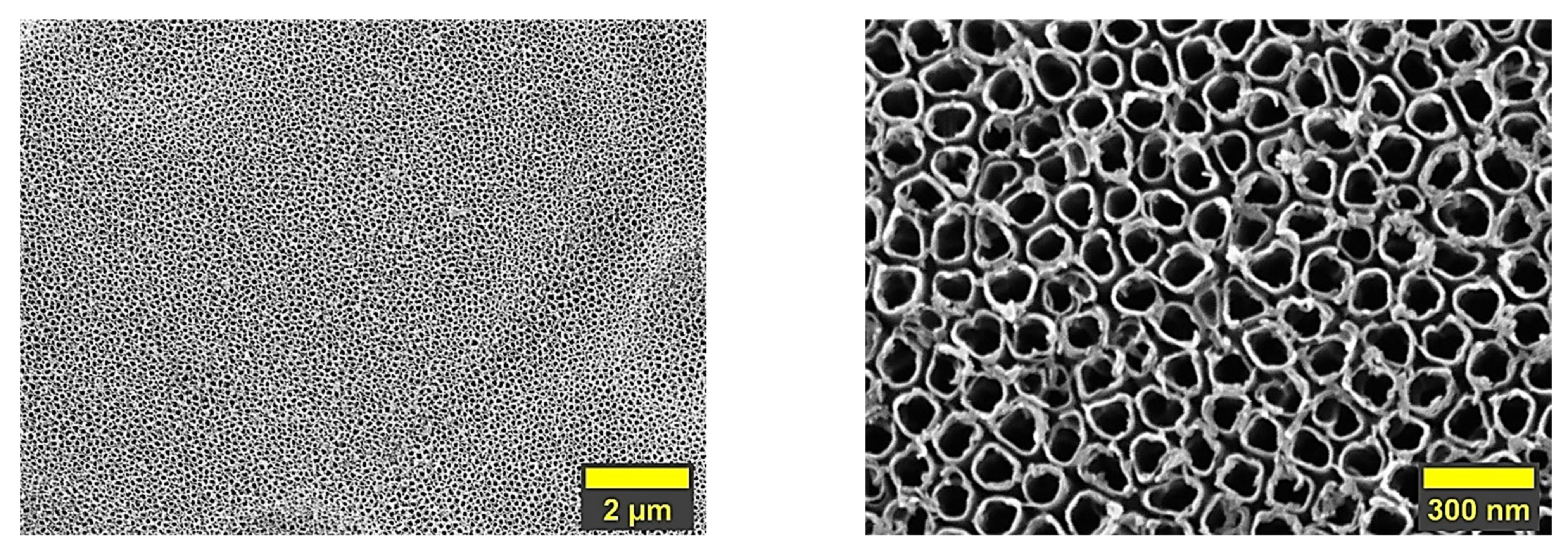
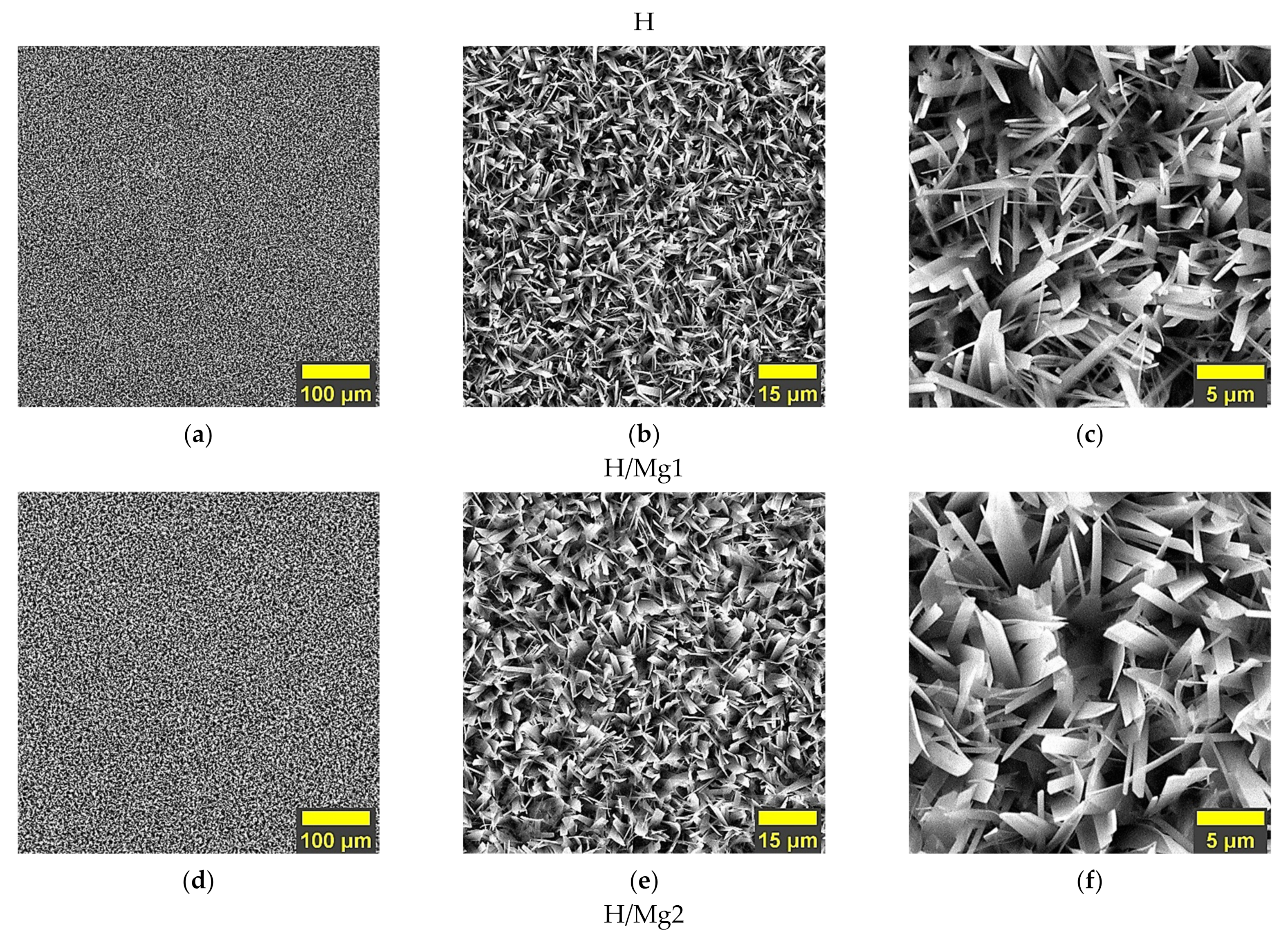
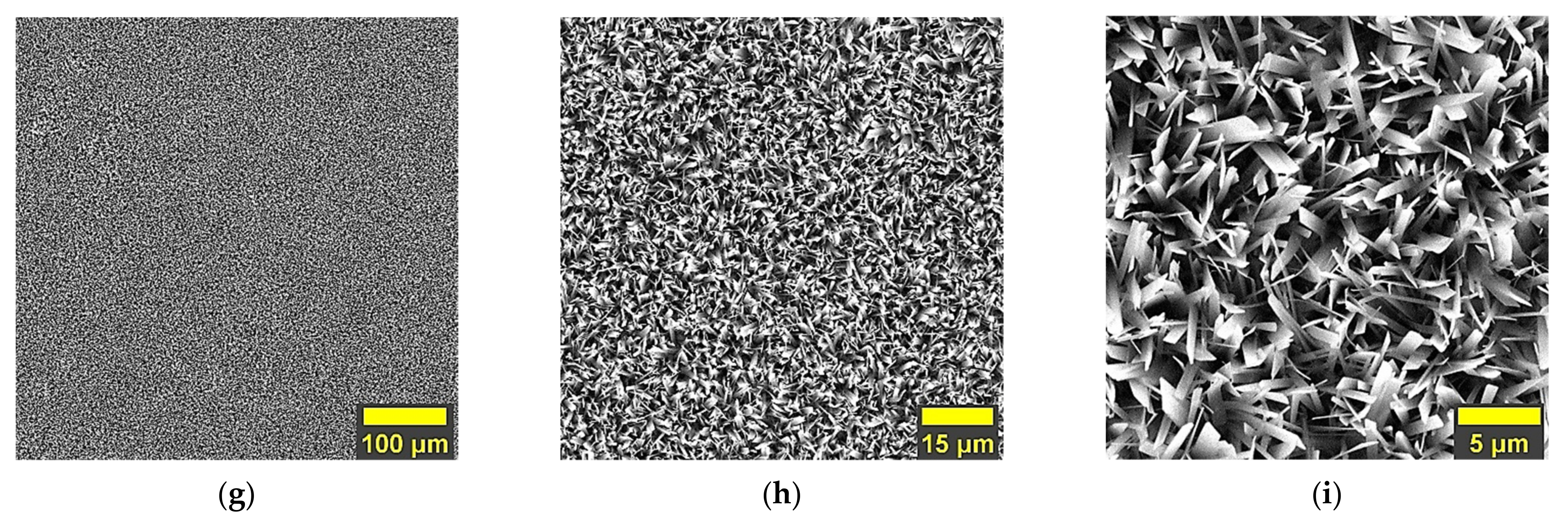
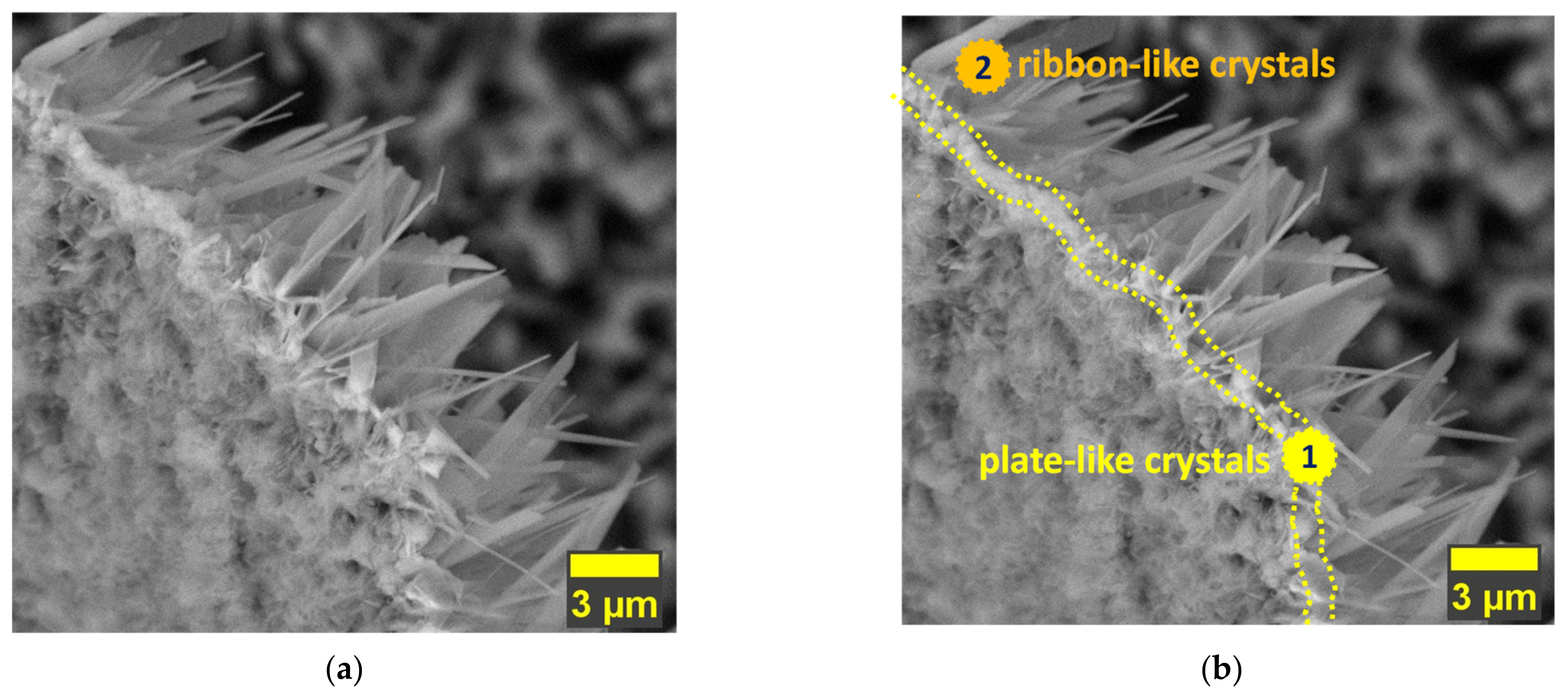
3.1. Morphology
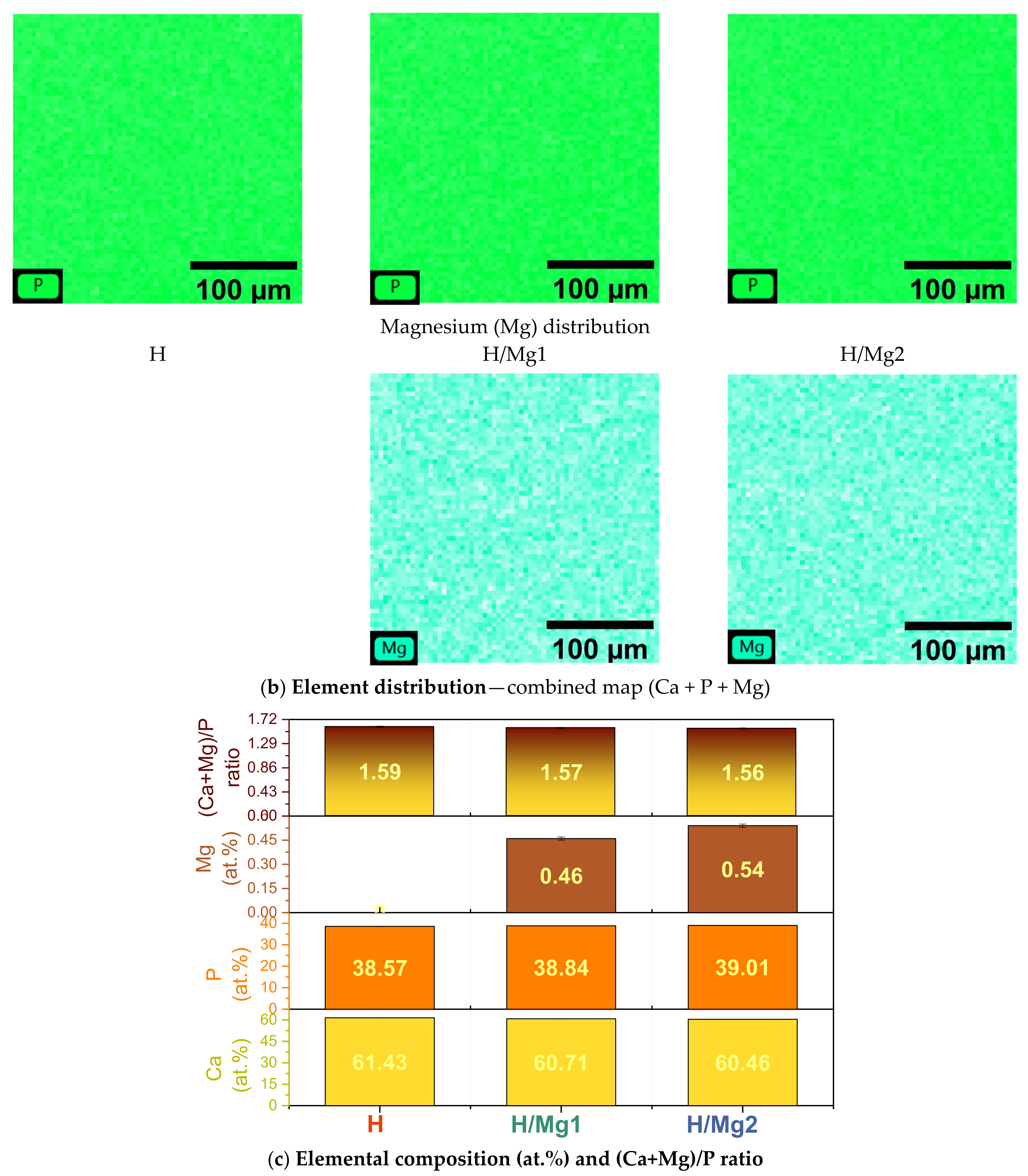
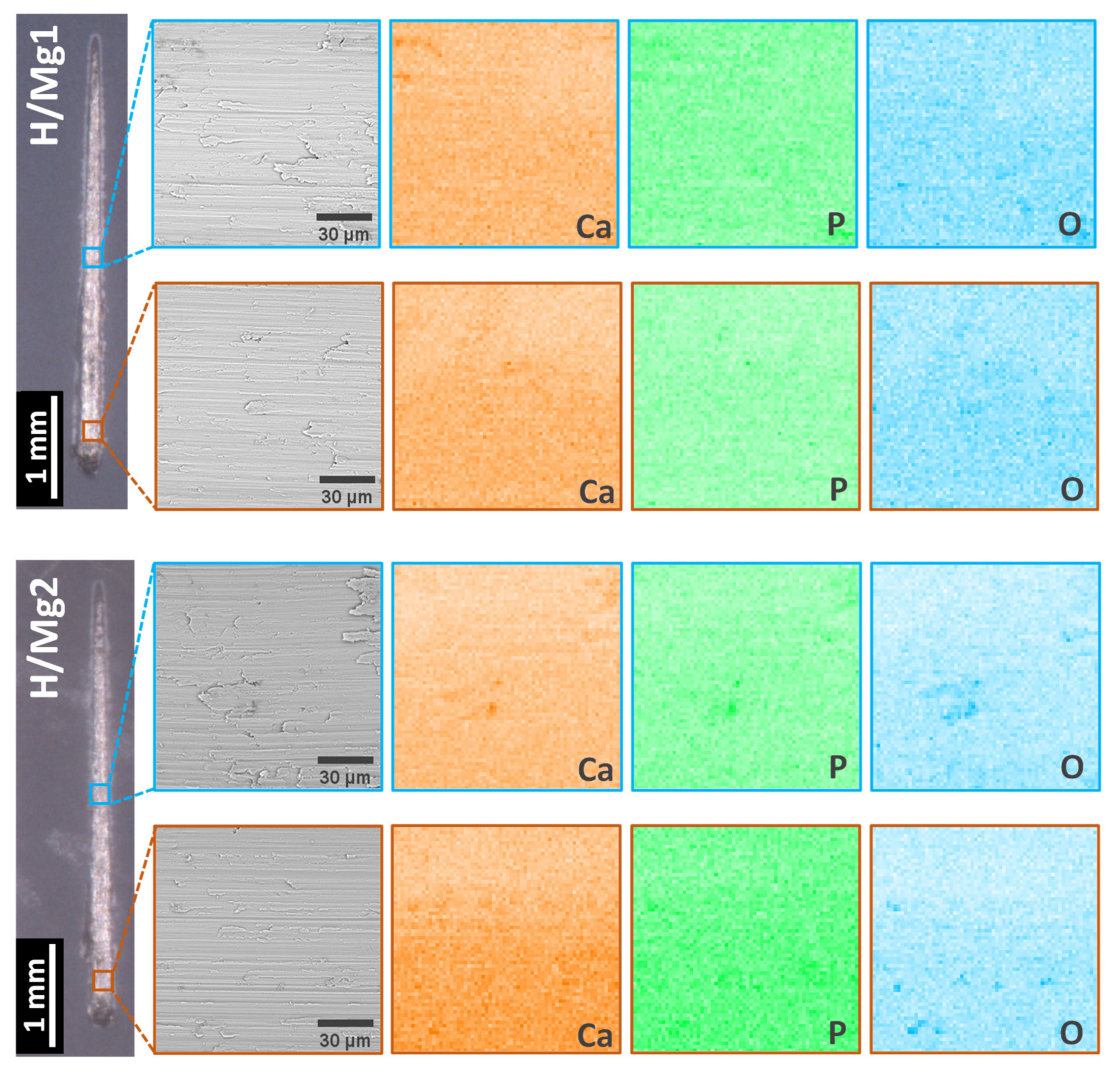
3.2. Elemental Composition
3.3. Phase Composition
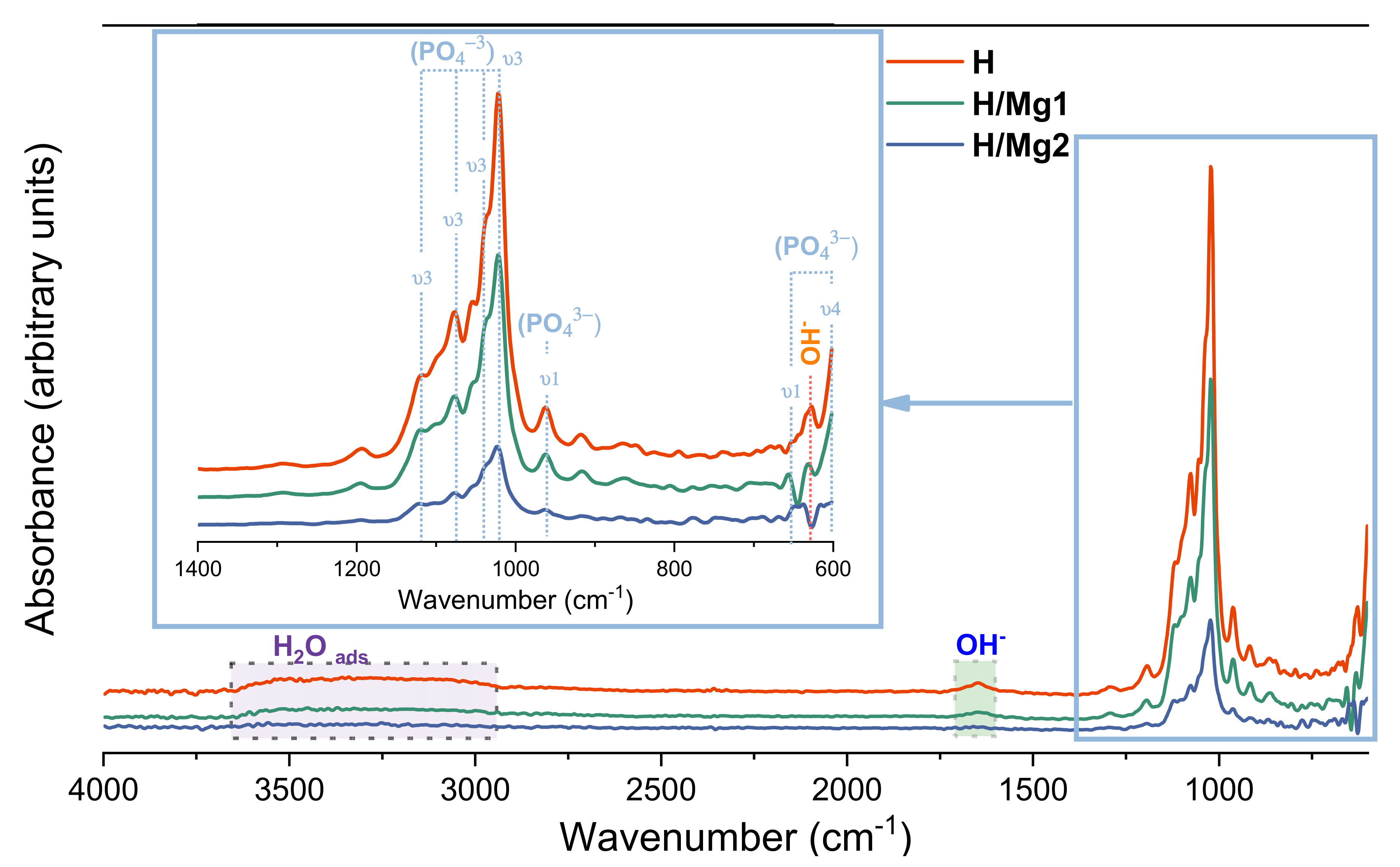
3.4. Chemical Bonds
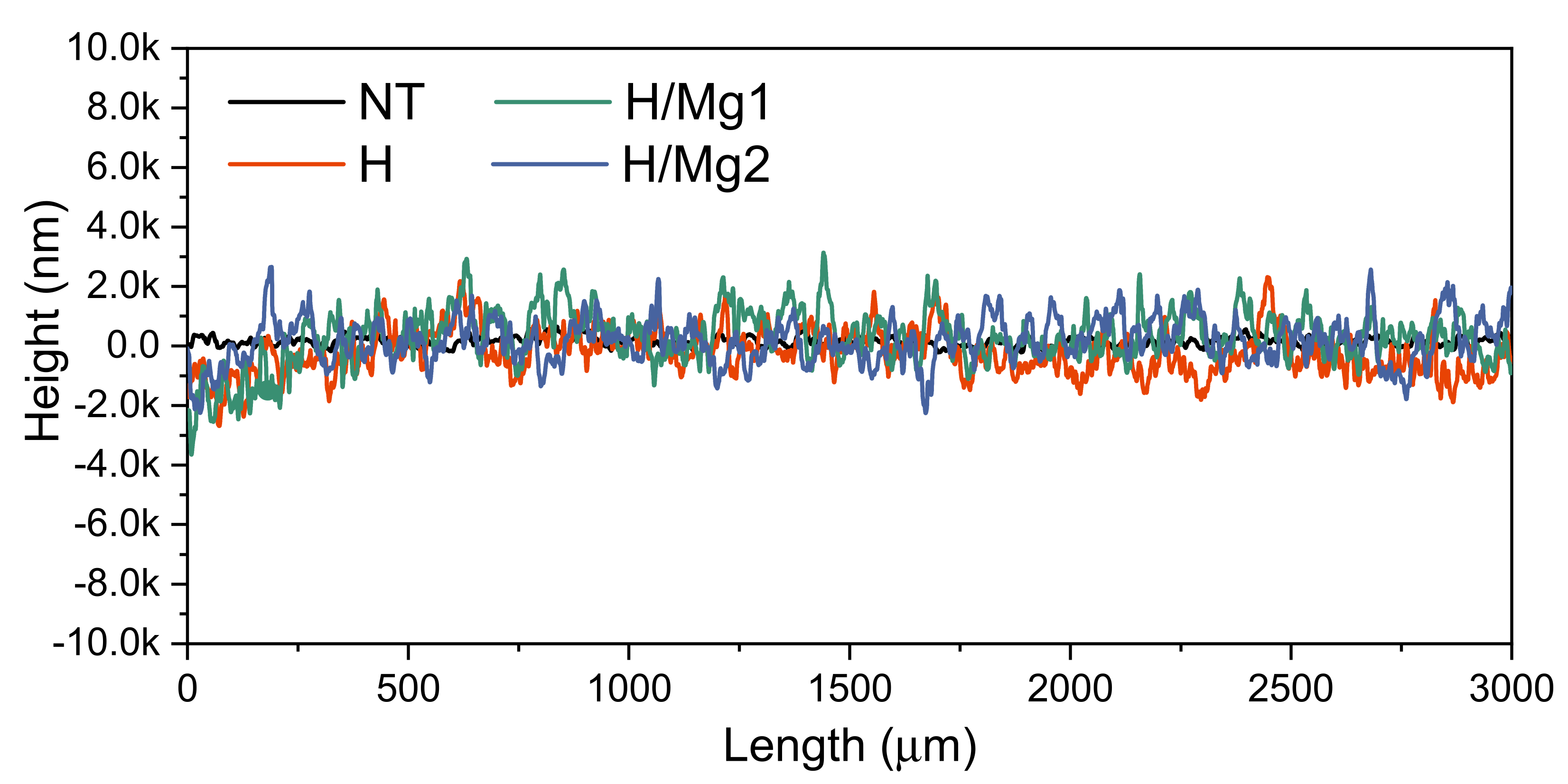
3.5. Surface Roughness
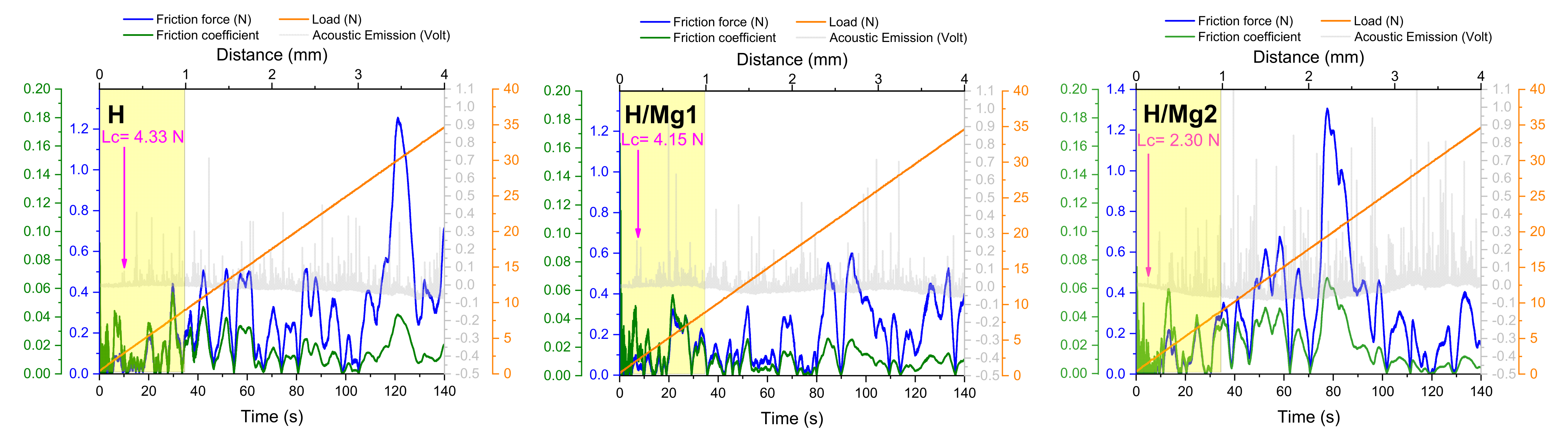
3.6. Adhesion
4. Conclusions
- Hydroxyapatite-based coatings undoped and doped with Mg in different concentrations were successfully deposited by electrochemical deposition in pulsed galvanostatic mode on a nanostructured surface made of titania nanotubes with a diameter of ~72 nm (±2);
- In terms of morphology, the present study showed that the undoped HAp and the HAp coatings obtained with a concentration of 1.5 mM of Mg in the electrolyte present similar morphologies, which are made of very thin and narrow ribbon-like crystals, while the morphology of the HAp coatings obtained with a concentration of 1 mM of Mg in the electrolyte, are made of wider and thicker ribbon-like crystals, suggesting that the growth mechanism of the crystals is influenced by the Mg concentration within the electrolyte used to obtain the coatings;
- EDS analysis revealed that by increasing the Mg concentration in the electrolyte from 1 mM to 1.5 mM, the quantity of Mg identified in the coatings is 0.44 at.% (0.31 wt.%) and 0.54 at.% (0.37 wt.%) which are within the values of Mg found in human hard bone tissue (0.30–0.72 wt.%); also, all elements (Ca, P, and Mg) are uniformly distributed within the coatings, indicating that the pulsed galvanostatic method is a reliable deposition technique;
- Compared with the undoped HAp, which registered a thickness of 9.6 μm, addition and increment of the Mg concentration in the electrolyte from 1 mM to 1.5 mM, led to thickness of 11.3 and 13.7 μm, indicating a strong association between the Mg amount and the deposition rate. Thus, a concentration of 1.5 mM of Mg in the electrolyte increases the deposition rate, while with a decrement of the Mg concentration, to a value of 1 mM, a less thick coating can be obtained. Based on this, it can be said that the coating thickness can also be controlled through a careful selection of the doping element concentration within the electrolyte. Of course, this should also be associated and correlated with other parameters involved in the electrochemical deposition, such as the deposition technique, pH value, deposition temperature, and so on to design a coating with enhanced and tunable features;
- The phase composition and chemical bonds analysis confirmed that all coatings consist of the HAp phase and that Mg2+ substituted some of the Ca2+ in the HAp structure. Moreover, the XRD analysis showed that the coatings have a preferred orientation toward the c-axis direction and that the addition of Mg in the proposed concentrations of 1 mM and 1.5 mM in the electrolyte can enhance the crystallinity of the HAp-based coatings;
- With respect to the Mg concentration in the electrolyte, it was observed that compared to the undoped HAp, the use of 1.5 mM Mg leads to an average roughness of 493 nm, while the use of a concentration of 1 mM Mg leads to an average roughness of 582 nm, suggesting that higher concentrations of Mg tend to smoothen the surface;
- The “tape test” and the micro-scratch tests highlighted that the adhesion of the HAp-based coatings is in strong correlation with the Mg concentration, indicating that an increment of the Mg can affect the adhesion of the coating even if the coatings are obtained on a nanostructured surface, which is known to enhance the adhesion of the coatings obtained by electrochemical techniques;
- moreover, the adhesion tests also showed that even if the coatings are being scratched, a small amount of coating will remain on the metallic substrate.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bannon, B.; Mild, E. Titanium Alloys for Biomaterial Application: An Overview. In Titanium Alloys in Surgical Implants; Luckey, H.A., Kubli, F.J., Eds.; ASTM International: West Conshohocken, PA, USA, 1983; pp. 7–9. [Google Scholar]
