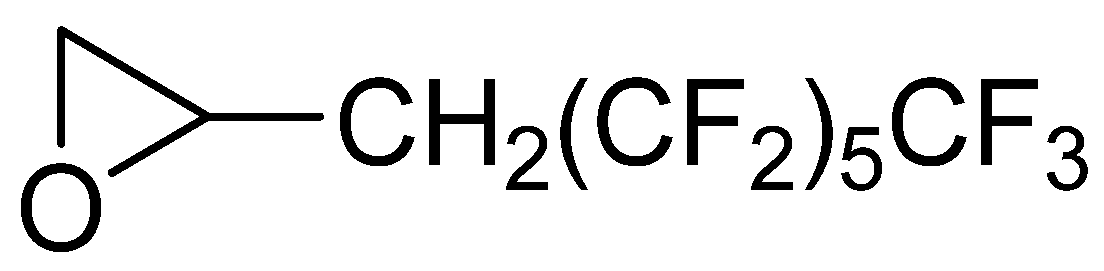3.1. Non-Fluorinated Oligoamides Synthesis
In the first step, the synthesis of non-fluorinated oligoamides was performed by condensation of an ester and an amine group through step-growth polymerization. The monomers were selected to obtain products with the structural characteristics required for their use as wood protective agents. When it was possible, the monomers recoverable from biomass were also chosen.
Dimethyl L-tartrate was chosen as one of the monomers for the production of oligoamides because it has two hydroxyl groups in the molecule, which can make the final product affine to a polar material as wood. The dextrorotatory enantiomer of (R,R)-L-(+)-tartaric acid is a renewable resource widely distributed in nature (in many plants, particularly grapes, bananas, and tamarinds as well as in other fruits).
Diethyl succinate or diethyl adipate were selected to improve the solubility in alcohol and the hydrophobicity of the final fluorinated derivatives. Diethyl succinate derives from succinic acid, which is a naturally occurring four-carbon dicarboxylic acid, naturally formed by most living cells as an outcome of anaerobic digestion, and it is a coproduct of particular interest in biorefineries production. Adipic acid can be obtained in biorefinery processes by the fermentation of sugars to muconic acid followed by hydrogenation, direct fermentation, or by the anaerobic oxidation of sugar to glucaric acid and subsequent hydrodeoxygenation of glucaric acid.
Ethylenediamine and diethylenetriamine were selected as the amine blocks for the synthesis to compare the role of two different amines in the performance of the final products.
The selected amines can react with esters of dicarboxylic acid to form the –CONH– functional group, which make the final product affine to polar materials by interacting with their components through dipolar interaction or hydrogen bonding. Moreover, the amide group is more stable than other polar groups such as the ester ones.
By combining different amine blocks with different esters of diacids, it is possible to modulate the molecular weights and the physical characteristics of the products, such as hydrophilic or hydrophobic properties and solubility.
In the diethylenetriamine, the secondary –NH– group, generally not involved in the amidation reaction, can interact with polar material with additional hydrogen bonding, providing different physical characteristics and properties.
In summary, the choice of monomers was made in order to balance the presence of polar groups, to favor interactions with polar substrates and hydrophobic parts.
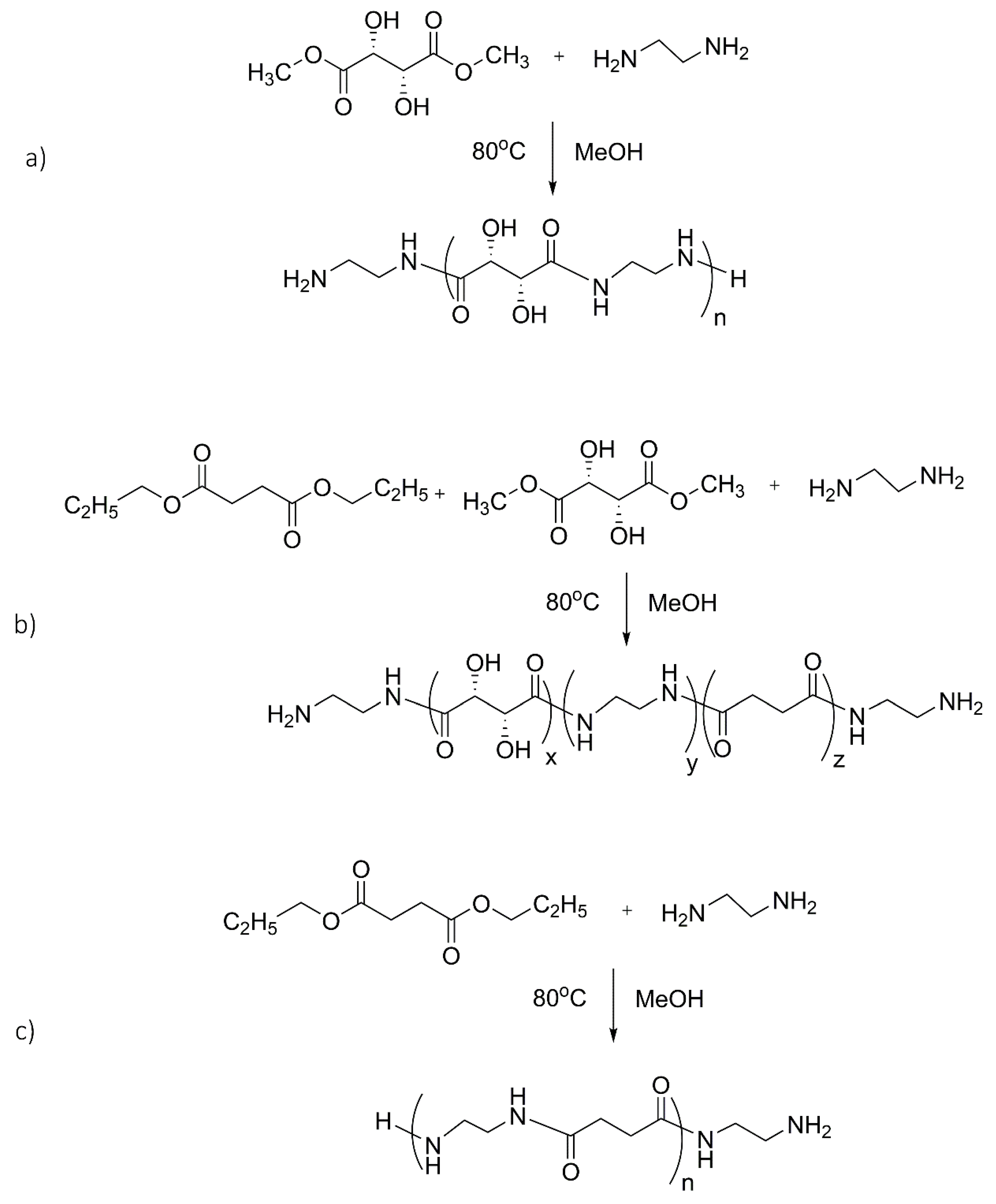
Synthetic procedures for the synthesis of oligoamides were designed to obtain products with low molecular weight and/or unwanted degradation products. In fact, in previous preliminary syntheses carried out in accordance with the industrial synthesis of common nylons (i.e., salts of hydroxylated monomers heated at high temperature), we obtained polymers with too-high molecular weights or a high percentage of byproducts. Therefore, esters of dicarboxylic acids were used for amides production in mild reaction conditions, avoiding salt formation between diacid and diamine, which requires higher temperatures for the condensation reaction. Starting from diesters, the low temperature (80 °C) avoids the alteration of the hydroxylated portions. In order to obtain oligoamides with terminal amine groups for successive reactions, the molar amount of amine was kept in slight excess with respect to the selected stoichiometric ratio diamine/diester (1:1 or 2:1). The syntheses of oligoamides were typically carried out in methanol as polar solvent, suitable for solubilizing all of the reagents.
