Test and Mesoscopic Analysis of Chloride Ion Diffusion of High-Performance-Concrete with Fly Ash and Silica Fume
Abstract
:1. Introduction
2. Experimental Programs
2.1. Detail of Specimens
2.2. Compressive Strength Test
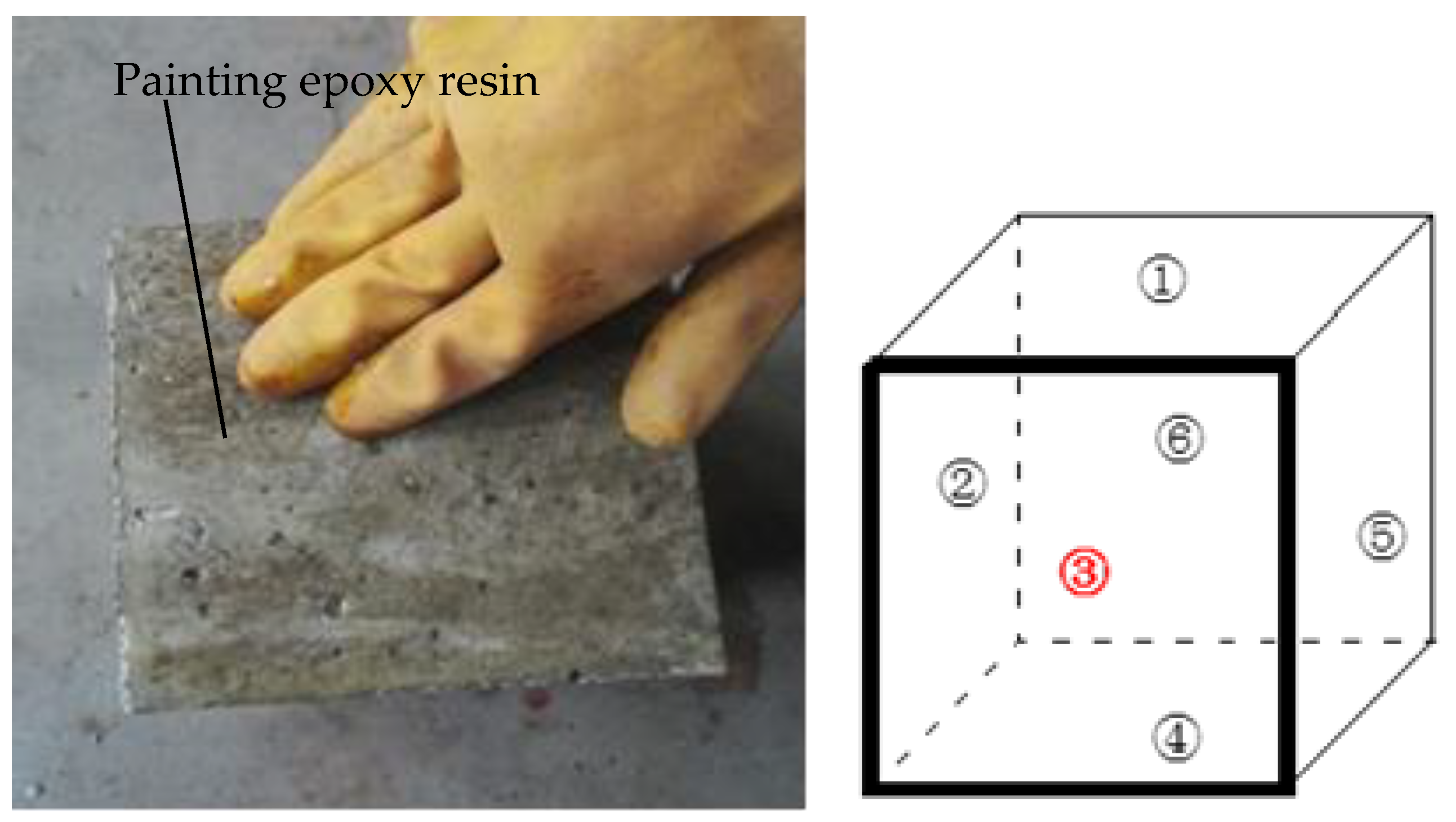
2.3. Chloride Penetration Test
3. Experimental Result Analysis
3.1. Influence of the W/B
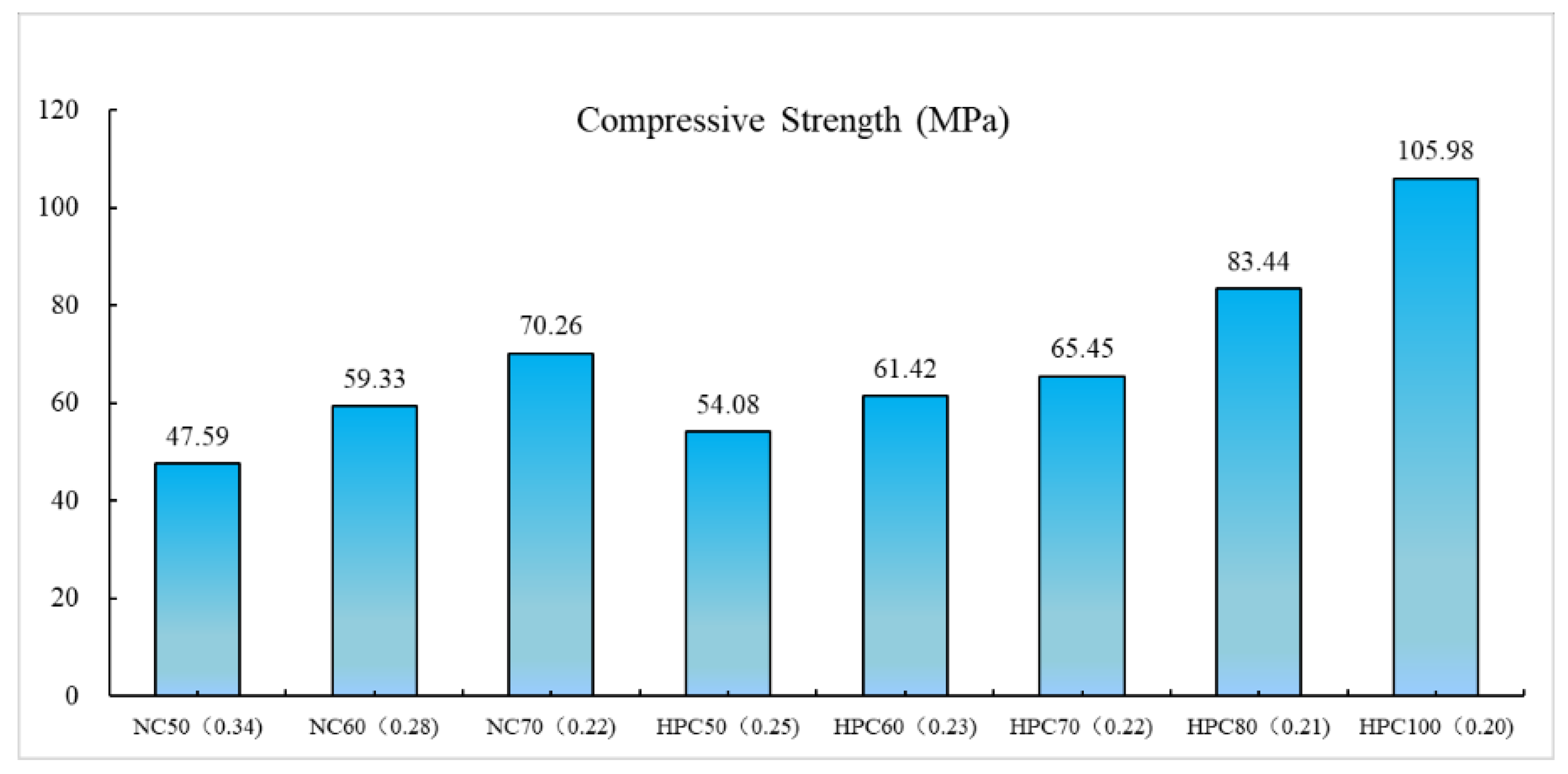
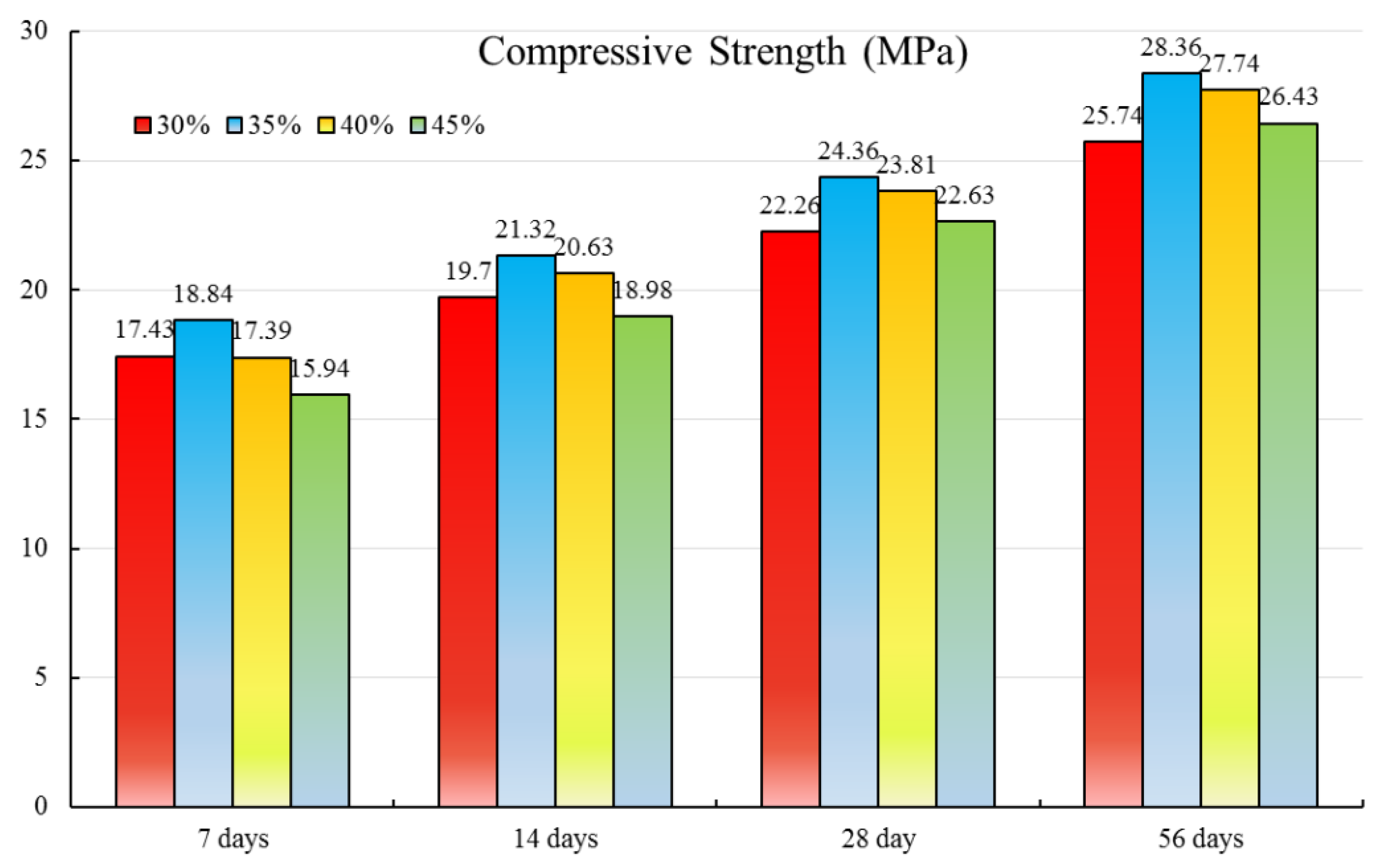
3.1.1. Concrete Compressive Strength
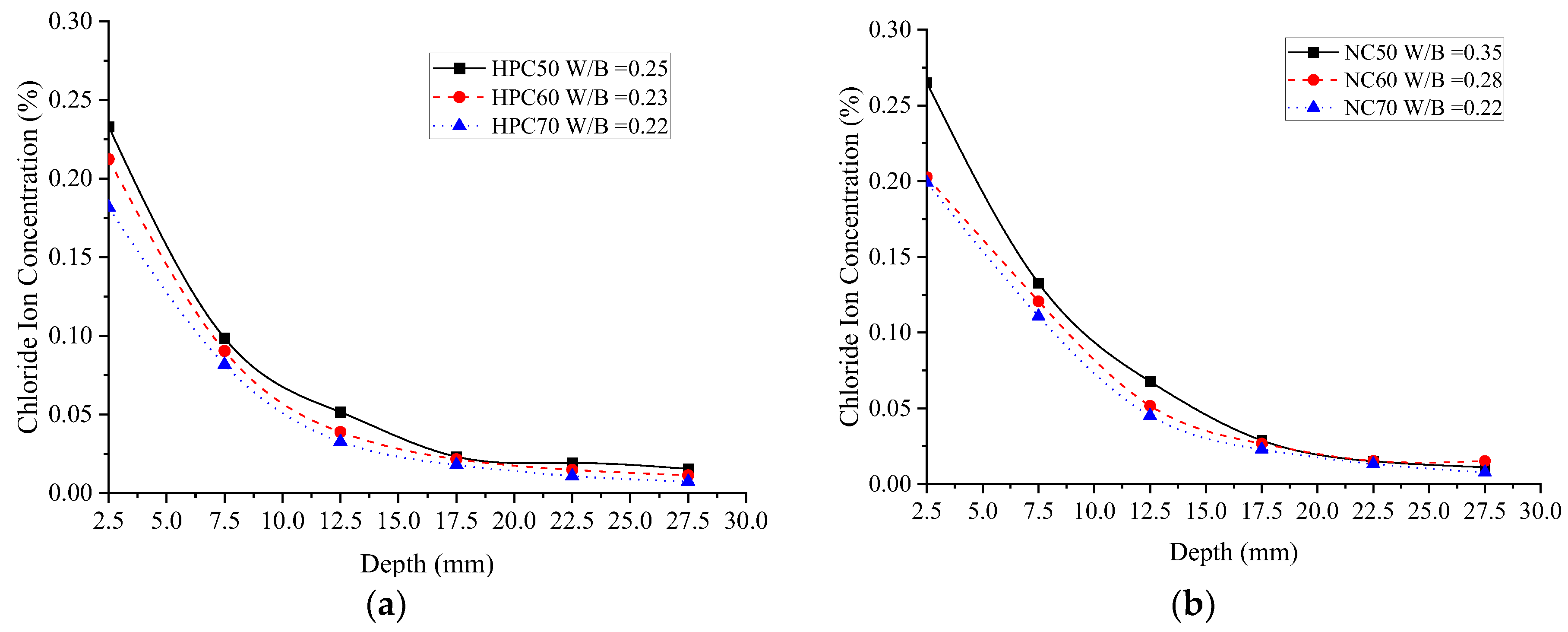
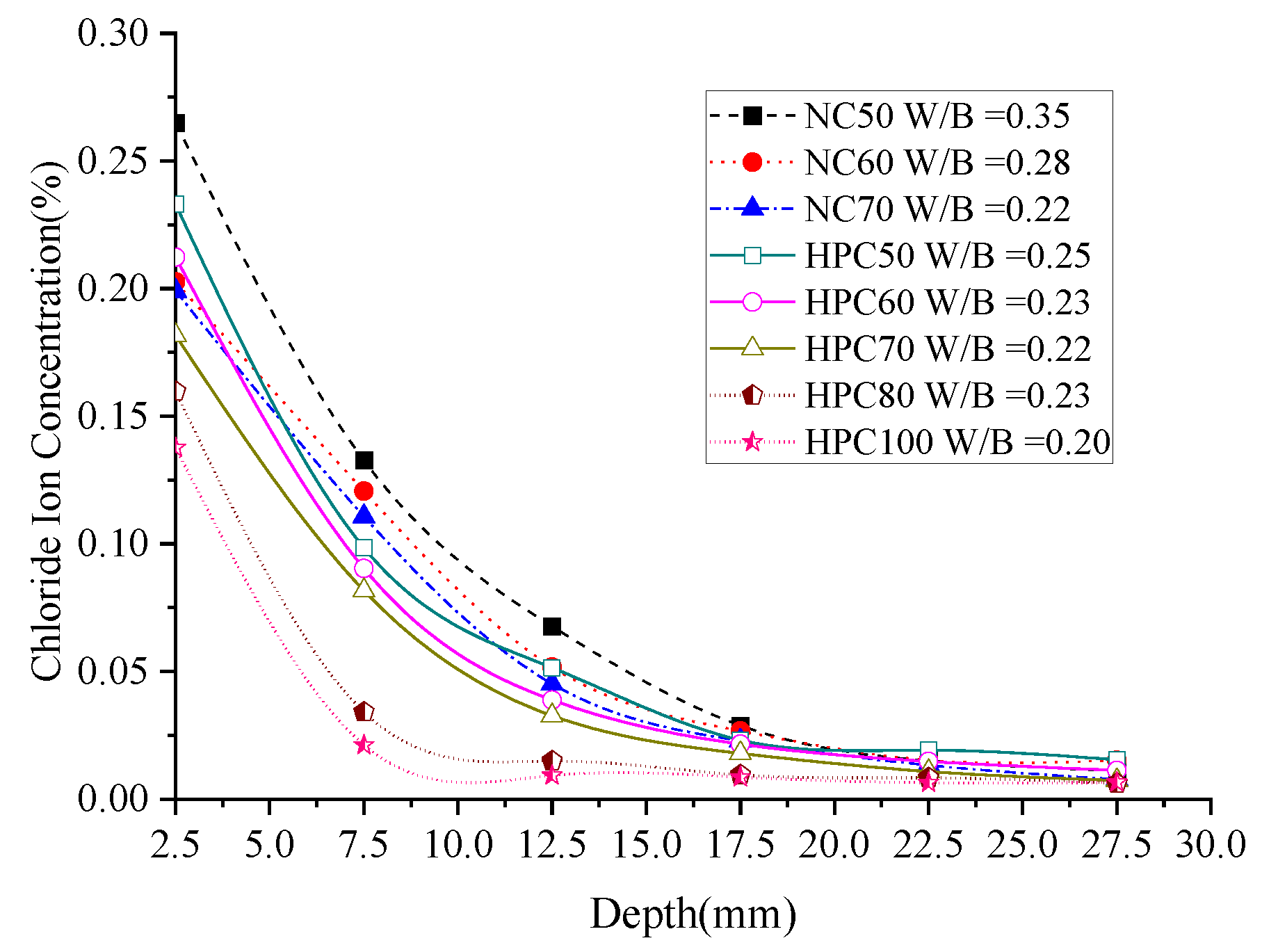
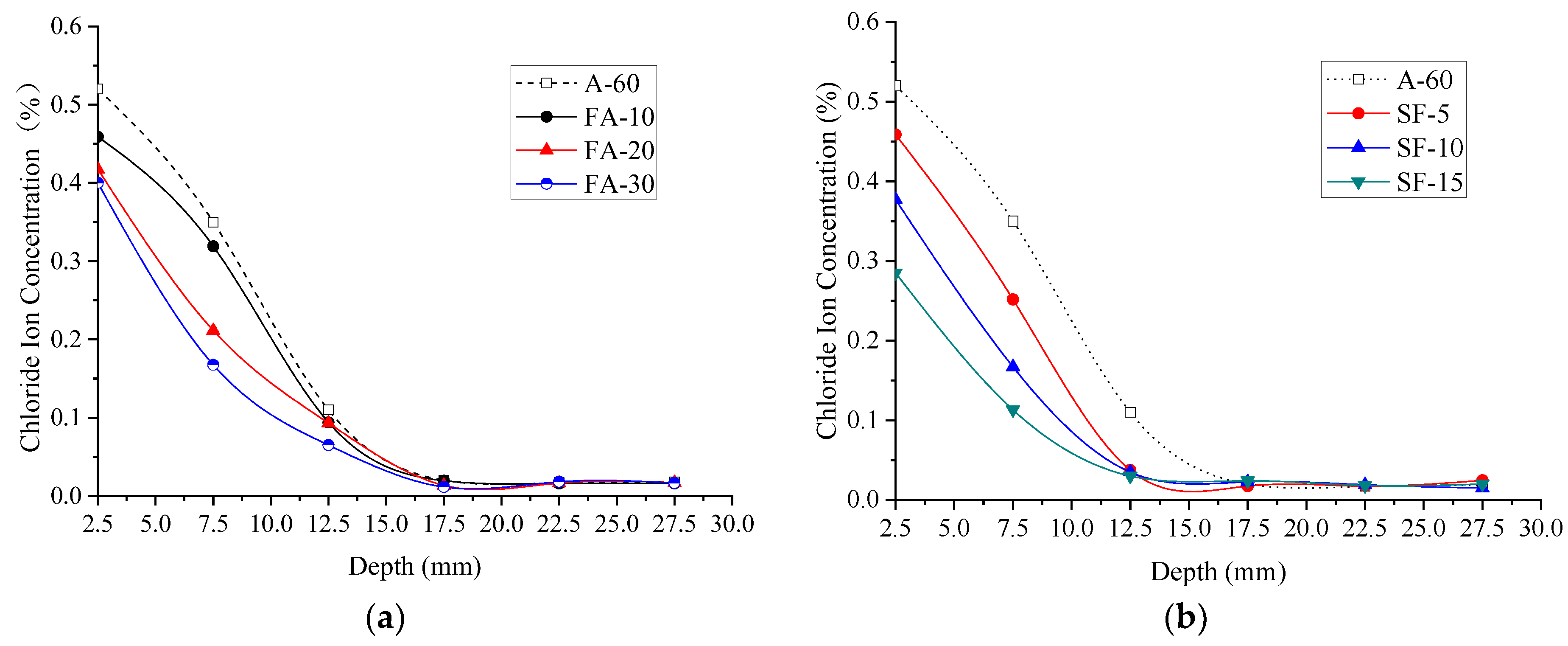
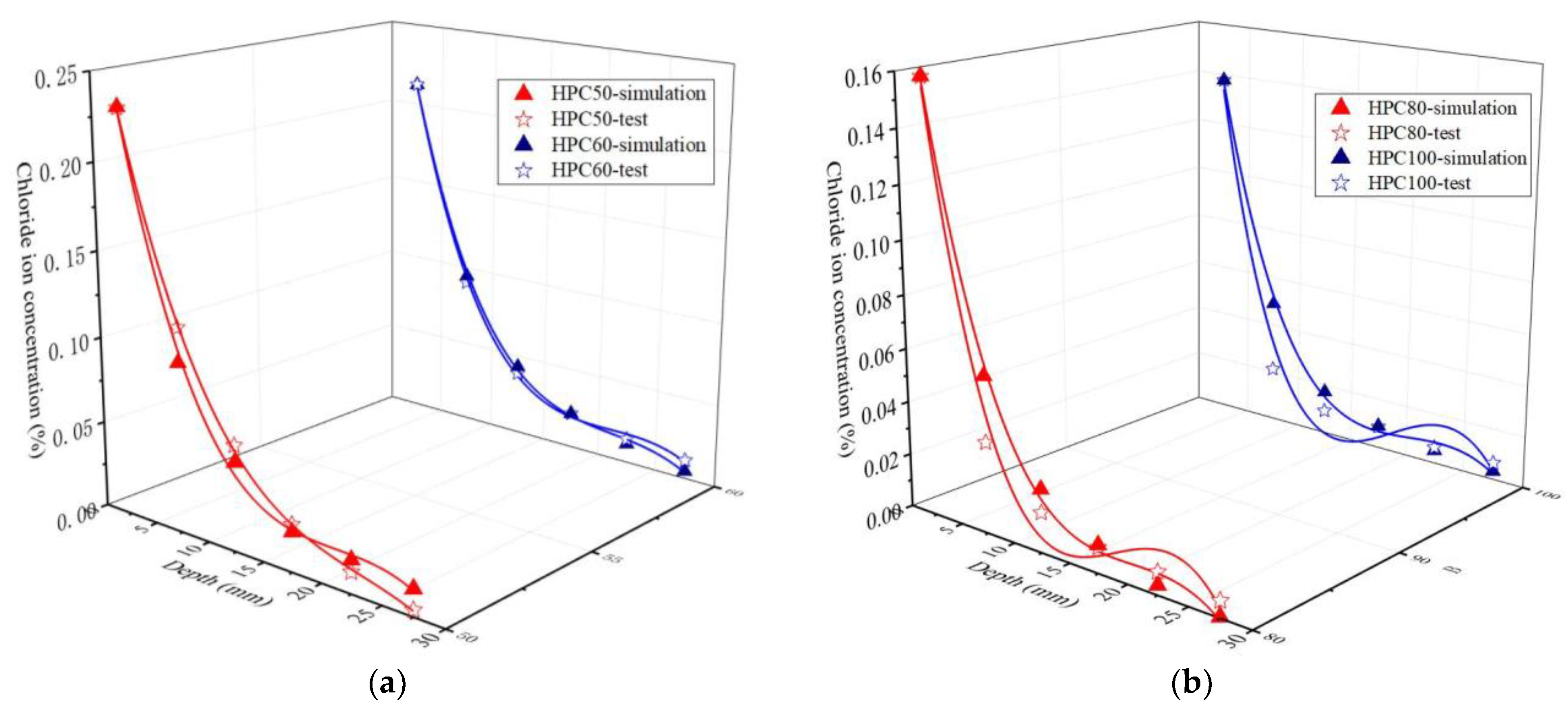
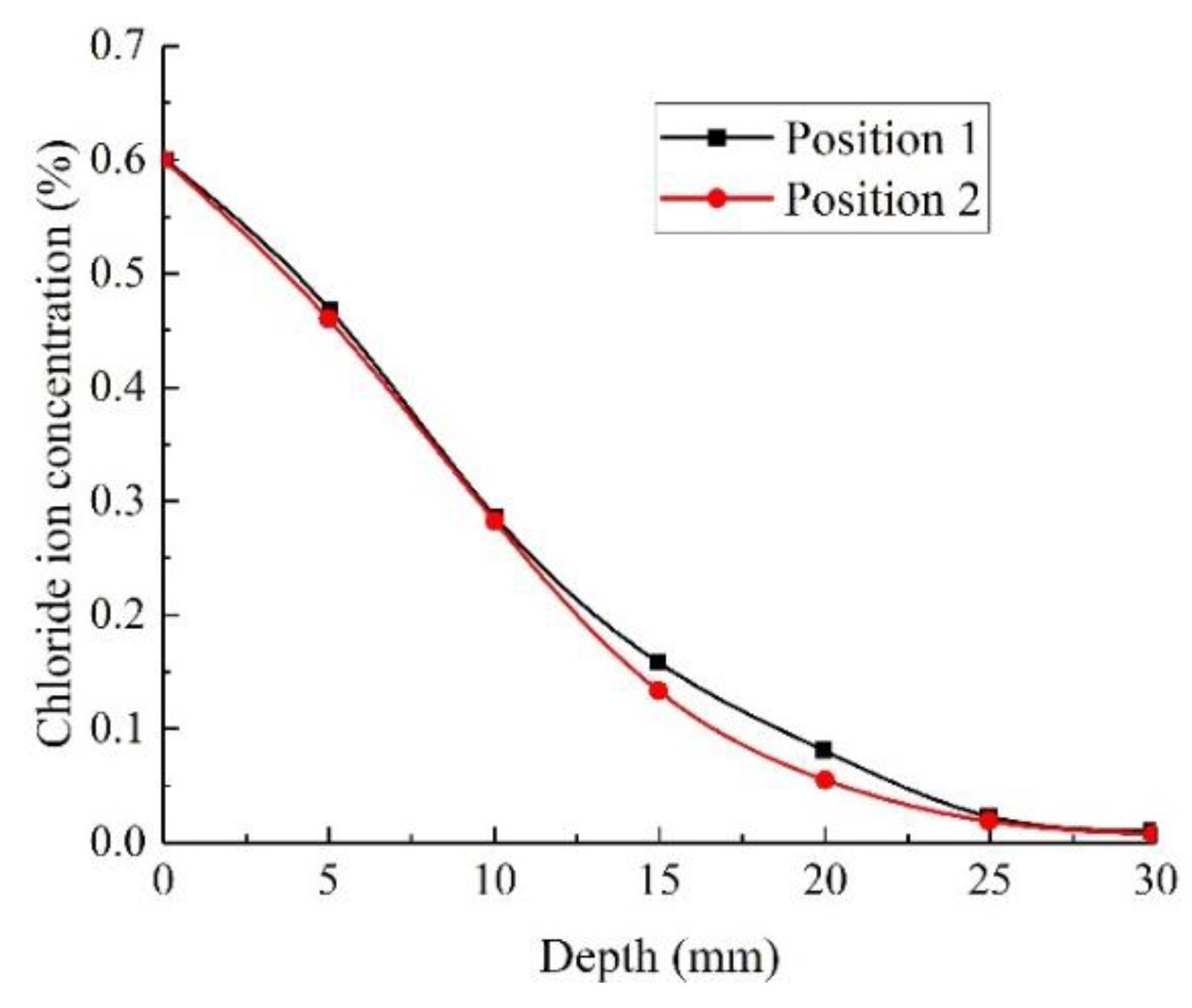