- Hanawa, T. Biofunctionalization of Titanium for Dental Implant. Jpn. Dent. Sci. Rev. 2010, 46, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in Orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraris, S.; Spriano, S. Antibacterial Titanium Surfaces for Medical Implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef]
- Baino, F.; Potestio, I. Orbital Implants: State-of-the-Art Review with Emphasis on Biomaterials and Recent Advances. Mater. Sci. Eng. C 2016, 69, 1410–1428. [Google Scholar] [CrossRef]
- Saini, M. Implant Biomaterials: A Comprehensive Review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hady Gepreel, M.; Niinomi, M. Biocompatibility of Ti-Alloys for Long-Term Implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef]
- Davis, R.; Singh, A.; Jackson, M.J.; Coelho, R.T.; Prakash, D.; Charalambous, C.P.; Ahmed, W.; da Silva, L.R.R.; Lawrence, A.A. A Comprehensive Review on Metallic Implant Biomaterials and Their Subtractive Manufacturing. Int. J. Adv. Manuf. Technol. 2022, 120, 1473–1530. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Z.; Xu, K.; Wang, X.; Wang, S.; Qiu, H.; Li, X.; Chen, J. Multifunctional Coatings of Titanium Implants Toward Promoting Osseointegration and Preventing Infection: Recent Developments. Front. Bioeng. Biotechnol. 2021, 9, 783816. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Chen, L.-Y.; Wang, L. Surface Modification of Titanium and Titanium Alloys: Technologies, Developments, and Future Interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Madhavi, G.; Kishan, N.; Raghavendra, C.R. A Review on Recent Approaches in the Field of Surface Coating. Mater. Today Proc. 2022, 52, 403–406. [Google Scholar] [CrossRef]
- Chen, L.; Song, X.; Xing, F.; Wang, Y.; Wang, Y.; He, Z.; Sun, L. A Review on Antimicrobial Coatings for Biomaterial Implants and Medical Devices. J. Biomed. Nanotechnol. 2020, 16, 789–809. [Google Scholar] [CrossRef] [PubMed]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenhoek, A.V. Microscopical Observations of the Structure of Teeth and Other Bones: Made and Communicated, in a Letter by Mr. Anthony Leeuwenhoeck. Philos. Trans. R. Soc. Lond. 1677, 12, 1002–1003. [Google Scholar] [CrossRef] [Green Version]
- Shear, M.J.; Kramer, B. Composition of Bone: I. Analytical Micro Methods. J. Biol. Chem. 1928, 79, 105–120. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; González-Calbet, J.M. Calcium Phosphates as Substitution of Bone Tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H. Calcium Phosphate-Based Coatings on Titanium and Its Alloys. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2008, 85, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Koutsopoulos, S. Synthesis and Characterization of Hydroxyapatite Crystals: A Review Study on the Analytical Methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Sengupta, S.; Saha, P.; Das, K.; Das, S. Synthesis of Calcium Hydrogen Phosphate and Hydroxyapatite Coating on SS316 Substrate through Pulsed Electrodeposition. Mater. Sci. Eng. C 2016, 69, 875–883. [Google Scholar] [CrossRef]
- Mihailescu, N.; Stan, G.E.; Duta, L.; Chifiriuc, M.C.; Bleotu, C.; Sopronyi, M.; Luculescu, C.; Oktar, F.N.; Mihailescu, I.N. Structural, Compositional, Mechanical Characterization and Biological Assessment of Bovine-Derived Hydroxyapatite Coatings Reinforced with MgF 2 or MgO for Implants Functionalization. Mater. Sci. Eng. C 2016, 59, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone Grafts and Biomaterials Substitutes for Bone Defect Repair: A Review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Dascălu, C.-A.; Maidaniuc, A.; Pandele, A.M.; Voicu, S.I.; Machedon-Pisu, T.; Stan, G.E.; Cîmpean, A.; Mitran, V.; Antoniac, I.V.; Miculescu, F. Synthesis and Characterization of Biocompatible Polymer-Ceramic Film Structures as Favorable Interface in Guided Bone Regeneration. Appl. Surf. Sci. 2019, 494, 335–352. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Antoniac, A.; Vasile, E.; Tecu, C.; Fosca, M.; Yankova, V.G.; Rau, J.V. In Vitro Characterization of Novel Nanostructured Collagen-Hydroxyapatite Composite Scaffolds Doped with Magnesium with Improved Biodegradation Rate for Hard Tissue Regeneration. Bioact. Mater. 