The different reactivity of each monomer affects chain growth, resulting in different molecular weights, different monomer unit ratios, and different conversions for different oligoamide syntheses. Solid products were generally obtained for all of the oligoamides.
The reaction conditions used in the various syntheses are reported in
Table 2 together with the yields, molecular weights, and the ratios between the monomer units. The reaction schemes for the synthesis of ES, EST, and ES are shown in
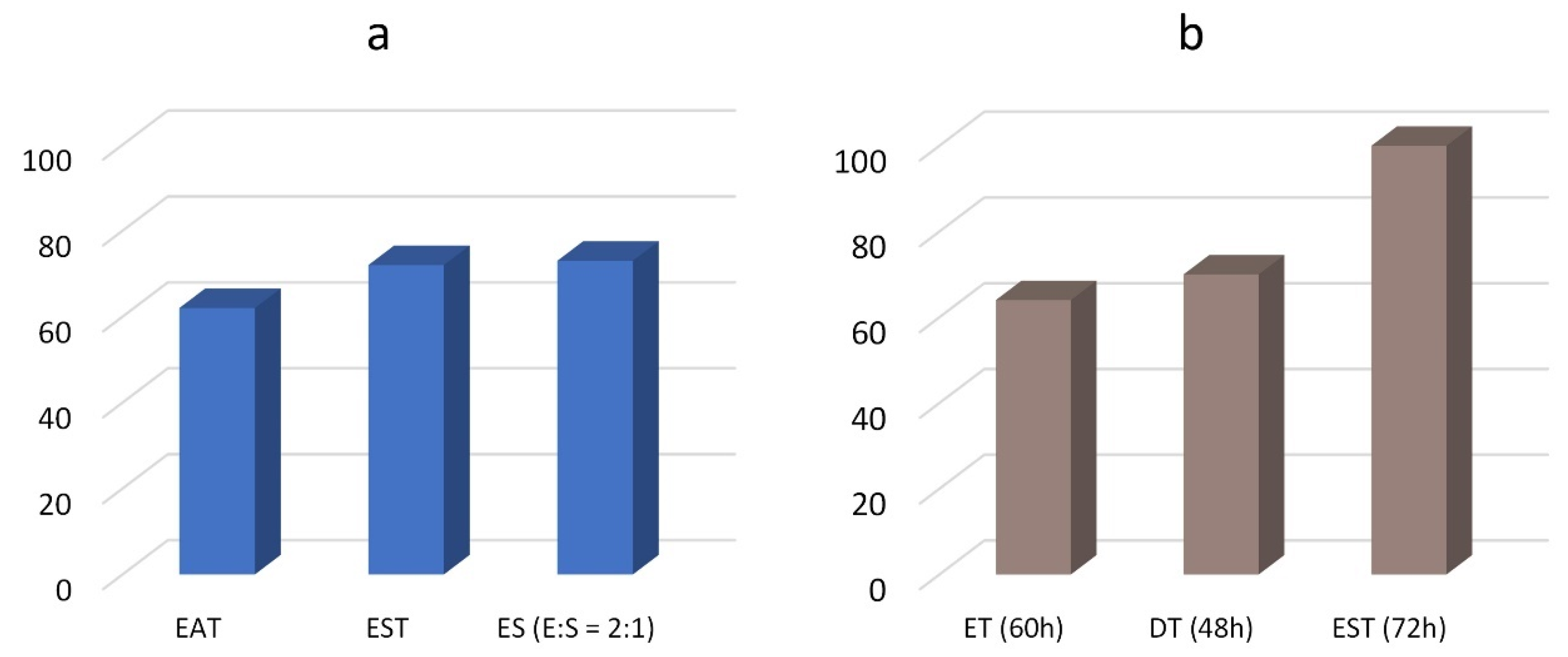
Figure 1. As expected, different monomers influence the reactivity and, consequently, the yield. The co-products present in the reaction mixture are typically unreacted products, rather than byproducts. In fact, by prolonging the reaction time, the yield increases, as observed for EST where the yield respectively passes from 72% to 100% after 24 and 72 h (
Table 2). In
Figure 2, the oligoamide yield for different monomers is compared after 24 h and after long reaction times.
The results show that diethylenetriamine is more reactive than ethylenediamine, while the reactivity of the aliphatic diesters is in the order succinate > adipate. As the literature reports that diesters with hetero atom groups (including hydroxylated diesters) show enhanced reactivity in mild conditions compared with aliphatic diesters [
32,
33], the higher reactivity of ES (yield 73% after 24 h) in respect to ET (64% after 60 h) may be explained with the different diamine:diester ratio (2.1 instead of 1:1). However, when aliphatic and hydroxylated esters (i.e., tartrate) are contemporarily present in the reaction mixture, the tartrate shows a greater reactivity. This behavior is evidenced by comparing the higher yield of EST (73%) after 24 h (
Figure 2a) with the lower yield of ET (64%) after 60 h. (
Figure 2b). Moreover, in agreement with the reported higher reactivity of diesters with hetero atom groups, a higher tartaric:succinic ratio is observed at low reaction times (
Table 2).
During the step-growth polymerization, the monomers initially form oligomers with low molecular weight, which grow gradually, but molecules of different lengths are synthesized and the final product, although at low average molecular weight, is composed of macromolecules with a different degree of polymerization. The
1H NMR spectral integration method was previously applied to estimate the number of average molecular weights (M
n) [
26,
34]. Similarly, in this study, specific equations were applied for each oligoamide in agreement with its structure, selecting suitable signals. First of all, the characterization of new compounds with the attribution of all of the signals present in the
1H and
13C NMR spectra was also performed using, when necessary, 2D NMR spectroscopy (gCOSY, gHSQC). In particular, using
1H and
13C NMR spectroscopy, the presence of amino groups at the end of the polymeric chain was determined (signals respectively at 2.70–3.00 ppm and near 40 ppm). The absence in the
1H NMR of a singlet signal in the 3.60–3.80 ppm range and a signal at 52 ppm in the
13C NMR, both attributable to the –OCH
3 in the terminal ester group, is in agreement for all products with the presence of two terminal amino groups. As the CH
2-NH
2 signal is present in a quite clean region of the
1H NMR spectrum, the corresponding integral was used as the reference value and set as 2 when one amino group was present at the end of chain, or 4 when both end groups were amino groups. Then, the integrals of the other signals were evaluated with respect to this value. Since the integral of the signal area is proportional to the number of protons, it is possible to calculate the number “x”, “y”, “z” of the repeating units of the oligoamide using the equations reported in
Table 3 for each compound. As an example, the
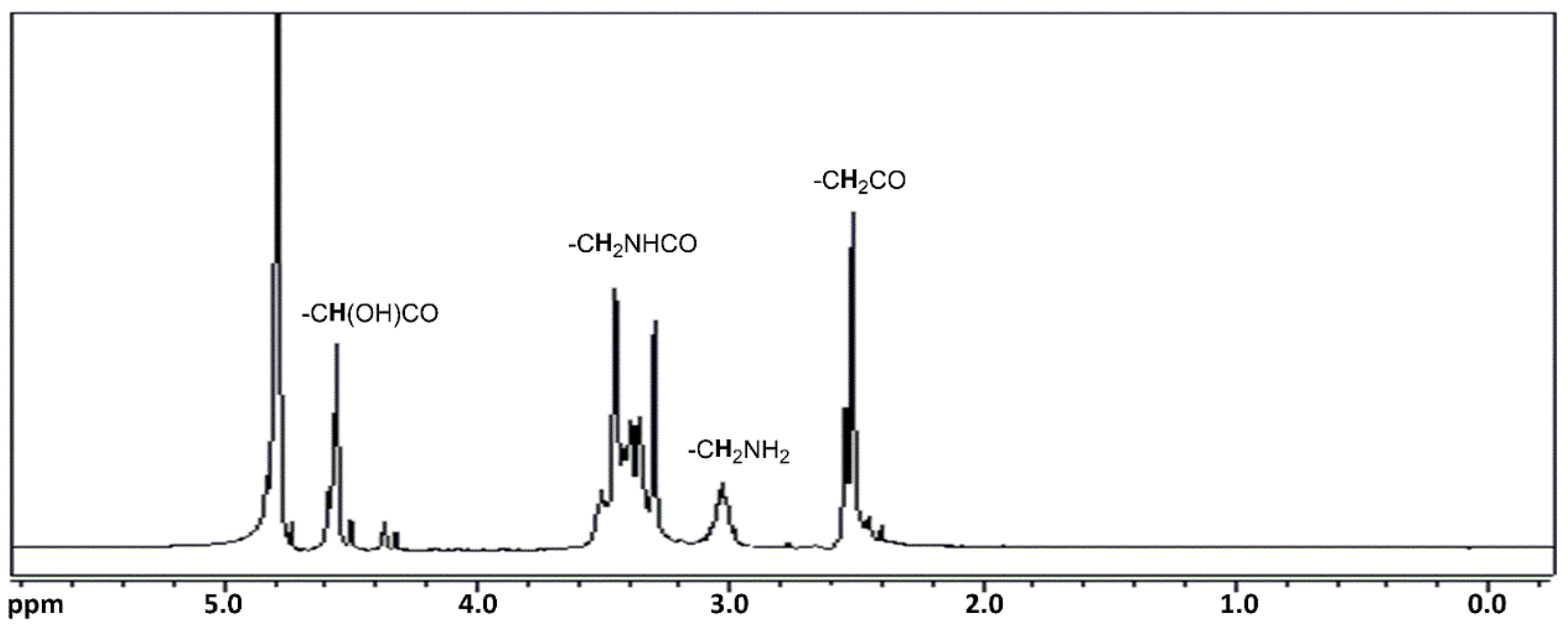
1H NMR spectrum of EST is shown in
Figure 3, while the other NMR and FT-IR spectra of all synthesized products are shown in the
Supplementary Materials (Figures S1–S36).