3.1.2. Chloride Ion Concentration (Permeability)
3.2. Influence of the Mixing Method of Mineral Admixtures
3.2.1. Concrete Compressive Strength
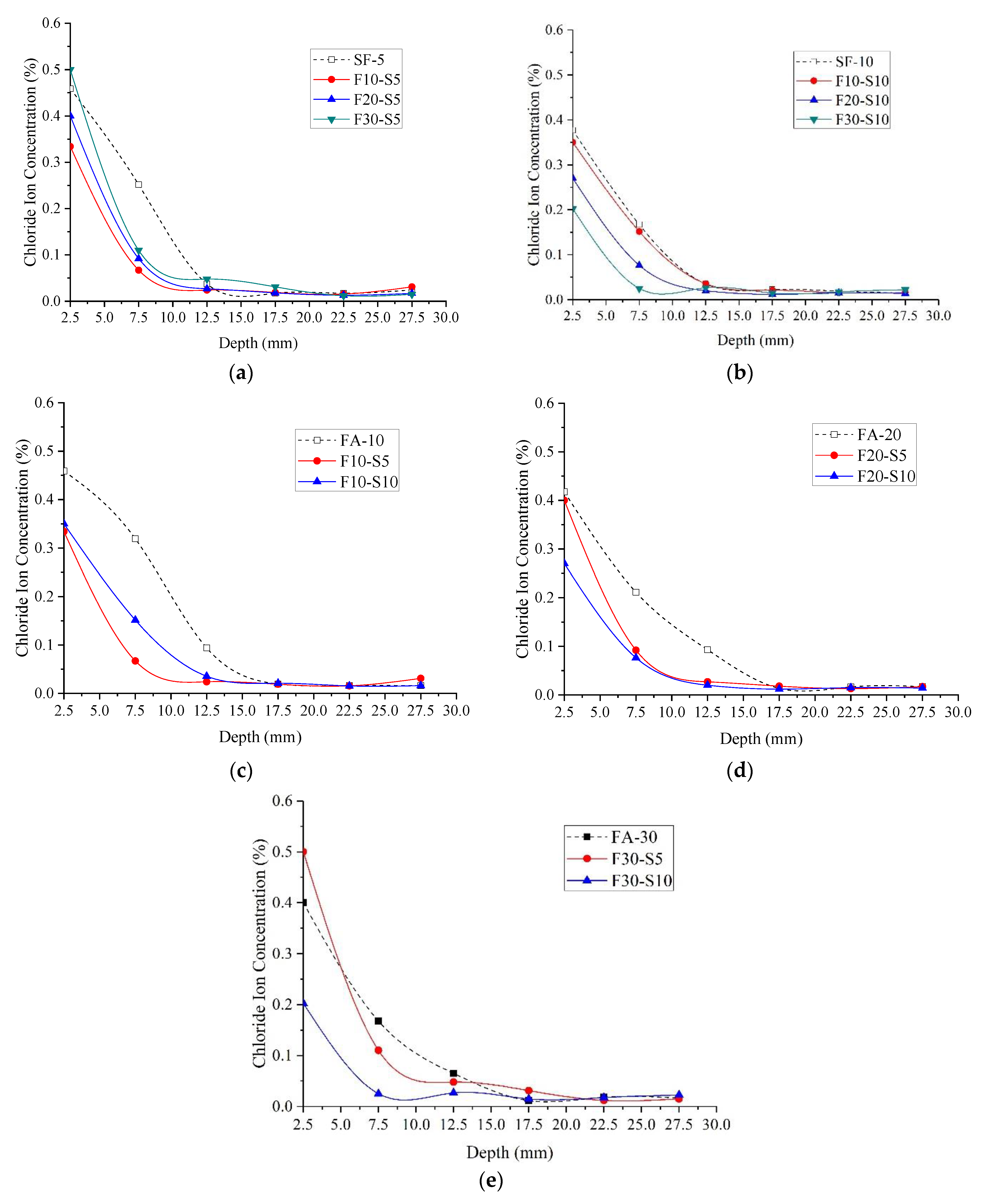
3.2.2. Chloride Ion Concentration
4. Modification of the Chloride Ion Diffusion Model
4.1. Ideal Model-Fick’s Second Law
4.2. Modified Diffusion Model
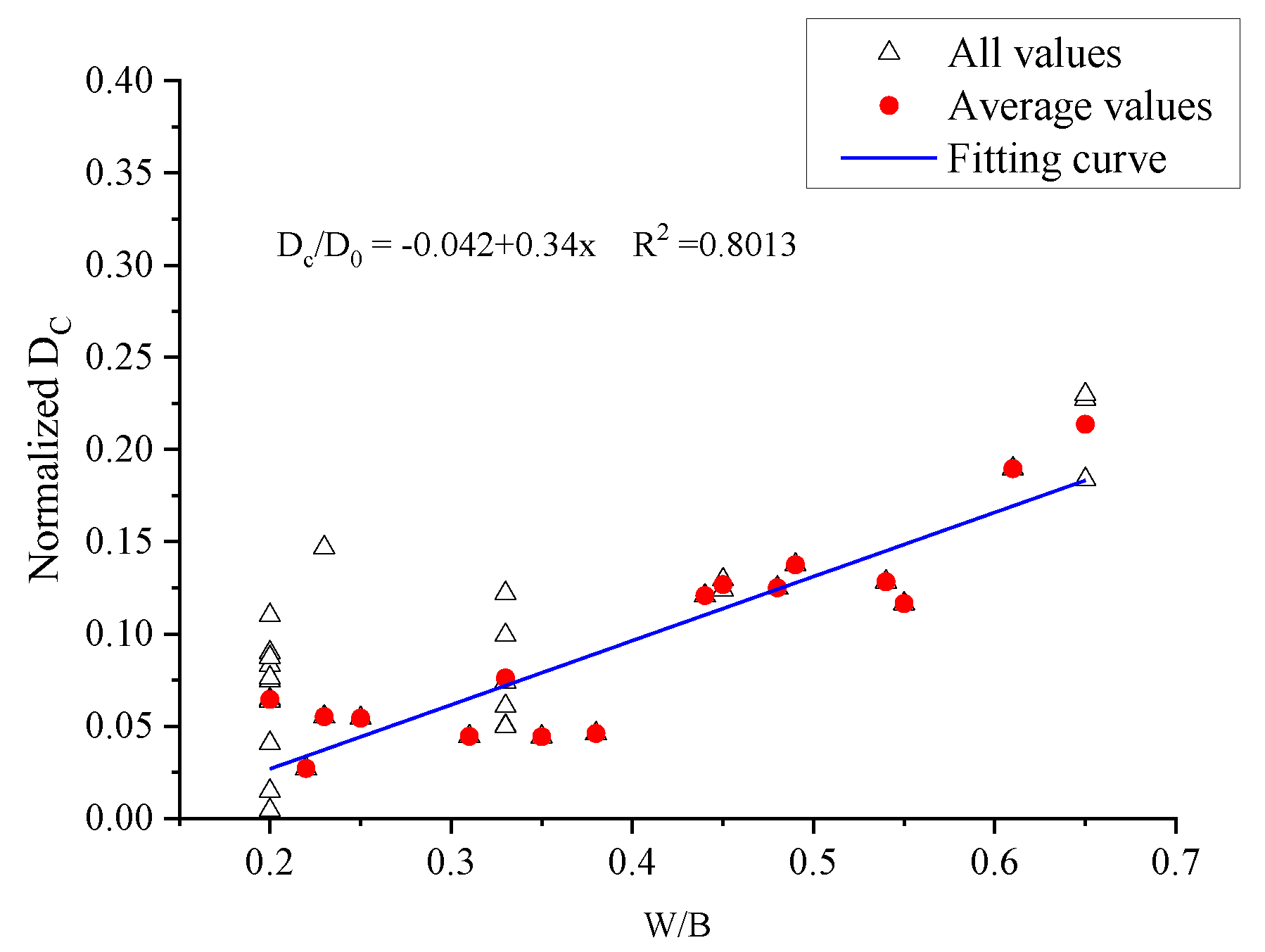
4.2.1. Relationship between the Diffusion Coefficient and W/B
4.2.2. Relationship between the Diffusion Coefficient and Time, Temperature
4.2.3. Relationship between the Diffusion Coefficient and Humidity
4.2.4. Initial Value Condition
4.2.5. Boundary Condition
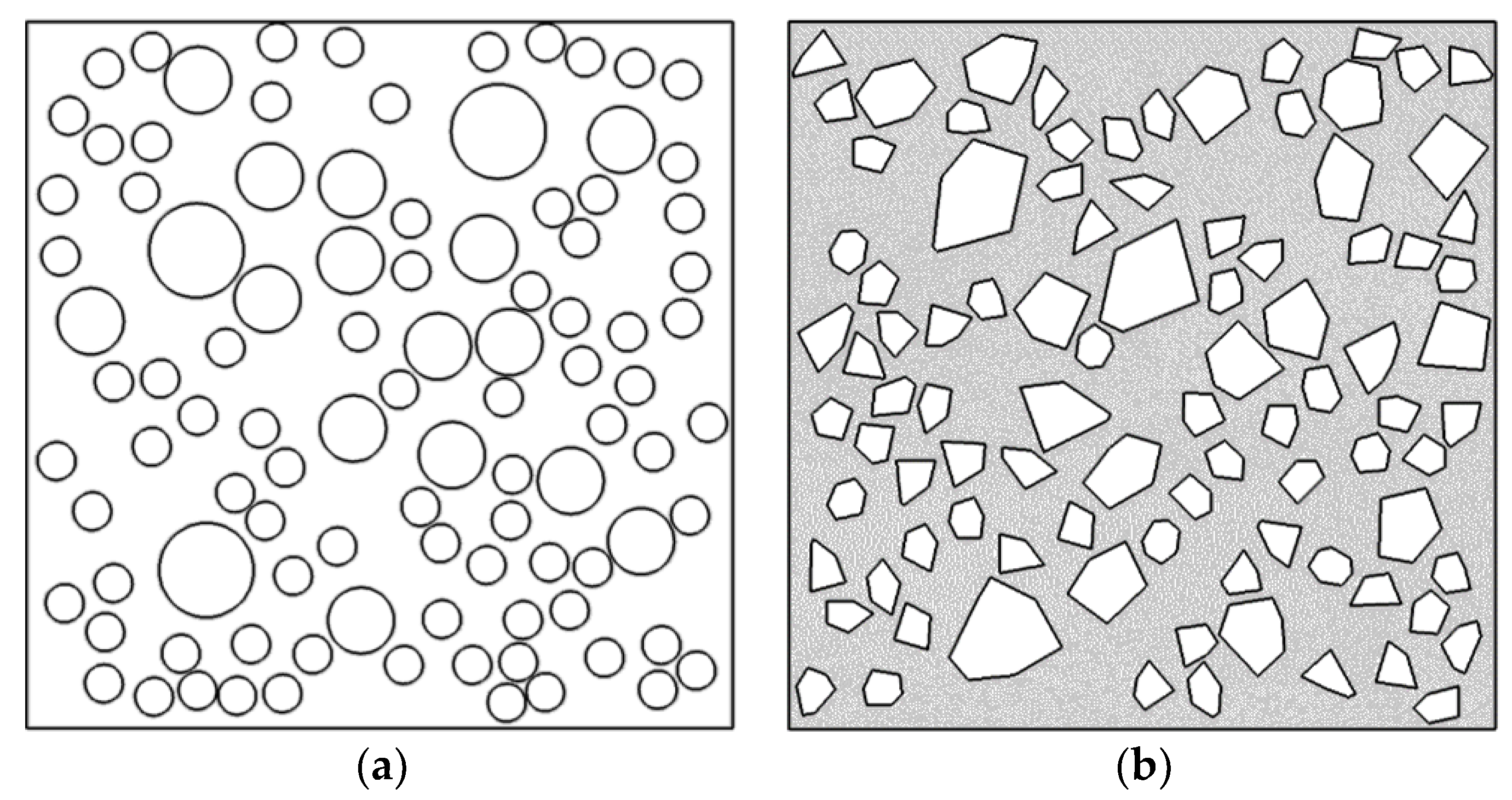
5. Random Aggregate Model of Concrete