2021, 6, 3383–3395. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Chen, H.; Zhao, R.; Deng, X.; Chen, G.; Yang, X.; Xiao, Z.; Aurora, A.; Iulia, B.A.; Zhang, K.; et al. Construction of a Magnesium Hydroxide/Graphene Oxide/Hydroxyapatite Composite Coating on Mg-Ca-Zn-Ag Alloy to Inhibit Bacterial Infection and Promote Bone Regeneration. Bioact. Mater. 2022, 18, 354–367. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, F.; Cotrut, C.; Ficai, A.; Rau, J.V.; Grosu, E.; Antoniac, A.; Tecu, C.; Cristescu, I. Controlling the Degradation Rate of Biodegradable Mg–Zn-Mn Alloys for Orthopedic Applications by Electrophoretic Deposition of Hydroxyapatite Coating. Materials 2020, 13, 263. [Google Scholar] [CrossRef] [Green Version]
- Bryington, M.S.; Hayashi, M.; Kozai, Y.; Vandeweghe, S.; Andersson, M.; Wennerberg, A.; Jimbo, R. The Influence of Nano Hydroxyapatite Coating on Osseointegration after Extended Healing Periods. Dent. Mater. 2013, 29, 514–520. [Google Scholar] [CrossRef]
- Gambardella, A.; Bianchi, M.; Kaciulis, S.; Mezzi, A.; Brucale, M.; Cavallini, M.; Herrmannsdoerfer, T.; Chanda, G.; Uhlarz, M.; Cellini, A.; et al. Magnetic Hydroxyapatite Coatings as a New Tool in Medicine: A Scanning Probe Investigation. Mater. Sci. Eng. C 2016, 62, 444–449. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic Substituted Hydroxyapatite for Bone Regeneration Applications: A Review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Shepherd, D.V.; Best, S.M. Substituted Hydroxyapatites for Bone Repair. J. Mater. Sci. Mater. Med. 2012, 23, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Zhang, S.; Khor, K.A.; Lye, S.W.; Zeng, X.; Weng, W.; Liu, C.; Venkatraman, S.S.; Ma, L.L. Osteoblastic Cell Response on Magnesium-Incorporated Apatite Coatings. Appl. Surf. Sci. 2008, 255, 304–307. [Google Scholar] [CrossRef]
- Bigi, A.; Foresti, E.; Gregorini, R.; Ripamonti, A.; Roveri, N.; Shah, J.S. The Role of Magnesium on the Structure of Biological Apatites. Calcif. Tissue Int. 1992, 50, 439–444. [Google Scholar] [CrossRef]
- Howlett, C.R.; Zreioat, H.; O’Dell, R.; Noorman, J.; Evans, P.; Dalton, B.A.; McFarland, C.; Steele, J.G. The Effect of Magnesium Ion Implantation into Alumina upon the Adhesion of Human Bone Derived Cells. J. Mater. Sci. Mater. Med. 1994, 5, 715–722. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, M.; Mănescu (Păltânea), V.; Stere, A.; Quan, P.H.; Păltânea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef] [PubMed]
- Peron, M.; Torgersen, J.; Berto, F. Mg and Its Alloys for Biomedical Applications: Exploring Corrosion and Its Interplay with Mechanical Failure. Metals 2017, 7, 252. [Google Scholar] [CrossRef] [Green Version]
- Uskoković, V. Ion-Doped Hydroxyapatite: An Impasse or the Road to Follow? Ceram. Int. 2020, 46, 11443–11465. [Google Scholar] [CrossRef]
- Ratha, I.; Datta, P.; Balla, V.K.; Nandi, S.K.; Kundu, B. Effect of Doping in Hydroxyapatite as Coating Material on Biomedical Implants by Plasma Spraying Method: A Review. Ceram. Int. 2021, 47, 4426–4445. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, Q.; Peel, S.; Wang, X.; He, F. Effects of Magnesium-Substituted Nanohydroxyapatite Coating on Implant Osseointegration. Clin. Oral Implant. Res. 2013, 24, 34–41. [Google Scholar] [CrossRef]
- Tampieri, A.; Celotti, G.C.; Landi, E.; Sandri, M. Magnesium Doped Hydroxyapatite: Synthesis and Characterization. Key Eng. Mater. 2004, 264–268, 2051–2054. [Google Scholar] [CrossRef]
- Ren, F.; Leng, Y.; Xin, R.; Ge, X. Synthesis, Characterization and Ab Initio Simulation of Magnesium-Substituted Hydroxyapatite. Acta Biomater. 2010, 6, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Lemos, I.A.F.; Rocha, J.H.G.; Ferreira, J.M.F. Synthesis and Characterization of Magnesium Substituted Biphasic Mixtures of Controlled Hydroxyapatite/β-Tricalcium Phosphate Ratios. J. Solid State Chem. 2005, 178, 3190–3196. [Google Scholar] [CrossRef]