Based on the 1H NMR characterization, the formation of the amide group is confirmed by the presence of signals between 3.2 and 3.5 ppm attributable to the CH2 group in α position to this group: CH2NHCO (2 methylene groups in the middle of the chain and the CH2 in the terminal amino groups CONHCH2CH2NH2). Similarly, in the 13C NMR spectrum, the amide group formation is confirmed by the presence of signals at 174.0 and 175.0 ppm (CONH) and 38.5 ppm (CH2NHCO).
The presence of different monomeric units is confirmed by the presence of characteristic signals. In particular, the signal at 4.56 ppm in the 1H NMR spectrum is attributable to protons of tartrate units COCH(OH)CH(OH)CO, while a signal at 72.3 ppm is present in the 13C NMR spectrum related to the CHOH. Signals between 2.30 and 2.55 ppm in the 1H NMR spectrum are attributable to protons of 2 methylene groups in the α position of each of the 2 carbonyl groups CH2CONH of the succinic or adipic units, while in the 13C NMR spectrum a signal is present at 31.0–35.5 ppm (CH2CONH). In the 1H NMR, a signal between 2.73 and 2.77 attributable to protons of CH2NHCH2 is present when diethylenetriamine has been used as monomer, while in the 13C NMR, the signals at 47.0 and 45.7 are attributable to CH2NHCH2. Finally, a signal at 1.61 ppm (24.7 ppm in the 13C NMR) is attributable to the presence of adipic units (CH2CH2CONH).
3.2. Partially Fluorinated Oligoamides Synthesis
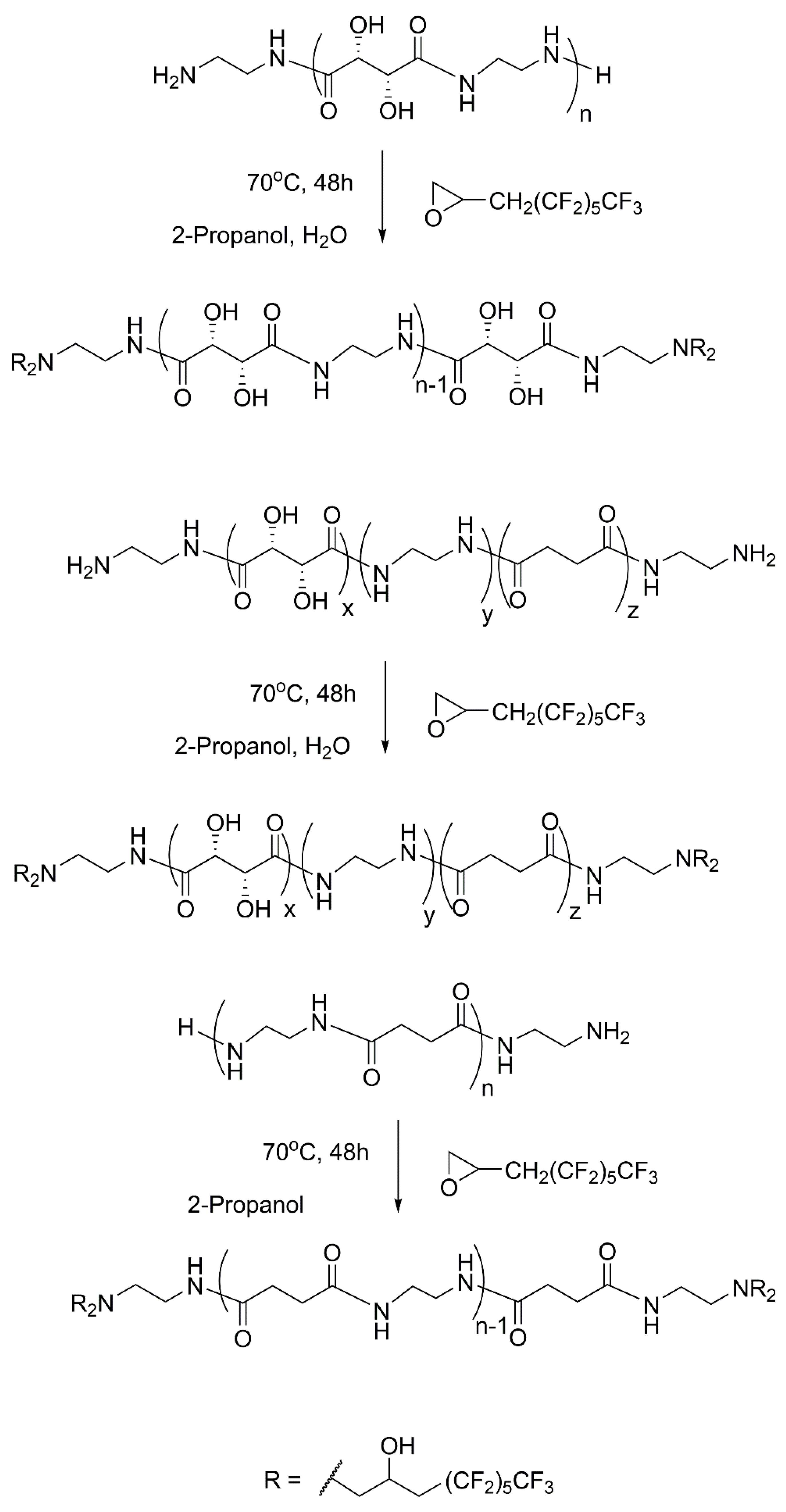
The synthesis of fluorinated derivatives was carried out by the nucleophilic ring-opening reaction between the terminal amino groups of oligoamides and a perfluorinated epoxy derivative (3-perfluorohexyl-1,2-epoxypropane, EC6F,
Figure 4). This fluorinated compound was selected in order to use a reagent with good solubility in alcoholic and hydro-alcoholic solvents and with a low environmental impact.