5.1. Basic Theory
5.1.1. Circular Aggregate Generation and Release Process
- (1)
- Defining the radius and the centroid coordinates of aggregate (xi, yi)The xi and yi need to satisfy the following requirements: , . Where, a and b are the side length of release area, respectively, a = b = 150 mm in this paper. η is a random number between 0 and 1.
- (2)
- Overlapping judgment.This process is determining whether the generated i aggregate overlaps with the generate i − 1 aggregate. If un-overlapped, the area of the aggregate is calculated and the information of the aggregate is recorded; if overlapped, turning back to the step (1) and recoding the value. The basis of overlap judgment is: If the interval length d between two circular aggregate centroid is larger than the sum of their radius, namely d > R1 + R2, it can be concluded that the circular aggregate with radius R1 does not overlap with the circular aggregate with radius R2.
- (3)
- Judging of the aggregate area.If the condition is not met, the researcher should be turn back to step (1) and continue to release. If the above condition is met, the aggregate is finished. Where A0 is the area fraction of the calculated aggregate.
5.1.2. Polygonal Aggregate Generation and Release Process
5.1.3. Examples of Random Aggregate
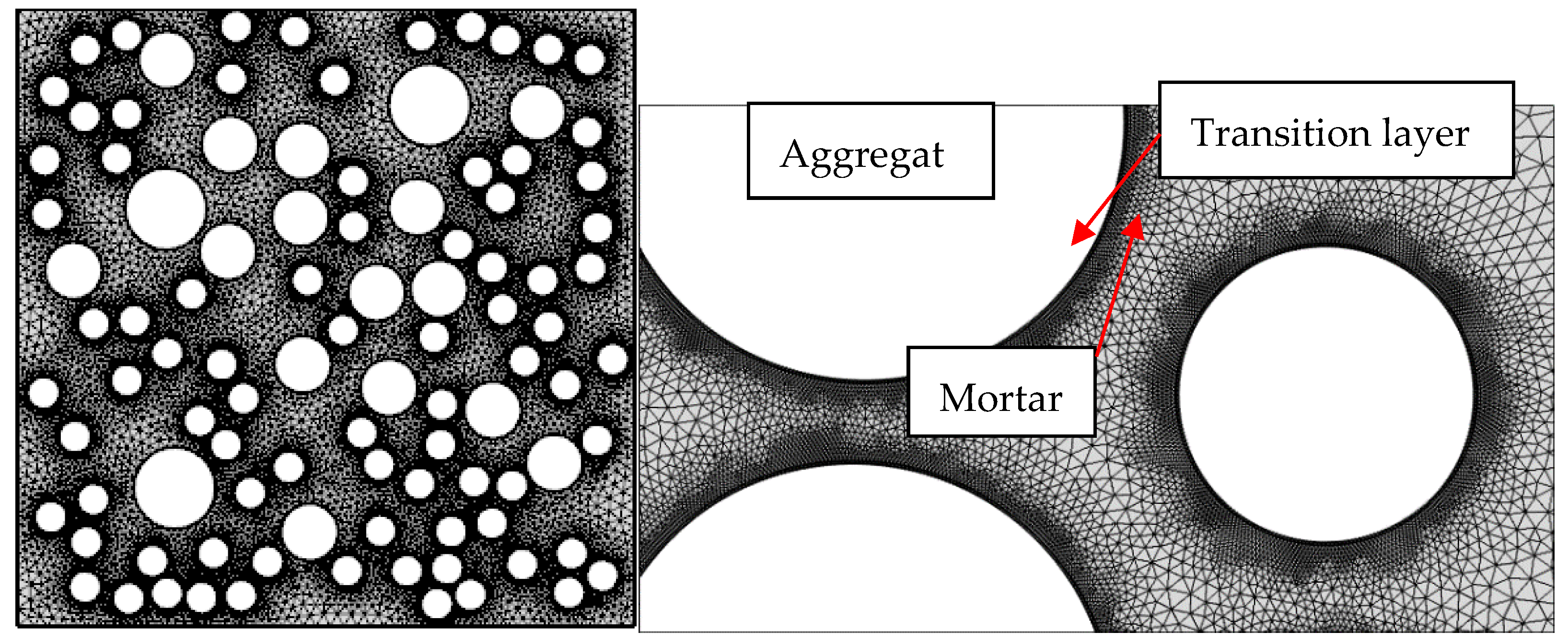
6. Mesoscopic Simulation of Chloride Ion Diffusion
Simulation Method
- i.
- Choosing the Transport of Diluted Species Interface in the COMSOL software and importing the two-dimensional concrete random aggregate geometric model proposed in Part 4 into the software and set the cell.
- ii.
- Defining the parameter value in transfer equation and diffusion coefficient model based on the Equation (11).
- iii.
- Defining the boundary condition Cs, diffusion coefficient and initial value condition C0.
- iv.
- Dividing the mesh.
- v.
- Defining a two-dimensional transversal and extracting the chloride ion concentration on the transversal lines of 2.5 mm, 7.5 mm, 12.5 mm, 17.5 mm, 22.5 mm, and 27.5 mm, respectively.