- Laurencin, D.; Almora-Barrios, N.; de Leeuw, N.H.; Gervais, C.; Bonhomme, C.; Mauri, F.; Chrzanowski, W.; Knowles, J.C.; Newport, R.J.; Wong, A.; et al. Magnesium Incorporation into Hydroxyapatite. Biomaterials 2011, 32, 1826–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchanek, W.L.; Byrappa, K.; Shuk, P.; Riman, R.E.; Janas, V.F.; TenHuisen, K.S. Preparation of Magnesium-Substituted Hydroxyapatite Powders by the Mechanochemical–Hydrothermal Method. Biomaterials 2004, 25, 4647–4657. [Google Scholar] [CrossRef] [PubMed]
- Mehrjoo, M.; Javadpour, J.; Shokrgozar, M.A.; Farokhi, M.; Javadian, S.; Bonakdar, S. Effect of Magnesium Substitution on Structural and Biological Properties of Synthetic Hydroxyapatite Powder. Mater. Express 2015, 5, 41–48. [Google Scholar] [CrossRef]
- Landi, E.; Logroscino, G.; Proietti, L.; Tampieri, A.; Sandri, M.; Sprio, S. Biomimetic Mg-Substituted Hydroxyapatite: From Synthesis to in Vivo Behaviour. J. Mater. Sci. Mater. Med. 2008, 19, 239–247. [Google Scholar] [CrossRef]
- Chirică, I.M.; Enciu, A.-M.; Tite, T.; Dudău, M.; Albulescu, L.; Iconaru, S.L.; Predoi, D.; Pasuk, I.; Enculescu, M.; Radu, C.; et al. The Physico-Chemical Properties and Exploratory Real-Time Cell Analysis of Hydroxyapatite Nanopowders Substituted with Ce, Mg, Sr, and Zn (0.5–5 at.%). Materials 2021, 14, 3808. [Google Scholar] [CrossRef]
- Chen, S.; Shi, Y.; Zhang, X.; Ma, J. Biomimetic Synthesis of Mg-substituted Hydroxyapatite Nanocomposites and Three-dimensional Printing of Composite Scaffolds for Bone Regeneration. J. Biomed. Mater. Res. Part A 2019, 107, 2512–2521. [Google Scholar] [CrossRef]
- He, L.Y.; Zhang, X.M.; Liu, B.; Tian, Y.; Ma, W.H. Effect of Magnesium Ion on Human Osteoblast Activity. Braz. J. Med. Biol. Res. 2016, 49, 1–6. [Google Scholar] [CrossRef]
- Jiao, M.-J.; Wang, X.-X. Electrolytic Deposition of Magnesium-Substituted Hydroxyapatite Crystals on Titanium Substrate. Mater. Lett. 2009, 63, 2286–2289. [Google Scholar] [CrossRef]
- Mróz, W.; Bombalska, A.; Burdyńska, S.; Jedyński, M.; Prokopiuk, A.; Budner, B.; Ślósarczyk, A.; Zima, A.; Menaszek, E.; Ścisłowska-Czarnecka, A.; et al. Structural Studies of Magnesium Doped Hydroxyapatite Coatings after Osteoblast Culture. J. Mol. Struct. 2010, 977, 145–152. [Google Scholar] [CrossRef]
- Adzila, S.; Kanasan, N.; Hassan, M.F.; Arifin, A.M.T.; Yunos, M.Z.; Rahman, M.N.A.; Haq, R.H.A. Synthesis and Characterization of Magnesium Doped Calcium Phosphatefor Bone Implant Application. ARPN J. Eng. Appl. Sci. 2016, 11, 8694–8697. [Google Scholar]
- Leena, M.; Rana, D.; Webster, T.J.; Ramalingam, M. Accelerated Synthesis of Biomimetic Nano Hydroxyapatite Using Simulated Body Fluid. Mater. Chem. Phys. 2016, 180, 166–172. [Google Scholar] [CrossRef]
- Ganvir, A.; Nagar, S.; Markocsan, N.; Balani, K. Deposition of Hydroxyapatite Coatings by Axial Plasma Spraying: Influence of Feedstock Characteristics on Coating Microstructure, Phase Content and Mechanical Properties. J. Eur. Ceram. Soc. 2021, 41, 4637–4649. [Google Scholar] [CrossRef]
- Bița, A.-I.; Antoniac, I.; Miculescu, M.; Stan, G.E.; Leonat, L.; Antoniac, A.; Constantin, B.; Forna, N. Electrochemical and In Vitro Biological Evaluation of Bio-Active Coatings Deposited by Magnetron Sputtering onto Biocompatible Mg-0.8Ca Alloy. Materials 2022, 15, 3100. [Google Scholar] [CrossRef]
- Vranceanu, D.M.; Cotrut, C.M.; Bramowicz, M.; Titorencu, I.; Kulesza, S.; Kiss, A.; Berbecaru, A.; Pruna, V.; Branzei, M.; Vladescu, A. Osseointegration of Sputtered SiC-Added Hydroxyapatite for Orthopaedic Applications. Ceram. Int. 2016, 42, 10085–10093. [Google Scholar] [CrossRef]
- Osuchukwu, O.A.; Salihi, A.; Abdullahi, I.; Obada, D.O. Synthesis and Characterization of Sol–Gel Derived Hydroxyapatite from a Novel Mix of Two Natural Biowastes and Their Potentials for Biomedical Applications. Mater. Today Proc. 2022, 62, 4182–4187. [Google Scholar] [CrossRef]
- Duta, L.; Oktar, F.N.; Stan, G.E.; Popescu-Pelin, G.; Serban, N.; Luculescu, C.; Mihailescu, I.N. Novel Doped Hydroxyapatite Thin Films Obtained by Pulsed Laser Deposition. Appl. Surf. Sci. 2013, 265, 41–49. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Filipescu, M.; Barbaro, K.; Bonciu, A.; Birjega, R.; Cotrut, C.M.; Galvano, E.; Fosca, M.; Fadeeva, I.V.; Vadalà, G.; et al. Iron Ion-Doped Tricalcium Phosphate Coatings Improve the Properties of Biodegradable Magnesium Alloys for Biomedical Implant Application. Adv. Mater. Interfaces 2020, 7, 2000531. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. Electrodeposited Hydroxyapatite-Based Biocoatings: Recent Progress and Future Challenges. Coatings 2021, 11, 110. [Google Scholar] [CrossRef]