Preliminarily, the reactivity of the fluorinated epoxide was studied with hydroxyl nucleophiles such as the OH groups of sodium tartrate, selected as a reference for the reactivity of the tartaric unit. The reactivity with water was also studied for possible use of alcohol/water mixtures as solvent, if necessary, for the dissolution of oligoamides containing tartrate units. Regarding the evaluation of reactivity with the hydroxyl groups of the tartrate, no reaction was observed in the presence of EC6F with a molar ratio tartrate/EC6F = 2:1 after heating 48 h at 70 °C with 2-propanol. On the contrary, in the presence of water as a co-solvent, the formation of the diol by opening the epoxy ring prevailed. As confirmation, the formation of diol was also obtained by reacting EC6F with water. In the
1H NMR spectrum (
Figure S1), new signals are present at 4.06 ppm (CHOH) and between 3.65 and 3.45 ppm attributable to CH
2OH. Therefore, the presence of water as a co-solvent requires an excess of reagents for the competitive reaction of the epoxy group, which produces the corresponding diol and the subsequent purification of the product from the diol.
On the other hand, no conversion of the epoxide is observed after heating for 48 h at 70 °C in 2-propanol, as confirmed by the presence of the characteristic signals of EC6F at 3.30, 2.85, and 2.60 ppm (
Figure S2) in the
1H NMR spectrum of the solid residue recovered at the end of the reaction.
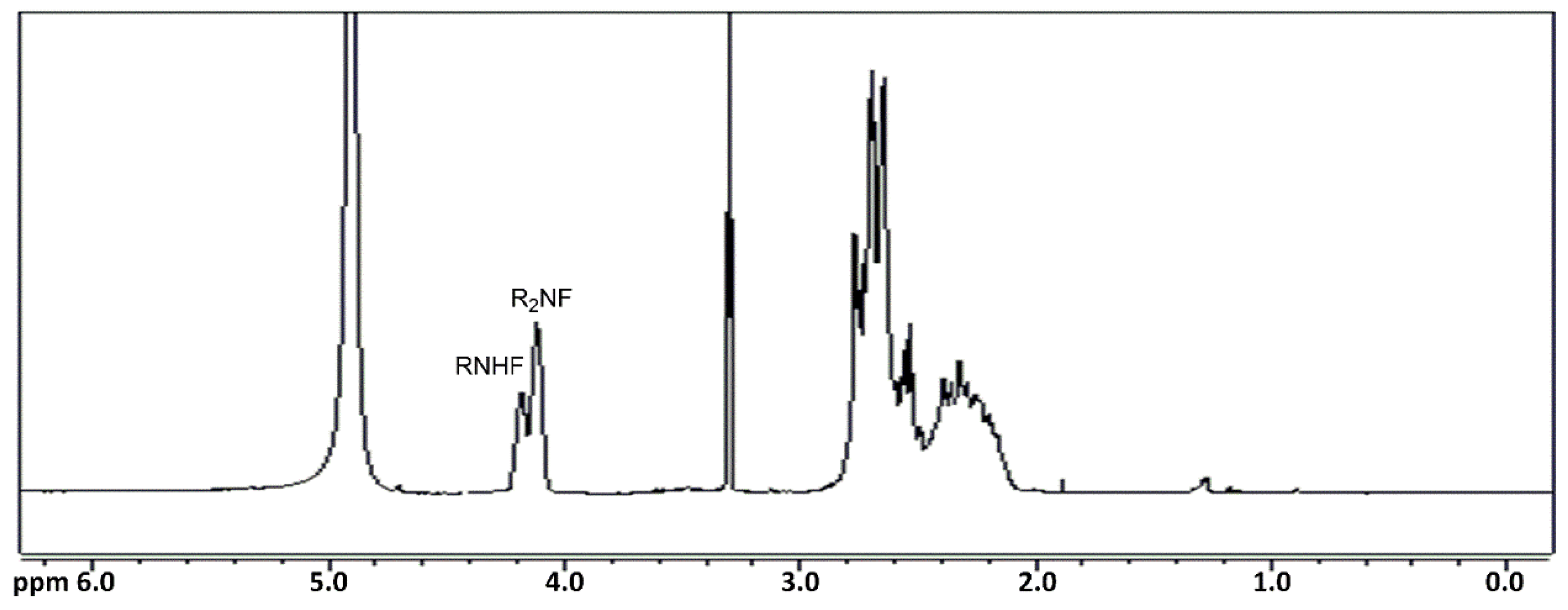
Similarly, to evaluate the reactivity of the epoxide with the amines, preliminary tests were carried out in 2-propanol using diethylenetriamine as a reagent with different molar ratios with respect to the epoxide (1:1, 1:2, 1:4, 1:6). In this way, it was possible to observe the formation of products (DF) with mono and di-substituted amino groups and to compare their behavior with respect to oligoamides.
In the
1H NMR spectrum (
Figure 5), the signals at 4.11 and 4.19 ppm are attributable to CH in the CH
2CH(OH)CH
2(CF
2)
5CF
3 respectively for the amino group with two fluorinated chains and for the mono-substituted one. In fact, the presence of different integral ratios between the two signals attributable to CH
2CH(OH)CH
2(CF
2)
5CF
3 agrees with the different molar ratio used between the two reagents (
Table 1). This integral ratio, along with the molar ratio of the reagents, allowed the identification of the signals at 4.11 ppm for the presence of amino groups with two fluorinated chains, while the signal at 4.19 ppm can be assigned to the presence of amino groups with only one substituent. Finally, the low signal at 4.25 pm can be attributed to the reaction of the secondary amino group with the epoxide. The gCOSY spectrum (
Figure S4) confirms the coupling between both signals at 4.11 or 4.19 ppm and signals between 2.07 and 2.83 ppm, which are attributable to NHCH
2CH(OH)CH
2(CF
2)
5CF
3 overlapped with the signals ascribable to CH
2NH.
On the basis of the preliminary results, the conditions for the reactions between oligoamides and epoxide were selected considering that the starting oligoamides show different solubility in pure alcoholic solvent depending on the monomers present and on the molecular weight. The reaction schemes for the synthesis of ES, EST, and ES are shown in
Figure 6. In particular, for oligoamides without tartaric units, 2-propanol was selected as the solvent, while oligoamides containing tartrate units are partially soluble in 2-propanol and soluble in water or a mixture of 2-propanol/water. Consequently, for the products containing tartrate units, the presence of undissolved oligoamide in the synthesis with 2-propanol can cause low conversion. On the contrary, the dissolution of both reactants can be achieved by working with a mixture of 2-propanol/water favoring the conversion. Then mixtures of water and 2-propanol in different ratios (1:1 or 1:3) were tested as a solvent to optimize the conversion, while different oligoamide/epoxide (OA/EC6F) molecular ratios between 1/1 and 1/12 were also studied to obtain different functionalization degrees. The most relevant results are shown in
Table 4.
The best reaction conditions resulted in a molar ratio OA/EC6F of 1:4 and heating at 70 °C for 48 h. Using a 2-propanol/water mixture as a solvent in the ratio 3:1, higher conversion and degree of substitution values were obtained compared to the 1:1 ratio. Furthermore, a lower quantity of diol was obtained in the presence of a smaller quantity of water, allowing for better purification. As observed for the synthesis of non-fluorinated oligoamides, the co-products were typically unreacted reagents, except for the reaction performed with 2-propanol/water mixture as a solvent in which the fluorinated diol was also formed.