Dividing the Mesh
7. Microscopic Model Validation and Parameters Analysis
7.1. Model Validation
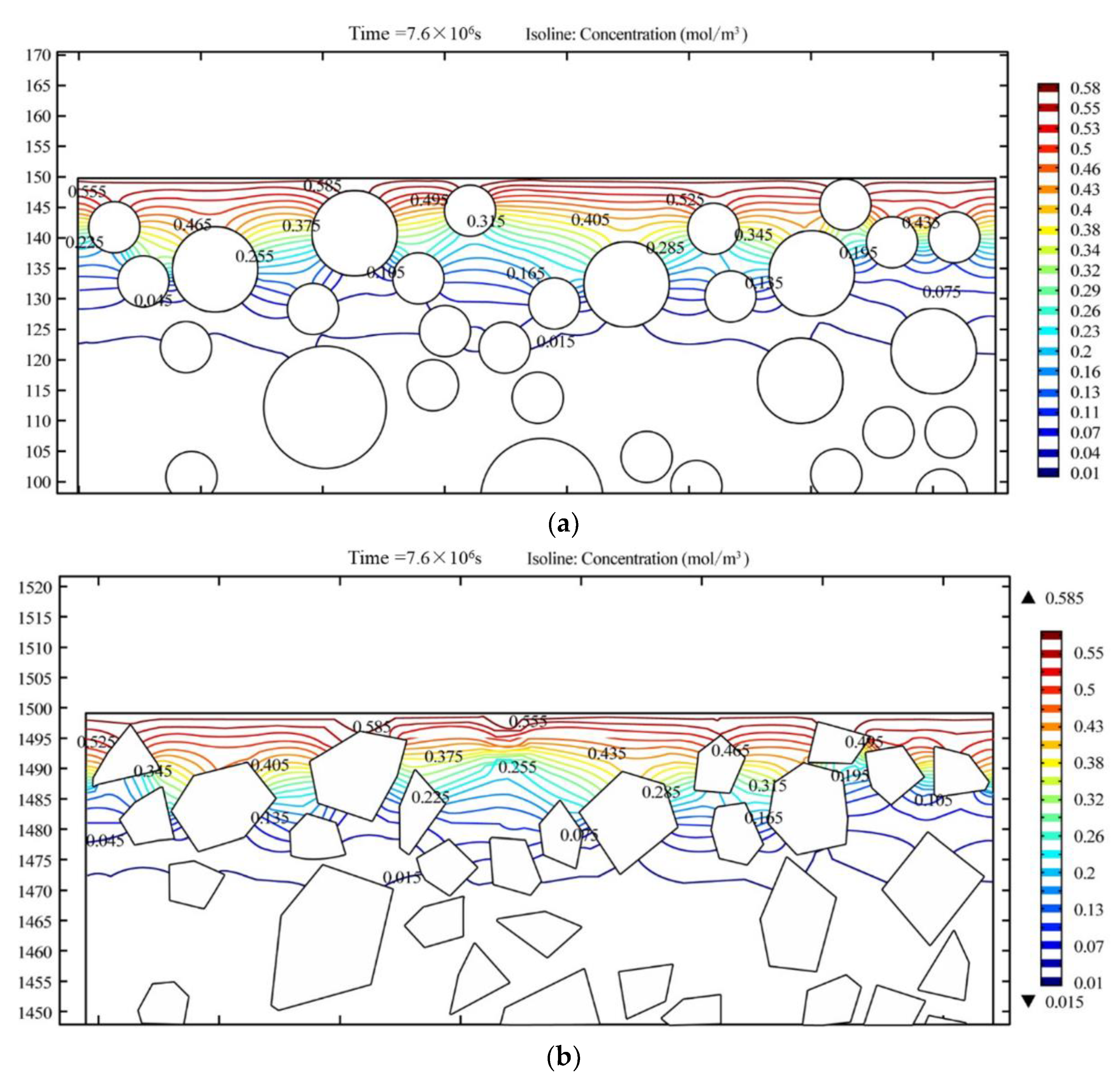
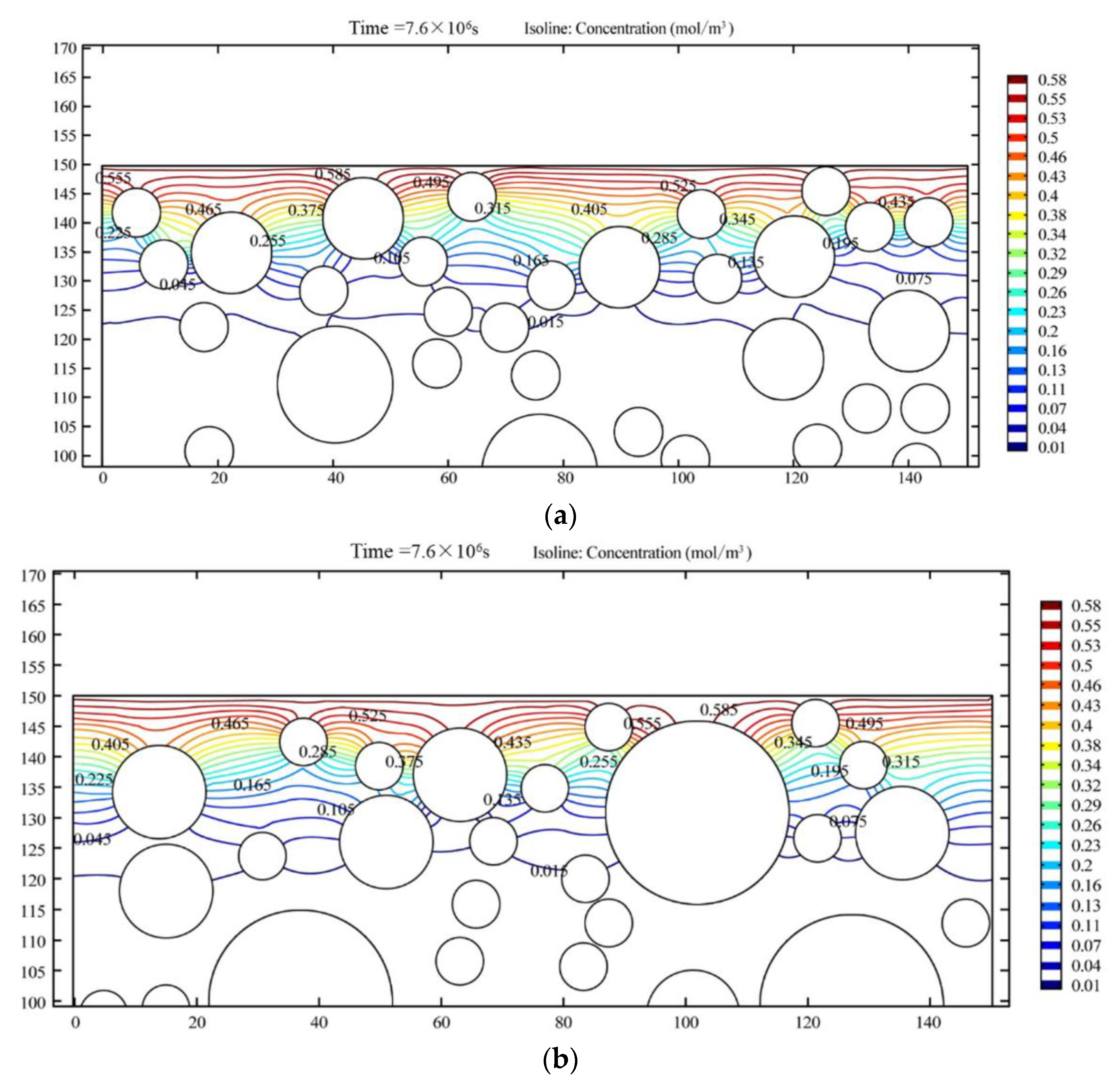
7.1.1. Effect of Aggregate Shape on Chloride Ion Permeability
7.1.2. Effect of Aggregate Distribution on Chloride Ion Permeability
7.1.3. Effect of Aggregate Size on Chloride Ion Permeability
8. Conclusions
- (1)
- The influence of the W/B on the concrete strength for normal concrete is obviously more than the HPC. The contents of the SF or FA developing the compressive strength is limited. The concrete compressive strength of ternary combination specimens decreases with the increase in FA or SF when the content of the other mineral admixture SF or FA keeps unchanged.
- (2)
- No matter normal concrete or the HPC, from depth 7.5 mm to 17.5 mm, the influence of W/B on the chloride ion concentration is most significant, under the same diffusion depth, the chloride concentration decreases with the increase in W/B. The ternary combination of PC-FA-SF is more efficient in prohibiting chlorides ingress inside the specimens than the binary combination of PC-FA or PC-SF.