- Mokabber, T.; Lu, L.Q.; van Rijn, P.; Vakis, A.I.; Pei, Y.T. Crystal Growth Mechanism of Calcium Phosphate Coatings on Titanium by Electrochemical Deposition. Surf. Coat. Technol. 2018, 334, 526–535. [Google Scholar] [CrossRef]
- Katić, J.; Metikoš-Huković, M.; Škapin, S.D.; Petravić, M.; Varašanec, M. The Potential-Assisted Deposition as Valuable Tool for Producing Functional Apatite Coatings on Metallic Materials. Electrochim. Acta 2014, 127, 173–179. [Google Scholar] [CrossRef]
- Aina, V.; Lusvardi, G.; Annaz, B.; Gibson, I.R.; Imrie, F.E.; Malavasi, G.; Menabue, L.; Cerrato, G.; Martra, G. Magnesium- and Strontium-Co-Substituted Hydroxyapatite: The Effects of Doped-Ions on the Structure and Chemico-Physical Properties. J. Mater. Sci. Mater. Med. 2012, 23, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Farzadi, A.; Bakhshi, F.; Solati-Hashjin, M.; Asadi-Eydivand, M.; abu Osman, N.A. Magnesium Incorporated Hydroxyapatite: Synthesis and Structural Properties Characterization. Ceram. Int. 2014, 40, 6021–6029. [Google Scholar] [CrossRef] [Green Version]
- Bauer, L.; Antunović, M.; Rogina, A.; Ivanković, M.; Ivanković, H. Bone-Mimetic Porous Hydroxyapatite/Whitlockite Scaffolds: Preparation, Characterization and Interactions with Human Mesenchymal Stem Cells. J. Mater. Sci. 2021, 56, 3947–3969. [Google Scholar] [CrossRef]
- Vranceanu, D.M.; Ionescu, I.C.; Ungureanu, E.; Cojocaru, M.O.; Vladescu, A.; Cotrut, C.M. Magnesium Doped Hydroxyapatite-Based Coatings Obtained by Pulsed Galvanostatic Electrochemical Deposition with Adjustable Electrochemical Behavior. Coatings 2020, 10, 727. [Google Scholar] [CrossRef]
- Yajing, Y.; Qiongqiong, D.; Yong, H.; Han, S.; Pang, X. Magnesium Substituted Hydroxyapatite Coating on Titanium with Nanotublar TiO2 Intermediate Layer via Electrochemical Deposition. Appl. Surf. Sci. 2014, 305, 77–85. [Google Scholar] [CrossRef]
- Robertson, S.F.; Bandyopadhyay, A.; Bose, S. Titania Nanotube Interface to Increase Adhesion Strength of Hydroxyapatite Sol-Gel Coatings on Ti-6Al-4V for Orthopedic Applications. Surf. Coat. Technol. 2019, 372, 140–147. [Google Scholar] [CrossRef]
- Kar, A.; Raja, K.S.; Misra, M. Electrodeposition of Hydroxyapatite onto Nanotubular TiO2 for Implant Applications. Surf. Coat. Technol. 2006, 201, 3723–3731. [Google Scholar] [CrossRef]
- Sul, Y.-T.; Johansson, C.B.; Jeong, Y.; Wennerberg, A.; Albrektsson, T. Resonance Frequency and Removal Torque Analysis of Implants with Turned and Anodized Surface Oxides. Clin. Oral Implant. Res. 2002, 13, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y. The Significance of the Surface Properties of Oxidized Titanium to the Bone Response: Special Emphasis on Potential Biochemical Bonding of Oxidized Titanium Implant. Biomaterials 2003, 24, 3893–3907. [Google Scholar] [CrossRef]
- Yao, C.; Slamovich, E.B.; Webster, T.J. Enhanced Osteoblast Functions on Anodized Titanium with Nanotube-like Structures. J. Biomed. Mater. Res. Part A 2008, 85, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.-K.; Lee, D.H.; Kim, T.; Jang, G.; Choi, S.; Oh, J.B.; Ye, G.; Lee, S. Modification of Titanium Implant and Titanium Dioxide for Bone Tissue Engineering. Nov. Biomater. Regen. Med. 2018, 1077, 355–368. [Google Scholar] [CrossRef]
- Popat, K.C.; Leoni, L.; Grimes, C.A.; Desai, T.A. Influence of Engineered Titania Nanotubular Surfaces on Bone Cells. Biomaterials 2007, 28, 3188–3197. [Google Scholar] [CrossRef]
- Wang, N.; Li, H.; Lü, W.; Li, J.; Wang, J.; Zhang, Z.; Liu, Y. Effects of TiO2 Nanotubes with Different Diameters on Gene Expression and Osseointegration of Implants in Minipigs. Biomaterials 2011, 32, 6900–6911. [Google Scholar] [CrossRef] [PubMed]