In the general work-up procedure, the solvent and the excess epoxide were removed by evaporation, and then water was used to wash the residue from the unreacted oligoamide. Finally, cold acetone (−20 °C) was used to wash the residue again in order to remove the diol formed as a byproduct of the reaction between the epoxy and water.
Oligoethylene-
L-tartaramide was used as the first oligoamide in order to highlight the influence of the -OH groups of the hydroxylated diacid on the reactivity with EC6F and on the properties of the final product (i.e., solubility, color, physical state). Using 2-propanol as a solvent and an OA:EC6F molar ratio of 1:2, no reaction was observed due the low solubility of the starting oligoamide. Low conversion was also obtained using 2-propanol:water 1:1 as solvent and the same OA/EC6F molar ratio, while a yield of 48% with a degree of substitution of 2.5 was achieved by increasing the concentration of EC6F up to a OA/EC6F molar ratio of 1:4 (
Table 4). Unfortunately, the fluorinated product shows a reduced solubility in all common solvents, and ethanol or methanol only dissolves 57% of the reaction solid residue. This behavior makes the product difficult to use for subsequent applications, and for this reason, ethylenediamine was replaced with diethylenetriamine to increase the polarity of the final product. As a result, a slightly higher conversion (56%) was observed compared with a better solubility in alcohol of the fluorinated product (68% instead of 57% of ETF) (
Table 4), confirming that the reactivity with the epoxide is mainly controlled by the reduced solubility of the oligotartaramide. The partial solubility of the fluorinated products is attributable to the presence of oligomers with different molecular weight and also with different degrees of functionalization, which give rise to fractions of product with different solubility.
To increase the solubility of the starting oligoamide and fluorinated products in alcoholic solvents, products containing units of succinic or adipic acid together with tartaric acid were synthesized. The reactivity of the ETA or EST oligoamides was subsequently tested with the EC6F epoxide.
Due to the few secondary interactions between the aliphatic chains and the consequent better affinity for the solvent, greater solubility in alcohol is expected for the fluorinated products when using adipic or succinic units.
As reported in
Table 4, the best results as conversion were obtained for ESTF. In this synthesis, the reaction was performed in the mixture 2-propanol:H
2O with a volume ratio of 1:1 or 1:3 for 48 at 70 °C and with a molar ratio OA:EC6F of 1:4. After the work-up procedure, the final product was obtained as a yellow solid with yields of 61% (in 2-propanol:water 1:1) or 70% (in 2-propanol:water 3:1) and was found to be soluble in 2-propanol or ethanol (8 mg/mL, 1%). However, the milky solution maintained for a long time (typically 1 day) at room temperature easily forms a fine solid. The spectroscopic characterization (
1H, gCOSY,
13C,
19F NMR) confirms the presence of fluorinated groups attached to the oligoamide with a degree of functionalization (DS) > 3.5. For this compound, DS was evaluated as the integral ratio between the signal at 4.10 (ascribing to 1H) and 4.47 ppm (ascribing to 2H for an average of 2.18 tartaric units on each oligoamide chain, value obtained by processing NMR data according to the equations reported in
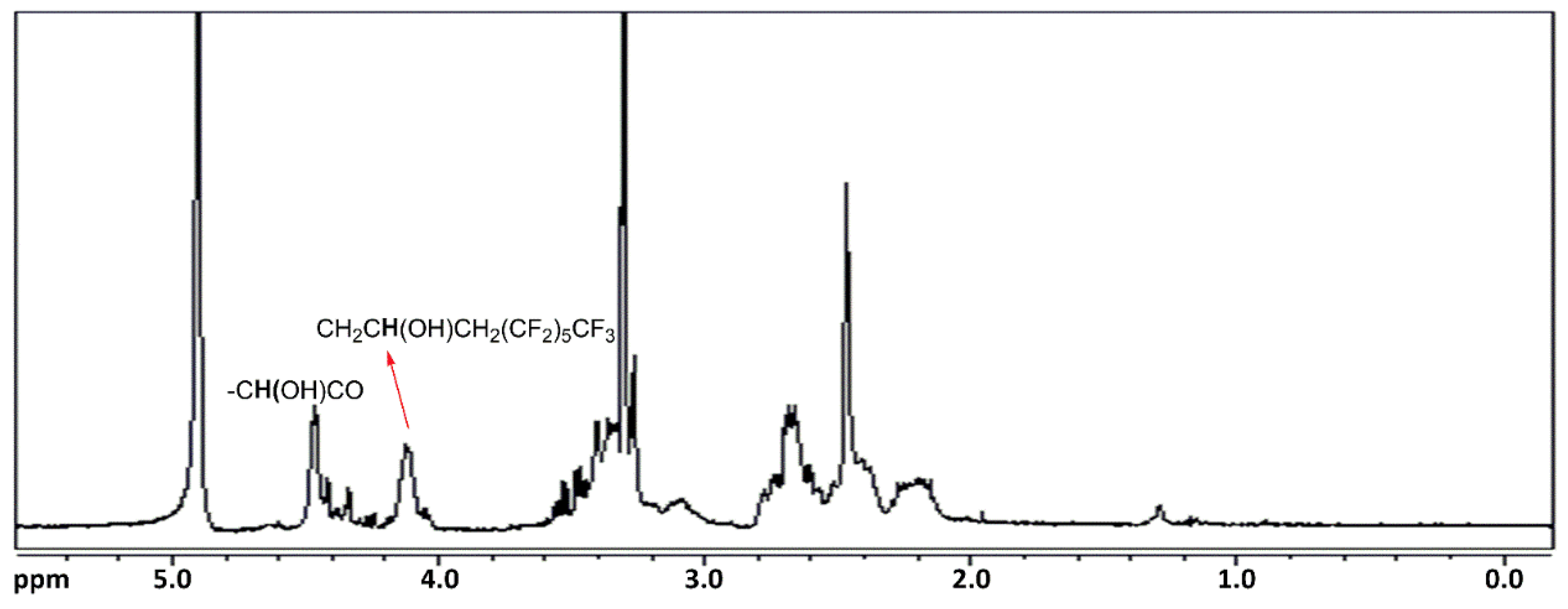
Table 3). In the
1H NMR spectrum recorded in CD
3OD (
Figure 7), the presence of the fluorine chain is confirmed by the signals between 2.10 ppm and 2.80 ppm attributable to CH
2 in CH
2NHCH
2CH(OH)CH
2(CF
2)
5CF
3, while the signal at 4.10 ppm is attributable to CH in the fragment CH
2CH(OH)CH
2(CF
2)
5CF
3, which is formed by the opening of the epoxy group during the reaction with the amino group. The signals ascribable to the hydroxylated oligoamide fragment are also present. The signals present in the
19F NMR spectrum confirm the presence of fluorinated chains (−82.5 (CF
3), −122.9, −124.0, −124.6 (CH
2(CF
2)
4CF
2CF
3) and −127.5 (CH
2(CF
2)
4CF
2CF
3) ppm).
The presence of succinic units improved the solubility of the final product and it therefore seemed interesting to synthesize the fluorinated derivative of oligoethylenesuccinamide to evaluate the effect of structural variations from an applicative point of view.
The molar ratio between the reagents and the reaction conditions were the same as those used for the ESTF synthesis, but pure 2-propanol was used as a solvent. In fact, the solubility of the starting oligoamides in alcohol increases when the ratio of alkyl groups increases in its structures and when tartaric units are not present. The fluoro-oligoamide ESF was obtained as a white solid with an 89% yield.
The signals in the 1H, 13C, 19F NMR, and gCOSY spectra confirm the presence of fluorinated groups bonded to oligoamide. However, the overlap between numerous signals does not allow an evaluation of the degree of functionalization for this compound, although also in this case, the presence of the signal at 4.11 ppm and the considerations reported on the characterization of the previous fluorinated products (ESTF and DF) allows one to hypothesize the presence of amino groups with two substituent groups and therefore a high degree of functionalization.