- (3)
- The mesoscopic simulation and the tested value of the chloride ion under the same depth is close, the average ratio of simulation value to tested value is 0.91. The aggregate shape and distribution have a negligible influence on chloride diffusivity in the HPC, but the chloride ion concentration increases with the increase in aggregate size.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bachtiar, E.; Rachim, F.; Makbul, R.; Tata, A.; Irfan-Ul-Hassan, M.; Kırgız, M.S.; Syarif, M.; De Sousa Galdino, A.G.; Khitab, A.; Benjeddou, O.; et al. Monitoring of chloride and Friedel’s salt, hydration components, and porosity in high-performance concrete. Case Stud. Constr. Mat. 2022, 17, e01208. [Google Scholar] [CrossRef]
- Xie, H.; Yang, L.; Zhang, Q.; Huang, C.; Chen, M.; Zhao, K. Research on energy dissipation and damage evolution of dynamic splitting failure of basalt fiber reinforced concrete. Constr. Build. Mater. 2022, 330, 127292. [Google Scholar] [CrossRef]
- Han, C.; Shen, W.; Ji, X.; Wang, Z.; Ding, Q.; Xu, G.; Lv, Z.; Tang, X. Behavior of high performance concrete pastes with different mineral admixtures in simulated seawater environment. Constr. Build. Mater. 2018, 187, 426–438. [Google Scholar] [CrossRef]
- Felekoğlu, B.; Ramyar, K.; Tosun, K.; Musal, B. Sulfate resistances of different types of Turkish Portland cements by selecting the appropriate test methods. Constr. Build. Mater. 2006, 20, 819–823. [Google Scholar] [CrossRef]
- Felekoğlu, B.; Sarıkahya, H. Effect of chemical structure of polycarboxylate-based superplasticizers on workability retention of self-compacting concrete. Constr. Build. Mater. 2008, 22, 1972–1980. [Google Scholar] [CrossRef]
- Seleem, H.E.-D.H.; Rashad, A.M.; El-Sabbagh, B.A. Durability and strength evaluation of high-performance concrete in marine structures. Constr. Build. Mater. 2010, 24, 878–884. [Google Scholar] [CrossRef]
- Sengul, O.; Tasdemir, M.A. Compressive strength and rapid chloride permeability of concretes with ground fly ash and slag. J. Mater. Civil. Eng. 2009, 21, 494–501. [Google Scholar] [CrossRef]
- Chalee, W.; Jaturapitakkul, C. Effects of W/B ratios and fly ash finenesses on chloride diffusion coefficient of concrete in marine environment. Mater. Struct. 2009, 42, 505–514. [Google Scholar] [CrossRef]
- Moffatt, E.G.; Thomas, M.D.A.; Fahim, A. Performance of high-volume fly ash concrete in marine environment. Cem. Concr. Res. 2017, 102, 127–135. [Google Scholar] [CrossRef]
- Cabrera, J.G.; Nwaubani, S.O. The microstructure and chloride ion diffusion characteristics of cements containing metakaolin and fly ash. ACI Symp. Public. 1998, 178, 385–400. [Google Scholar]
- Thomas, B.S.; Gupta, R.C.; Mehra, P.; Kumar, S. Performance of high strength rubberized concrete in aggressive environment. Constr. Build. Mater. 2015, 83, 320–326. [Google Scholar] [CrossRef]
- Hooton, R.; Gruber, K.; Boddy, A. The chloride penetration resistance of concrete containing high reactivity metakaolin. In Proceedings of the PCI/FHWA International Symposium on High Performance Concrete, Orlando, FL, USA, 25–27 September 2001; pp. 172–183. [Google Scholar]
- Coleman, N.J.; Page, C.L. Aspects of the pore solution chemistry of hydrated cement pastes containing metakaolin. Cem. Concr. Res. 1997, 27, 147–154. [Google Scholar] [CrossRef]
- Geetha, S.; Madhavan, S. High Performance Concrete with Copper slag for Marine Environment. Mater. Today Proc. 2017, 4, 3525–3533. [Google Scholar] [CrossRef]
- Harilal, M.; Rathish, V.R.; Anandkumar, B.; George, R.P.; Mohammed, M.S.H.S.; Philip, J.; Amarendra, G. High performance green concrete (HPGC) with improved strength and chloride ion penetration resistance by synergistic action of fly ash, nanoparticles and corrosion inhibitor. Constr. Build. Mater. 2019, 198, 299–312. [Google Scholar] [CrossRef]
- Jiang, J.; Zheng, X.; Wu, S.; Liu, Z.; Zheng, Q. Nondestructive experimental characterization and numerical simulation on self-healing and chloride ion transport in cracked ultra-high performance concrete. Constr. Build. Mater. 2019, 198, 696–709. [Google Scholar] [CrossRef]
- Ding, Q.; Geng, J.; Hu, S.; Sun, J.; Sun, B. Different effects of fly ash and slag on anti-rebar corrosion ability of concrete with chloride ion. Wuhan Univ. J. Nat. Sci. 2009, 14, 355–361. [Google Scholar] [CrossRef]
- Khan, M.I. Comparison of chloride ion penetration and diffusion of high-performance concrete. Ksce. J. Civ. Eng. 2012, 16, 779–784. [Google Scholar] [CrossRef]
- Wang, L.; Dai, L.; Bian, H.; Ma, Y.; Zhang, J. Concrete cracking prediction under combined prestress and strand corrosion. Struct. Infrastruct. Eng. 2019, 15, 285–295. [Google Scholar] [CrossRef]
- Dai, L.; Bian, H.; Wang, L.; Potier-Ferry, M.; Zhang, J. Prestress Loss Diagnostics in Pre-tensioned Concrete Structures with Corrosive Cracking. J. Struct. Eng. 2019, 146, 04020013. [Google Scholar] [CrossRef]
- Peng, J.; Tang, H.; Zhang, J. Structural Behavior of Corroded Reinforced Concrete Beams Strengthened with Steel Plate. J. Perform. Constr. Facil. 2017, 31, 04017013. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Luo, Y.; Wang, L. Interface shear strength between self-compacting concrete and carbonated concrete. J. Mater. Civil. Eng. 2020, 32, 04020113. [Google Scholar] [CrossRef]
- Tang, H.; Peng, J.; Zhang, J. Influence of further corrosion on structural behavior of corroded reinforced concrete beam strengthened with steel plate using different strengthening schemes. J. Perform. Constr. Facil. 2020, 34, 04019117. [Google Scholar] [CrossRef]
- Xiao, L.; Peng, J.; Zhang, J.; Ma, Y.; Cai, C.S. Comparative assessment of mechanical properties of HPS between electrochemical corrosion and spray corrosion. Constr. Build. Mater. 2020, 237, 117735. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, L.; Jianren, Z.; Cai, C.S.; Wang, L. Flexural behavior of corroded HPS beams. Eng. Struct. 2019, 195, 274–287. [Google Scholar] [CrossRef]
- Zheng, J.-J.; Zhou, X.-Z.; Wu, Y.-F.; Jin, X.-Y. A numerical method for the chloride diffusivity in concrete with aggregate shape effect. Constr. Build. Mater. 2012, 31, 151–156. [Google Scholar] [CrossRef]
- Du, X.L.; Jin, L.; Ma, G.W. A meso-scale numerical method for the simulation of chloride diffusivity in concrete. Finite Elem. Anal. Des. 2014, 85, 87–100. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, A.; Ruan, X. Spatial variability of chloride and its influence on thickness of concrete cover: A two-dimensional mesoscopic numerical research. Eng. Struct. 2015, 95, 154–169. [Google Scholar] [CrossRef]
- Hu, S.; Peng, J.; Zhang, J.; Cai, C.S. Influences of Time, Temperature, and Humidity on Chloride Diffusivity: Mesoscopic Numerical Research. J. Mater. Civil. Eng. 2017, 29, 04017223. [Google Scholar] [CrossRef]
- Peng, J.; Hu, S.; Zhang, J.; Cai, C.S.; Li, L.-y. Influence of cracks on chloride diffusivity in concrete: A five-phase mesoscale model approach. Constr. Build. Mater. 2019, 197, 587–596. [Google Scholar] [CrossRef]
- Guo, L.-P.; Carpinteri, A.; Roncella, R.; Spagnoli, A.; Sun, W.; Vantadori, S. Fatigue damage of high performance concrete through a 2D mesoscopic lattice model. Comp. Mater. Sci. 2009, 44, 1098–1106. [Google Scholar] [CrossRef]
- Huespe, A.E.; Oliver, J.; Mora, D.F. Computational modeling of high performance steel fiber reinforced concrete using a micromorphic approach. Comput. Mech. 2013, 52, 1243–1264. [Google Scholar] [CrossRef] [Green Version]
- Forquin, P.; Zinszner, J.L.; Lukic, B. Mesoscopic Modelling of Ultra-High Performance Fiber Reinforced Concrete Under Dynamic Loading. In Dynamic Behavior of Materials; Springer International Publishing: Cham, Switzerland, 2017; Volume 1, pp. 123–129. [Google Scholar]
- Feng, T.; Jia, M.; Xu, W.; Wang, F.; Li, P.; Wang, X.; Tan, Y.; Jiang, J. Three-dimensional mesoscopic investigation of the compression mechanical properties of ultra-high performance concrete containing coarse aggregates. Cem. Concr. Compos. 2022, 133, 104678. [Google Scholar] [CrossRef]
- MOHURD (Ministry of Housing and Urban-Rural Development). GB50010-2010: Code for Design of Concrete Structures; Ministry of Housing and Urban-Rural Development of the People’s Republic of China (MOHURD): Beijing, China, 2010.