- Von Wilmowsky, C.; Bauer, S.; Roedl, S.; Neukam, F.W.; Schmuki, P.; Schlegel, K.A. The Diameter of Anodic TiO2 Nanotubes Affects Bone Formation and Correlates with the Bone Morphogenetic Protein-2 Expression in Vivo. Clin. Oral Implant. Res. 2012, 23, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Mo, A. A Review on the Electrochemically Self-Organized Titania Nanotube Arrays: Synthesis, Modifications, and Biomedical Applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Macak, J.M.; Hildebrand, H.; Marten-Jahns, U.; Schmuki, P. Mechanistic Aspects and Growth of Large Diameter Self-Organized TiO2 Nanotubes. J. Electroanal. Chem. 2008, 621, 254–266. [Google Scholar] [CrossRef]
- Ribeiro, B.; Offoiach, R.; Rossetti, S.; Salatin, E.; Lekka, M.; Fedrizzi, L. On Growth and Morphology of TiO2 Nanotubes on CP-Ti by Anodic Oxidation in Ethylene Glycol Electrolyte: Influence of Electrolyte Aging and Anodization Parameters. Materials 2022, 15, 3338. [Google Scholar] [CrossRef]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The Relationship between the Nanostructure of Titanium Surfaces and Bacterial Attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef]
- Ercan, B.; Taylor, E.; Alpaslan, E.; Webster, T.J. Diameter of Titanium Nanotubes Influences Anti-Bacterial Efficacy. Nanotechnology 2011, 22, 295102. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Flora, S.J.S. Effects of Fluoride on the Tissue Oxidative Stress and Apoptosis in Rats: Biochemical Assays Supported by IR Spectroscopy Data. Toxicology 2008, 254, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, S.; Wang, D.; Feng, G.; Bao, C.; Hu, J. The Effect of Hydrofluoric Acid Treatment on Titanium Implant Osseointegration in Ovariectomized Rats. Biomaterials 2010, 31, 3266–3273. [Google Scholar] [CrossRef] [PubMed]
- Isa, Z.M.; Schneider, G.B.; Zaharias, R.; Seabold, D.; Stanford, C.M. Effects of Fluoride-Modified Titanium Surfaces on Osteoblast Proliferation and Gene Expression. Int. J. Oral Maxillofac. Implant. 2006, 21, 203–211. [Google Scholar]
- Cooper, L.; Zhou, Y.; Takebe, J.; Guor, J.; Abron, A.; Holmen, A.; Ellingsen, J. Fluoride Modification Effects on Osteoblast Behavior and Bone Formation at TiO2 Grit-Blasted c.p. Titanium Endosseous Implants. Biomaterials 2006, 27, 926–936. [Google Scholar] [CrossRef]
- Ellingsen, J.E.; Johansson, C.B.; Wennerberg, A.; Holmén, A. Improved Retention and Bone-to Lmplant Contact with Fluoride-Modified Titanium Implants. Int. J. Oral Maxillofac. Implant. 2004, 19, 659–666. [Google Scholar]
- Abrahamsson, I.; Albouy, J.-P.; Berglundh, T. Healing at Fluoride-Modified Implants Placed in Wide Marginal Defects: An Experimental Study in Dogs. Clin. Oral Implant. Res. 2008, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Gazzano, E.; Bergandi, L.; Riganti, C.; Aldieri, E.; Doublier, S.; Costamagna, C.; Bosia, A.; Ghigo, D. Fluoride Effects: The Two Faces of Janus. Curr. Med. Chem. 2010, 17, 2431–2441. [Google Scholar] [CrossRef]
- Bačáková, L.; Mareš, V.; Lisá, V.; Švorčıík, V. Molecular Mechanisms of Improved Adhesion and Growth of an Endothelial Cell Line Cultured on Polystyrene Implanted with Fluorine Ions. Biomaterials 2000, 21, 1173–1179. [Google Scholar] [CrossRef]
- Li, B.; Logan, B.E. Bacterial Adhesion to Glass and Metal-Oxide Surfaces. Colloids Surf. B Biointerfaces 2004, 36, 81–90. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Spiliopoulou, I.; Dowling, D.P.; Missirlis, Y.F. Adhesion of Slime Producing Staphylococcus Epidermidis Strains to PVC and Diamond-like Carbon/Silver/Fluorinated Coatings. J. Mater. Sci. Mater. Med. 2006, 17, 679–689. [Google Scholar] [CrossRef]
- Shinonaga, Y.; Arita, K. Antibacterial Effect of Acrylic Dental Devices after Surface Modification by Fluorine and Silver Dual-Ion Implantation. Acta Biomater. 2012, 8, 1388–1393. [Google Scholar] [CrossRef]
- ARITA, K.; SHINONAGA, Y.; NISHINO, M. Plasma-Based Fluorine Ion Implantation into Dental Materials for Inhibition of Bacterial Adhesion. Dent. Mater. J. 2006, 25, 684–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, D.; Peng, Q.; Lin, J.; Wen, C. Biocompatibility of Nanoscale Hydroxyapatite Coating on TiO2 Nanotubes. Materials 2019, 12, 1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathyunes, L.; Khalil-Allafi, J.; Sheykholeslami, S.O.R.; Moosavifar, M. Biocompatibility Assessment of Graphene Oxide-Hydroxyapatite Coating Applied on TiO 2 Nanotubes by Ultrasound-Assisted Pulse Electrodeposition. Mater. Sci. Eng. C 2018, 87, 10–21. [Google Scholar] [CrossRef]