3.3. Treatments Evaluation
In this study, a specific protocol was developed to evaluate the efficacy of the synthesized products as hydrophobic agents and to evaluate the usefulness of their use on a highly hydrophilic and inhomogeneous material such as wood. To define the tests for the protocol, the need to obtain preliminary information to optimize the design of a suitable protective was also taken into consideration. However, based on the results obtained in this preliminary phase of the research, it will have to be optimized.
Diagnostic protocols for the evaluation of wood treatment generally concern the field of painted artefacts or preservative products, and are only partially regulated. However, this study aims to evaluate the behavior of materials capable of minimally altering the aesthetic appearance of the wood, but modifying its wettability and moisture content (mainly vapor absorbed to reach equilibrium with environmental humidity). Therefore, this specific diagnostic protocol is intended to be applied to those products that assume high importance in the conservation of materials of historical and artistic interest, such as the compounds developed in this work.
To the best of our knowledge, research in this area has not provided a very extensive previous background and few studies or patents cover this field of interest [
8,
9,
10,
11,
12,
35].
For sake of clarity, we remember that wood is a highly inhomogeneous material whose physical properties change anisotropically and its aesthetic appearance, even for the same wood species, is also affected by its different origin. The properties of wood are also influenced by manufacturing processes and environmental conditions. Consequently, the choice of the wood species, the type of cut and surface processing, and the presence or absence of concentrated extractives on the surface are the parameters that must firstly be taken into consideration for the selection criteria of the samples for the tests. The weight (moisture content variation) and other chemical–physical characteristics are finally influenced by the ambient humidity with which the wood is quickly balanced and a monitor of the behavior as a function of different RH values is also required.
Therefore, to reduce errors in the different measurement tests, and to understand the influence of some of the peculiarities of the wood, we decided to test two types of wood, beech and oak, with the same type of cut, two types of processing (planed and polished), and virgin and pre-extracted samples (i.e., wood extracted in ethanol for the removal of extractives).
On the basis of the data on the solubility in 2-propanol, the oligoamides ETAF, ESTF, ESF, and DF were selected for wood treatments.
The tested products, as expected and desired, did not show film-forming behavior, but they could vary the aesthetic appearance of the wood from a colorimetric point of view. The surface color variations, performed on the same position of each specimen before and after the treatment and expressed as ∆L*, ∆a*, ∆b*, and ∆E, are reported in
Table 5. Only for the samples treated with ESF was the chromatic variation below the detection limit of the human eye (∆E = 3). For the other samples, appreciable chromatic variations mainly influenced by the L* parameter were observed (
Table 5). However, it should be noted that the aesthetic appearance of the wood is not significantly altered compared to the typical and variable color of each species for all tested products, as can be seen in
Figure 8, where the samples treated with ESF, DF, or ESTF are compared with the wood without a coating as a reference.
The hydrophobic properties of the synthesized compounds were evaluated by detecting the wettability change of the coated wood surface through contact angle (CA) measurements. However, due to the inhomogeneity of wood, as previously mentioned, a great variability of the CA values is expected, even on different areas of the same surface. For this reason, to estimate the degree of variability, preliminary measurements of CA were carried out on both untreated (virgin) and ethanol-extracted (for the removal of extractives) wood samples. As expected, the preliminary measurements obtained on different positions of the same surface of each sample showed a great variability. In fact, the contact angle of the uncoated wood, evaluated after one second from the deposition of the water drop, varied between 50° and 105°, whether the measurement was performed in different positions of the same sample or on different samples of the same wood species. After 5 s from the deposition of the water drop, the CA becomes generally non-evaluable. These results confirm the need to adopt the strategy of always measuring the contact angle in the same area (before and after the coating) to obtain reliable data on the hydrophobic effect of the protective agent. Moreover, to provide statistical data, at each step of the test (i.e., before coating, after coating, or after aging), it is necessary to repeat the CA measurement several times, in different moments and after the complete evaporation of the previous drop.
Furthermore, the wood extractives could influence the CA values. In fact, the extraction treatment with ethyl alcohol decreased the natural hydrophobicity of wood attributable to the presence of extractives on the surface. For a reference oak sample, the contact angle changed from 105.9° ± 1.2° to 84.5° ± 0.9°, after extraction in ethanol. As these compounds are soluble in alcohols, their dissolution and migration during the application of the alcoholic solution of the coating is expected. For this reason, some coatings were also applied to oak wood after extraction with alcohol. Similar to the extraction treatment, a decrease in the contact angle can be observed after abrasion of the surface (59.2° ± 0.1°), while the simultaneous abrasion and extraction with alcohol determines a contrasting effect of the two actions on wettability (69.3° ± 1.1°). In fact, the abrasion of the wood surface likely removes the extractives concentrated on the surface, while it can expose a new surface where the extractives were not completely removed during the extraction process. Furthermore, abrasion with varying roughness can influence the hydrophobic behavior of the surfaces. Finally, it is necessary to consider the natural variability of the wood when different samples are used as a reference. In all cases, after extraction with alcohol and/or abrasion, the values of the contact angles are considerably lower than those of raw wood and always less than 90°.
Based on these observations, the comparison of the performance between different products was carried out in the following conditions: (a) by using wood samples with or without the preliminary extraction with ethanol; (b) by evaluating the CA several times, over time, in a well-defined position of each sample after 1 and 5 s. In the end, a final evaluation of the CA was carried out after 8 min, although this measurement could be affected by the minimal evaporation of water. The results are reported in
Table 6.
Treatment with fluorinated oligoamides containing tartaric unit show low CA values, always lower than 90° and generally lower than the initial value of the wood sample before treatment. This result highlights a negative effect of the presence of hydroxyl groups on the oligoamide chain, which can be attributed to a concomitant hydrophilic effect or to a greater ability to penetrate into the surface layers of the wood. In the latter case, the greater affinity with the polysaccharides constituting the wood instead of favoring the anchoring on the surface would favor its penetration and/or do not favor the distribution of fluorinated fragment towards the outside. The use of 2-propanol as a solvent for the deposition can also act on the surface of the wood favoring the migration of the extractives if not previously removed.
On the contrary, after treatment with ESF and DF, the wood samples show higher contact angle values, with a greater increase in the contact angle for the samples pre-treated with ethanol. The best hydrophobicity was founded in the sample without pre-treatment with ethanol and treated with ESF (138.2°). The presence of a non-functionalized NH group in the DF compound can favor anchoring to the support, but could reduce the hydrophobicity deriving from the presence of the fluorinated chain. However, the values obtained without pre-extraction in ethanol are around 132°. It is interesting to observe the comparison of these data with the contact angle observed in the presence of a commercial product such as Paraloid B72, a product widely used in the Cultural Heritage field. The application of equal amounts of product under the same conditions used for the fluorinated products shows a contact angle of (66 ± 4)° on wood as it is, and (63 ± 4)° on wood pre-extracted with ethanol. With this acrylic product, the color variation is relatively low (ΔE respectively 2.3 and 4.3), but the poor stability of the product reduces the interest in acrylic products.
After 5 s from the deposition of the water drop, a significant change in the contact angle is observed for samples with hydrophilic behavior (ϑc < 90°) compared to those with initial ϑc > 90°, in agreement with the hydrophobic properties and a reduced ease of water absorption of the wood specimens coated with ESF and DF. Finally, the contact angle was assessed after 8 min, but it was only possible for the treatments with ESF and DF.