- ASTM (American Society for Testing and Materials). Standard Test Method for Electrical Indication of Concretes Ability to Resist Chloride Ion Penetration; ASTM C1202; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- AASHT (American Association of State Highway and Transportation Officials). Standard Method of Test for Sampling and Testing for Chloride Ion in Concrete and Concrete Raw Material; AASHTOT260-97; AASHT: Washington, DC, USA, 1997. [Google Scholar]
- Chindaprasirt, P.; Jaturapitakkul, C.; Sinsiri, T. Effect of fly ash fineness on compressive strength and pore size of blended cement paste. Cem. Concr. Comp 2005, 27, 425–428. [Google Scholar] [CrossRef]
- Jerath, S.; Hanson, N. Effect of Fly Ash Content and Aggregate Gradation on the Durability of Concrete Pavements. J. Mater. Civil. Eng. 2007, 19, 367–375. [Google Scholar] [CrossRef]
- Leng, F.; Feng, N.; Lu, X. An experimental study on the properties of resistance to diffusion of chloride ions of fly ash and blast furnace slag concrete. Cem. Concr. Res. 2000, 30, 989–992. [Google Scholar] [CrossRef]
- Bamforth, P. Spreadsheet model for reinforcement corrosion in structures exposed to chlorides. Concr. Sev. Cond. 1998, 2, 64–75. [Google Scholar]
- Mangat, P.S.; Limbachiya, M.C. Effect of initial curing on chloride diffusion in concrete repair materials. Cem. Concr. Res. 1999, 29, 1475–1485. [Google Scholar] [CrossRef]
- Thomas, M.; Bamforth, P.B. Modelling chloride diffusion in concrete: Effect of fly ash and slag. Cem. Concr. Res. 1999, 29, 487–495. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Matthews, J.D. Performance of pfa concrete in a marine environment—10-year results. Cem. Concr. Comp 2004, 26, 5–20. [Google Scholar] [CrossRef]
- Shafiq, N. Effects of fly ash on chloride migration in concrete and calculation of cover depth required against the corrosion of embedded steel reinforcement. Struct. Concr. 2004, 5, 5–9. [Google Scholar] [CrossRef]
- Mangat, P.S.; Molloy, B.T. Prediction of long term chloride concentration in concrete. Mater. Struct. 1994, 27, 338–346. [Google Scholar] [CrossRef]
- Peng, J.; Tang, H.; Xiao, L.; Chen, X.; Zhang, J. Chloride diffusion and time to corrosion initiation of reinforced concrete structures. Mag. Concr. Res. 2021, 73, 757–770. [Google Scholar] [CrossRef]
- Bitaraf, M.; Mohammadi, S. Analysis of chloride diffusion in concrete structures for prediction of initiation time of corrosion using a new meshless approach. Constr. Build. Mater. 2008, 22, 546–556. [Google Scholar] [CrossRef]
- Liu, T.; Weyers, R.W. Modeling the Dynamic Corrosion Process in Chloride Contaminated Concrete Structures. Cem. Concr. Res. 1998, 28, 365–379. [Google Scholar] [CrossRef]
- Walraven, J.C.; Reinhardt, H.W. Concrete mechanics. Part A: Theory and experiments on the mechanical behavior of cracks in plain and reinforced concrete subjected to shear loading. Nasa Sti/Recon Tech. Rep. N 1981, 82, 25417. [Google Scholar]
- Basheer, L.; Kropp, J.; Cleland, D.J. Assessment of the durability of concrete from its permeation properties: A review. Constr. Build. Mater. 2001, 15, 93–103. [Google Scholar] [CrossRef]





| Specimen | Water (kg/m3) | Cement (kg/m3) | FA (kg/m3) | SF (kg/m3) | Fine Aggregate (kg/m3) | Coarse Aggregate (kg/m3) | Super Plasticizer (kg/m3) | FA Ratio | SF Ratio | W/B |
|---|---|---|---|---|---|---|---|---|---|---|
| NC50 1 | 136.35 | 396 | 0 | 0 | 837.4 | 964.25 | 7.13 | ― | ― | 0.34 |
| NC60 | 122.27 | 444 | 0 | 0 | 791.82 | 1008.91 | 8.44 | ― | ― | 0.28 |
| NC70 | 108.145 | 488 | 0 | 0 | 747.3 | 1052.56 | 9.76 | ― | ― | 0.22 |
| HPC50 2 | 123.135 | 396 | 64 | 35 | 795 | 1005.86 | 8.91 | 13% | 7% | 0.25 |
| HPC60 | 119.63 | 409 | 66 | 36 | 783.34 | 1017.03 | 9.71 | 13% | 7% | 0.23 |
| HPC70 | 115.215 | 421 | 69 | 37 | 769.56 | 1030.22 | 10.54 | 13% | 7% | 0.22 |
| HPC80 | 144 | 501 | 81 | 44 | 647 | 1096 | 8.76 | 13% | 7% | 0.21 |
| HPC100 | 129 | 516 | 84 | 45 | 607 | 1137 | 9.03 | 13% | 7% | 0.20 |
| Specimen | Water (kg/m3) | Cement (kg/m3) | FA (kg/m3) | SF (kg/m3) | Fine Aggregate (kg/m3) | Coarse Aggregate (kg/m3) | Super Plasticizer (kg/m3) | FA Ratio | SF Ratio | W/B |
|---|---|---|---|---|---|---|---|---|---|---|
| A-60 | 97.6 | 488 | 0 | 0 | 745 | 1044.5 | 10.5 | ― | ― | 0.2 |
| FA-10 | 97.6 | 439.2 | 48.8 | 0 | 745 | 1044.5 | 10.5 | 10% | ― | 0.2 |
| FA-20 | 97.6 | 390.5 | 97.6 | 0 | 745 | 1044.5 | 10.5 | 20% | ― | 0.2 |
| FA-30 | 97.6 | 341.3 | 147 | 0 | 745 | 1044.5 | 10.5 | 30% | ― | 0.2 |
| SF-5 | 97.6 | 463.2 | 0 | 24.6 | 745 | 1044.5 | 10.5 | ― | 5% | 0.2 |
| SF-10 | 97.6 | 439.5 | 0 | 49.2 | 745 | 1044.5 | 10.5 | ― | 10% | 0.2 |
| SF-15 | 97.6 | 414.2 | 0 | 73.8 | 745 | 1044.5 | 10.5 | ― | 15% | 0.2 |
| F10-S5 | 97.6 | 415.0 | 48.8 | 24.4 | 745 | 1044.5 | 10.5 | 10% | 5% | 0.2 |
| F20-S5 | 97.6 | 366.2 | 97.8 | 24.3 | 745 | 1044.5 | 10.5 | 20% | 5% | 0.2 |
| F30-S5 | 97.6 | 316.8 | 146.8 | 24.6 | 745 | 1044.5 | 10.5 | 30% | 5% | 0.2 |
| F10-S10 | 97.6 | 390.3 | 48.9 | 48.8 | 745 | 1044.5 | 10.5 | 10% | 10% | 0.2 |
| F20-S10 | 97.6 | 341.3 | 98.2 | 48.5 | 745 | 1044.5 | 10.5 | 20% | 10% | 0.2 |
| F30-S10 | 97.6 | 292.7 | 146.7 | 48.6 | 745 | 1044.5 | 10.5 | 30% | 10% | 0.2 |
| Specimen | Mass of Aggregate | Volume of Concrete | Volume of Aggregate | Proportion of Aggregate Volume |
|---|---|---|---|---|
| NC50 | 6.08056875 | 0.003375 | 0.002252063 | 0.67 |
| NC60 | 6.07746375 | 0.003375 | 0.002250913 | 0.67 |
| NC70 | 6.074510625 | 0.003375 | 0.002249819 | 0.67 |
| HPC50 | 6.077919375 | 0.003375 | 0.002251081 | 0.67 |
| HPC60 | 6.07624875 | 0.003375 | 0.002250463 | 0.67 |
| HPC70 | 6.074274375 | 0.003375 | 0.002249731 | 0.67 |
| HPC80 | 5.882625 | 0.003375 | 0.00217875 | 0.65 |
| HPC100 | 5.886 | 0.003375 | 0.00218 | 0.65 |
| Specimen | D0 | 22 | 18 | 12 | 5 |
|---|---|---|---|---|---|
| NC50 | D0/Dmax | 1 | 0.81 | 0.55 | 0.23 |
| Pc | 0.65 | 0.61 | 0.51 | 0.33 | |
| HPC80 | D0/Dmax | 1 | 0.81 | 0.55 | 0.23 |
| Pc | 0.63 | 0.59 | 0.49 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Yang, Y.; Peng, J.; Liu, P.; Zhang, J. Test and Mesoscopic Analysis of Chloride Ion Diffusion of High-Performance-Concrete with Fly Ash and Silica Fume. Coatings 2022, 12, 1095. https://doi.org/10.3390/coatings12081095
Tang H, Yang Y, Peng J, Liu P, Zhang J. Test and Mesoscopic Analysis of Chloride Ion Diffusion of High-Performance-Concrete with Fly Ash and Silica Fume. Coatings. 2022; 12(8):1095. https://doi.org/10.3390/coatings12081095
Chicago/Turabian StyleTang, Huang, Yiming Yang, Jianxin Peng, Peng Liu, and Jianren Zhang. 2022. "Test and Mesoscopic Analysis of Chloride Ion Diffusion of High-Performance-Concrete with Fly Ash and Silica Fume" Coatings 12, no. 8: 1095. https://doi.org/10.3390/coatings12081095
APA StyleTang, H., Yang, Y., Peng, J., Liu, P., & Zhang, J. (2022). Test and Mesoscopic Analysis of Chloride Ion Diffusion of High-Performance-Concrete with Fly Ash and Silica Fume. Coatings, 12(8), 1095. https://doi.org/10.3390/coatings12081095