- Shahmohammadi, P.; Khazaei, B.A. Characterization of Zn/Mg-Enriched Calcium Phosphate Coating Produced by the Two-Step Pulsed Electrodeposition Method on Titanium Substrate. Surf. Interfaces 2021, 22, 100819. [Google Scholar] [CrossRef]
- Vranceanu, D.M.; Ungureanu, E.; Ionescu, I.C.; Parau, A.C.; Kiss, A.E.; Vladescu, A.; Cotrut, C.M. Electrochemical Surface Biofunctionalization of Titanium through Growth of TiO2 Nanotubes and Deposition of Zn Doped Hydroxyapatite. Coatings 2022, 12, 69. [Google Scholar] [CrossRef]
- Blackwood, D.J.; Seah, K.H.W. Electrochemical Cathodic Deposition of Hydroxyapatite: Improvements in Adhesion and Crystallinity. Mater. Sci. Eng. C 2009, 29, 1233–1238. [Google Scholar] [CrossRef]
- Sa, Y.; Guo, Y.; Feng, X.; Wang, M.; Li, P.; Gao, Y.; Yang, X.; Jiang, T. Are Different Crystallinity-Index-Calculating Methods of Hydroxyapatite Efficient and Consistent? New J. Chem. 2017, 41, 5723–5731. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S. Densification Behaviour and Mechanisms of Synthetic Hydroxyapatites. J. Eur. Ceram. Soc. 2000, 20, 2377–2387. [Google Scholar] [CrossRef]
- Narayanan, R.; Kwon, T.-Y.; Kim, K.-H. Direct Nanocrystalline Hydroxyapatite Formation on Titanium from Ultrasonated Electrochemical Bath at Physiological PH. Mater. Sci. Eng. C 2008, 28, 1265–1270. [Google Scholar] [CrossRef]
- ASTM D3359-17; Standard Test Methods for Rating Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2017; pp. 1–9. [CrossRef]
- Mokabber, T.; Zhou, Q.; Vakis, A.I.; van Rijn, P.; Pei, Y.T. Mechanical and Biological Properties of Electrodeposited Calcium Phosphate Coatings. Mater. Sci. Eng. C 2019, 100, 475–484. [Google Scholar] [CrossRef]
- Eliaz, N.; Eliyahu, M. Electrochemical Processes of Nucleation and Growth of Hydroxyapatite on Titanium Supported by Real-Time Electrochemical Atomic Force Microscopy. J. Biomed. Mater. Res.—Part A 2007, 80, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.A.; Moldovan, M.S.; Jacomine, L.; Mateescu, M.; Werckmann, J.; Anselme, K.; Mille, P.; Pelletier, H. Oriented Hydroxyapatite Single Crystals Produced by the Electrodeposition Method. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2010, 169, 138–144. [Google Scholar] [CrossRef]
- Saremi, M.; Golshan, B.M. Microstructural Study of Nano Hydroxyapatite Coating Obtained by Pulse Electrodeposition Process on Ti–6Al–4V. Trans. IMF 2007, 85, 99–102. [Google Scholar] [CrossRef]
- Hong, Z.; Luan, L.; Paik, S.-B.; Deng, B.; Ellis, D.E.; Ketterson, J.B.; Mello, A.; Eon, J.G.; Terra, J.; Rossi, A. Crystalline Hydroxyapatite Thin Films Produced at Room Temperature—An Opposing Radio Frequency Magnetron Sputtering Approach. Thin Solid Film. 2007, 515, 6773–6780. [Google Scholar] [CrossRef]
- López-Tomàs, L.; Claret, J.; Sagués, F. Quasi-Two-Dimensional Electrodeposition under Forced Fluid Flow. Phys. Rev. Lett. 1993, 71, 4373–4376. [Google Scholar] [CrossRef]
- Wang, J.; Chao, Y.; Wan, Q.; Zhu, Z.; Yu, H. Fluoridated Hydroxyapatite Coatings on Titanium Obtained by Electrochemical Deposition. Acta Biomater. 2009, 5, 1798–1807. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, Q.; Pang, X.; Han, S.; Yan, Y. Corrosion Behavior and Biocompatibility of Strontium and Fluorine Co-Doped Electrodeposited Hydroxyapatite Coatings. Appl. Surf. Sci. 2013, 282, 456–462. [Google Scholar] [CrossRef]
- Liu, D.; Savino, K.; Yates, M.Z. Coating of Hydroxyapatite Films on Metal Substrates by Seeded Hydrothermal Deposition. Surf. Coat. Technol. 2011, 205, 3975–3986. [Google Scholar] [CrossRef]
- Perry, A.J. The Structure and Colour of Some Nitride Coatings. Thin Solid Film. 1986, 135, 73–85. [Google Scholar] [CrossRef]
- Kim, K.H.; Chun, J.S. X-Ray Studies of SnO2 Prepared by Chemical Vapour Deposition. Thin Solid Film. 1986, 141, 287–295. [Google Scholar] [CrossRef]
- ISO 13779-3:2018 (E); Implants for Surgery-Hydroxyapatite-Part 3: Chemical Analysis and Characterization of Crystallinity and Phase Purity. International Organization for Standardization: Geneva, Switzerland, 2018.