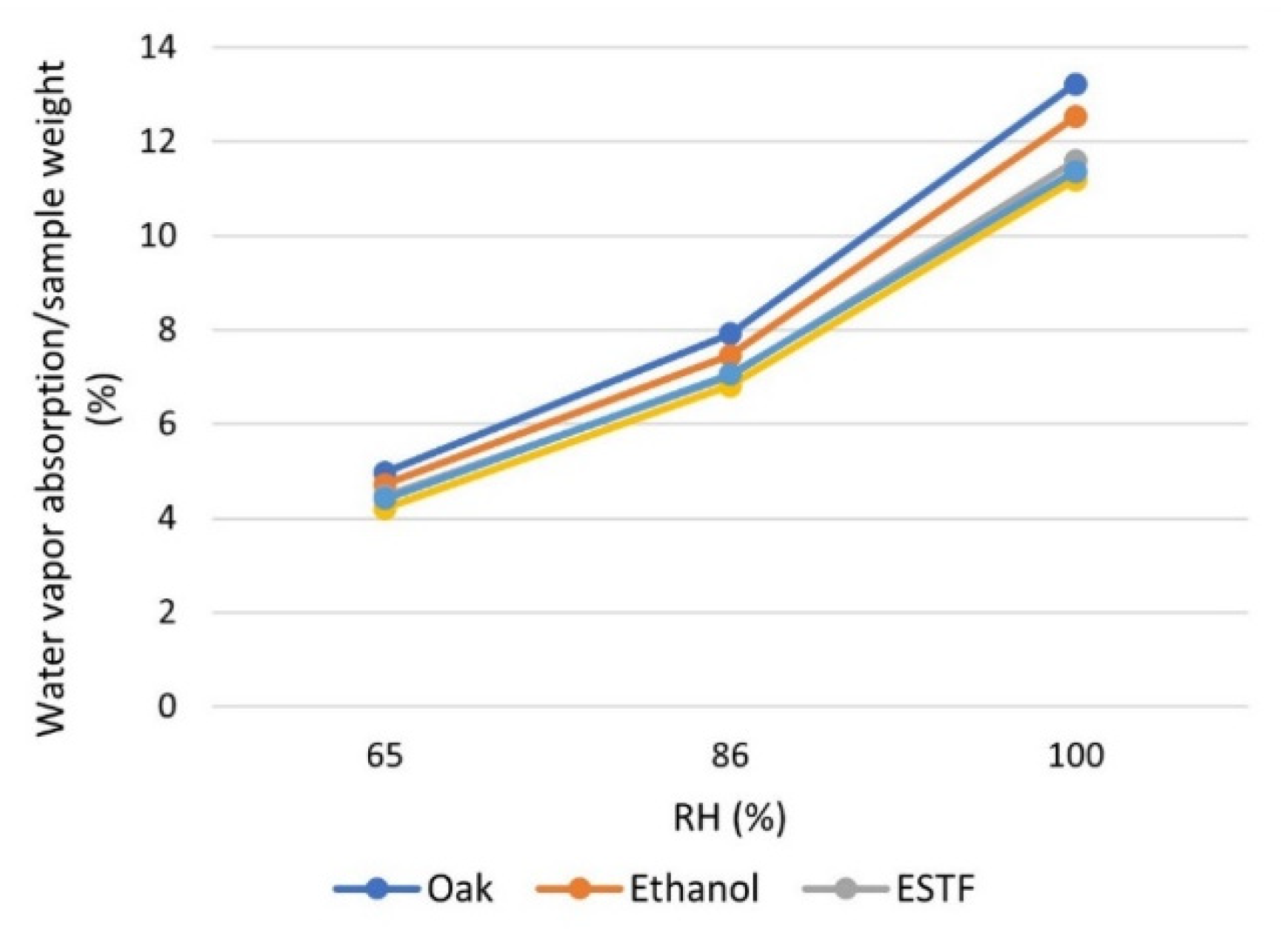
The water vapor absorption test was performed using oak wood samples treated with ESTF, ESF, and DF on all surfaces with a suitable quantity of product to completely cover them (40 g/m
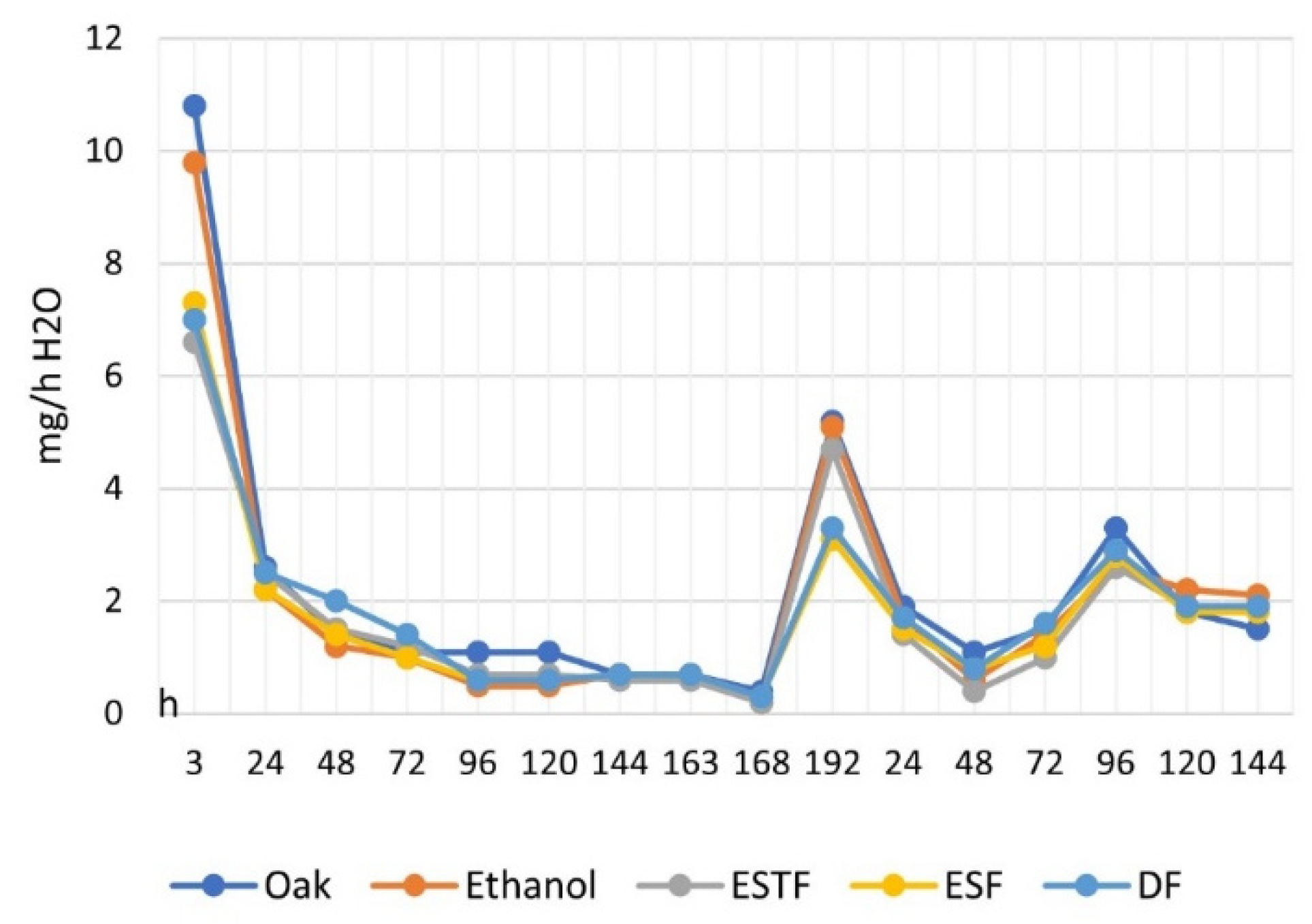
2). All of the samples were preliminarily extracted in ethanol, and after coating, they were kept in a desiccator for 72 h until a constant weight was reached. The weight variations observed by keeping the coated samples in the presence of progressively increasing humidity values (RH 65%, 86%, and 100%) were compared with those obtained in similar uncoated samples (one raw and one pre-extracted). For all humidity values, high water absorption is observed in the initial phase, which progressively decreases to zero in correspondence with the reached equilibrium, with a higher absorption rate on anhydrous samples (uncoated samples) (
Figure 9). The mass of water absorbed per unit mass of the specimen is lower for the samples coated with the fluorinated products, in the order ESF < DF < ESTF, compared to the one observed in the uncoated wood, with or without pre-extraction in ethanol (
Table 7 and
Figure 10).
The increase in the surface wettability of wood (i.e., lower CA,
Table 6) and the reduction in vapor absorption (i.e., lower mass of vapor absorbed,
Table 7) for the specimens extracted in ethanol, compared to the raw wood, can be explained with the removal of saccharide components and other compounds. The extraction in ethanol of saccharide components of different complexity is confirmed both by
1H-NMR (
Figure S37), for the presence of the signals attributable to the saccharide skeleton at 3.40–4.00 ppm, and by FT-IR (
Figure S38), for the signals at 3390 (O–H stretching), 1113, 1076, 1049 cm
−1 (C–O stretching). Low-intensity signals at 2927 (C–H stretching) and 1732 cm
−1 (C=O stretching), attributable to the presence of triglycerides and/or free fatty acids, were also present in the FT-IR spectrum. A different mobility of the various extractive components can be speculated, which can determine a lower concentration of hydrophobic components (e.g., triglycerides, free fatty acids) on the surface after extraction with ethanol, while the saccharide components are probably removed mainly from the wood mass, thus reducing its affinity towards water.
Finally, the stability of the synthetic partially fluorinated oligoamides and amine, deposed on glass slides, was estimated by means of accelerated aging tests exploiting UV radiations (wavelength of 365 nm). Their behavior was followed via 1H NMR, FT-IR spectroscopy and colorimetric measurements. The weight change was also recorded, in order to monitor the decomposition or oxidation (mainly absorption of oxygen) reactions.
The colorimetric measurements were made before and after irradiation to evaluate the color variations due to the interaction between the partially perfluorinated compounds and the UV radiation. No appreciable weight changes were noted for any product. The results of the colorimetric measurements on UV irradiated glass slices are shown in the
Table 8.
DF, which contains diethylenetriamine units, shows the highest ΔE* (9.14) after 254 h of irradiation among the three irradiation products tested, and is much higher than the threshold limit imperceptible to the human eye (ΔE* = 3). In particular, DF has a significant increase in Δb*, which means the samples turned yellow under UV radiations. On the contrary, both ESF and ESTF, which contain ethylenediamine units, show ΔE* and Δb* values around 1.
The FT-IR and the
1H NMR spectra of ESF after irradiation do not show changes, while the FT-IR of the irradiated ESTF displays slight shifts of the signals at about 3300 cm
−1 (–OH and –NH stretching) and 1550 cm
−1 (–NH bending, Amide II) compared to the non-irradiated ESTF. This modification may be justified with hydrogen bonding involving mainly –OH and –NH groups. ESTF did not show a significant yellowing after irradiation; however, in the
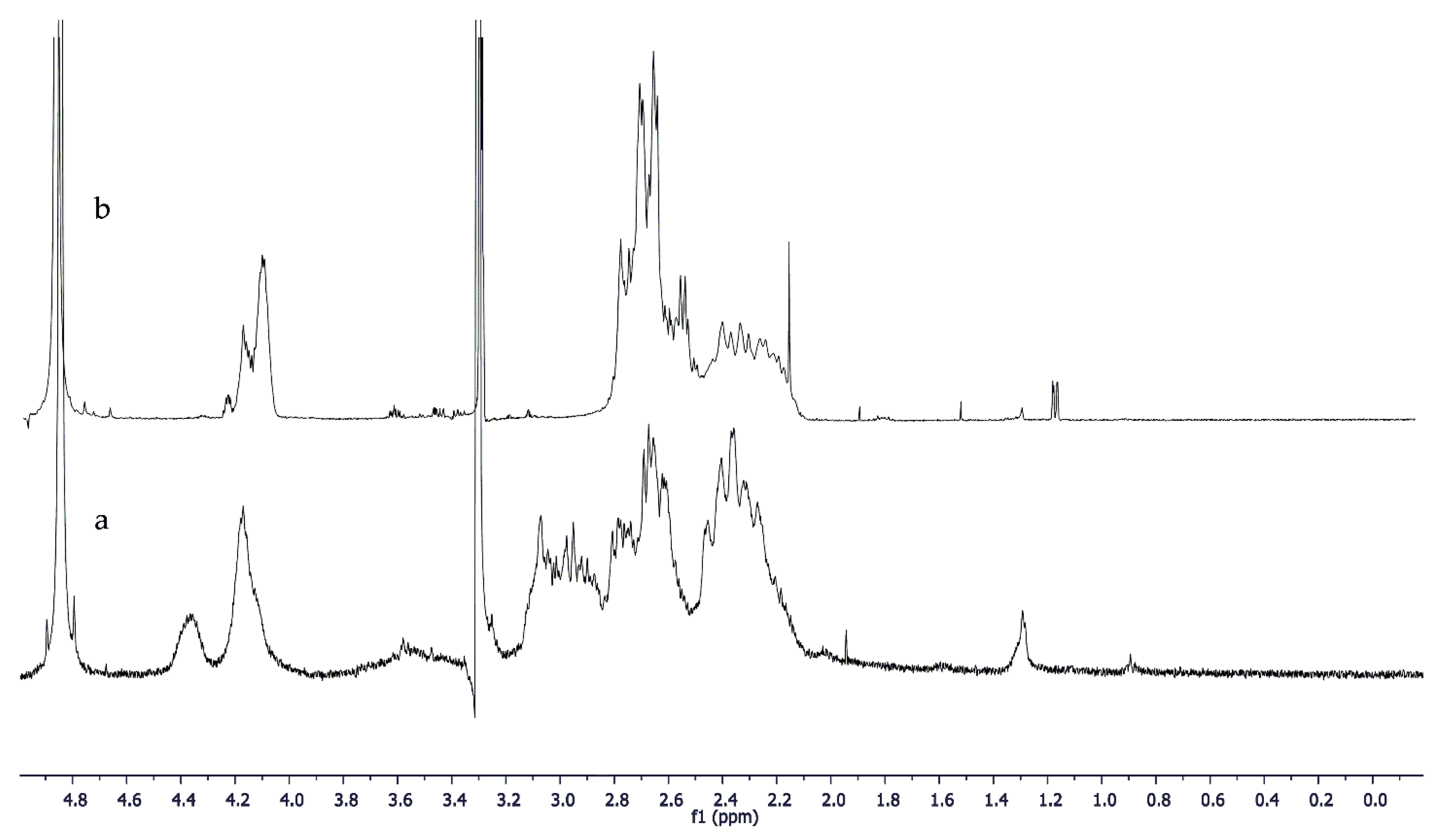
1H NMR spectrum, the reduction of signal intensity to 4.11 ppm and of signals between 2.6 and 3.0 ppm were observed (
Figure 11). These variations, as for the FT-IR results, are in agreement with a reduction in solubility due to hydrogen bonds between the OH and NH groups present in the compound.
The FT-IR spectrum of irradiated DF (
Figure S39) shows changes mainly in the region 1600–1700 cm
−1, probably attributable to a partial oxidation on the triamine units. In the
1H NMR spectrum (
Figure 12), the variation of the shape and relative intensity of the signals of CHNH in the 2–3 ppm zone can be observed. In particular, a new signal at 3.0 ppm appeared and the shape of the original CHNH area also changed.