- Jastrzębski, W.; Sitarz, M.; Rokita, M.; Bułat, K. Infrared Spectroscopy of Different Phosphates Structures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Stoch, A.; Jastrzȩbski, W.; Brozek, A.; Trybalska, B.; Cichocińska, M.; Szarawara, E. FTIR Monitoring of the Growth of the Carbonate Containing Apatite Layers from Simulated and Natural Body Fluids. J. Mol. Struct. 1999, 511–512, 287–294. [Google Scholar] [CrossRef]
- Ye, H.; Liu, X.Y.; Hong, H. Characterization of Sintered Titanium/Hydroxyapatite Biocomposite Using FTIR Spectroscopy. J. Mater. Sci. Mater. Med. 2009, 20, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanifi, A.; Fathi, M.H.; Mir Mohammad Sadeghi, H.; Varshosaz, J. Mg2+ Substituted Calcium Phosphate Nano Particles Synthesis for Non Viral Gene Delivery Application. J. Mater. Sci. Mater. Med. 2010, 21, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, T.; Kreis, S.; Behr, M.; Buergers, R. The Influence of Surface Texture and Wettability on Initial Bacterial Adhesion on Titanium and Zirconium Oxide Dental Implants. Int. J. Implant Dent. 2017, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [Green Version]
- Parcharoen, Y.; Kajitvichyanukul, P.; Sirivisoot, S.; Termsuksawad, P. Hydroxyapatite Electrodeposition on Anodized Titanium Nanotubes for Orthopedic Applications. Appl. Surf. Sci. 2014, 311, 54–61. [Google Scholar] [CrossRef]




| Surface Treatment | Material | Sample Codification | |
|---|---|---|---|
| 1 | Anodic oxidation | cp-Ti with nanostructured surface consisting in titania nanotubes | NT |
| 2 | Electrochemical deposition in pulsed galvanostatic mode | Functionalization of NT with undoped hydroxyapatite coatings | H |
| 3 | Functionalization of NT with Mg (concentration 1) doped hydroxyapatite coatings | H/Mg1 | |
| 4 | Functionalization of NT with Mg (concentration 2) doped hydroxyapatite coatings | H/Mg2 |
| 1 cycle | Activation | iON = −0.85 mA/cm2, tON = 1 s |
| Relaxation | iOFF = 0.00 mA/cm2, tOFF = 1 s | |
| Cycles number | 1200 | |
| Temperature (°C) | 75 °C | |
| Samples | Chemical Composition (mM) | (Ca + Mg)/P Ratio | ||
|---|---|---|---|---|
| Ca(NO3)2∙4H2O | NH4H2PO4 | Mg(NO3)2∙6H2O | ||
| H | 10 | 6 mM | 0 | (Ca + Mg)/P = 1.67 |
| H/Mg1 | 9 | 1 | ||
| H/Mg2 | 8.5 | 1.5 | ||
| Coating | TC(hkl) | Degree of Preferred Orientation σ | |||
|---|---|---|---|---|---|
| TC(002) | TC(211) | TC(112) | TC(213) | ||
| H | 2.96 | 0.21 | 0.35 | 0.48 | 1.14 |
| H/Mg1 | 3.23 | 0.15 | 0.25 | 0.36 | 1.29 |
| H/Mg2 | 3.48 | 0.10 | 0.17 | 0.26 | 1.43 |
| NT | H | H/Mg1 | H/Mg2 | |
|---|---|---|---|---|
| Ra (nm) | 132.2 (±9.1) | 535.6 (±22.8) | 582.6 (±34.6) | 493.5 (±61.4) |
| Rq(nm) | 154.5 (±12.9) | 689.8 (±49.3) | 741.9 (±49.4) | 644.7 (±59.6) |
| Coating Thickness (μm) | - | 9.6 (±0.9) | 11.3 (±0.6) | 13.7 (±1.1) |
| Sample | EDS Mapping on Area inside the Scratch | Concentration (at. %) | |||
|---|---|---|---|---|---|
| Ca | P | O | Ti | ||
| H | Area 1 * | 2.43 | 2.23 | 41.45 | Balance |
| Area 2 ** | 1.91 | 1.78 | 39.38 | Balance | |
| H/Mg1 | Area 1 * | 2.23 | 2.02 | 40.39 | Balance |
| Area 2 ** | 1.91 | 1.84 | 38.16 | Balance | |
| H/Mg2 | Area 1 * | 2.01 | 1.9 | 39.6 | Balance |
| Area 2 ** | 1.76 | 1.68 | 38.88 | Balance | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotrut, C.M.; Ungureanu, E.; Ionescu, I.C.; Zamfir, R.I.; Kiss, A.E.; Parau, A.C.; Vladescu, A.; Vranceanu, D.M.; Saceleanu, A. Influence of Magnesium Content on the Physico-Chemical Properties of Hydroxyapatite Electrochemically Deposited on a Nanostructured Titanium Surface. Coatings 2022, 12, 1097. https://doi.org/10.3390/coatings12081097
Cotrut CM, Ungureanu E, Ionescu IC, Zamfir RI, Kiss AE, Parau AC, Vladescu A, Vranceanu DM, Saceleanu A. Influence of Magnesium Content on the Physico-Chemical Properties of Hydroxyapatite Electrochemically Deposited on a Nanostructured Titanium Surface. Coatings. 2022; 12(8):1097. https://doi.org/10.3390/coatings12081097
Chicago/Turabian StyleCotrut, Cosmin Mihai, Elena Ungureanu, Ionut Cornel Ionescu, Raluca Ioana Zamfir, Adrian Emil Kiss, Anca Constantina Parau, Alina Vladescu, Diana Maria Vranceanu, and Adriana Saceleanu. 2022. "Influence of Magnesium Content on the Physico-Chemical Properties of Hydroxyapatite Electrochemically Deposited on a Nanostructured Titanium Surface" Coatings 12, no. 8: 1097. https://doi.org/10.3390/coatings12081097
APA StyleCotrut, C. M., Ungureanu, E., Ionescu, I. C., Zamfir, R. I., Kiss, A. E., Parau, A. C., Vladescu, A., Vranceanu, D. M., & Saceleanu, A. (2022). Influence of Magnesium Content on the Physico-Chemical Properties of Hydroxyapatite Electrochemically Deposited on a Nanostructured Titanium Surface. Coatings, 12(8), 1097. https://doi.org/10.3390/coatings12081097






.jpg)


