Corrosion Behavior of TiNi Alloy Fabricated by Selective Laser Melting in Simulated Saliva
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microstructure and Characterization
2.3. Electrochemical Measurements
3. Results
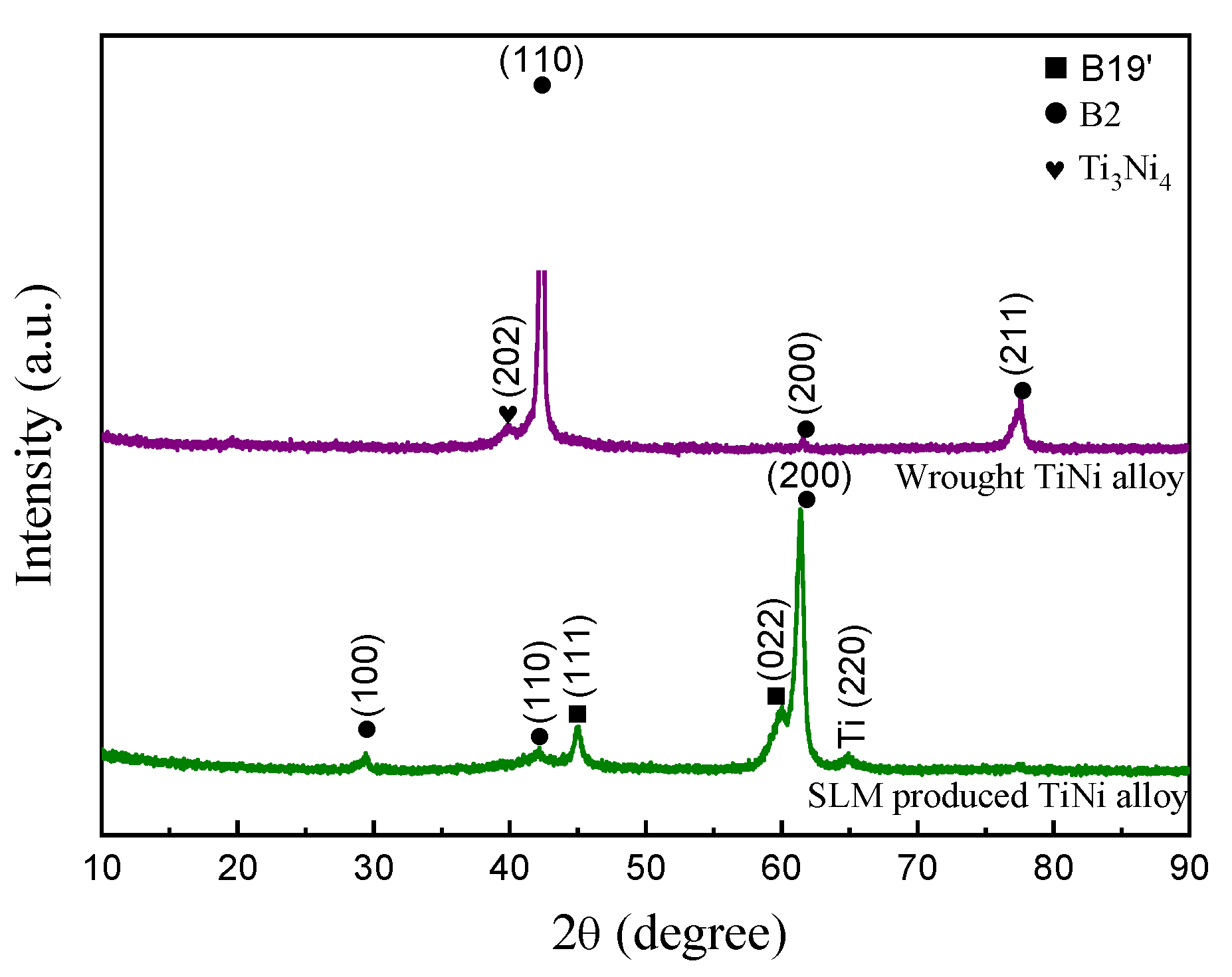
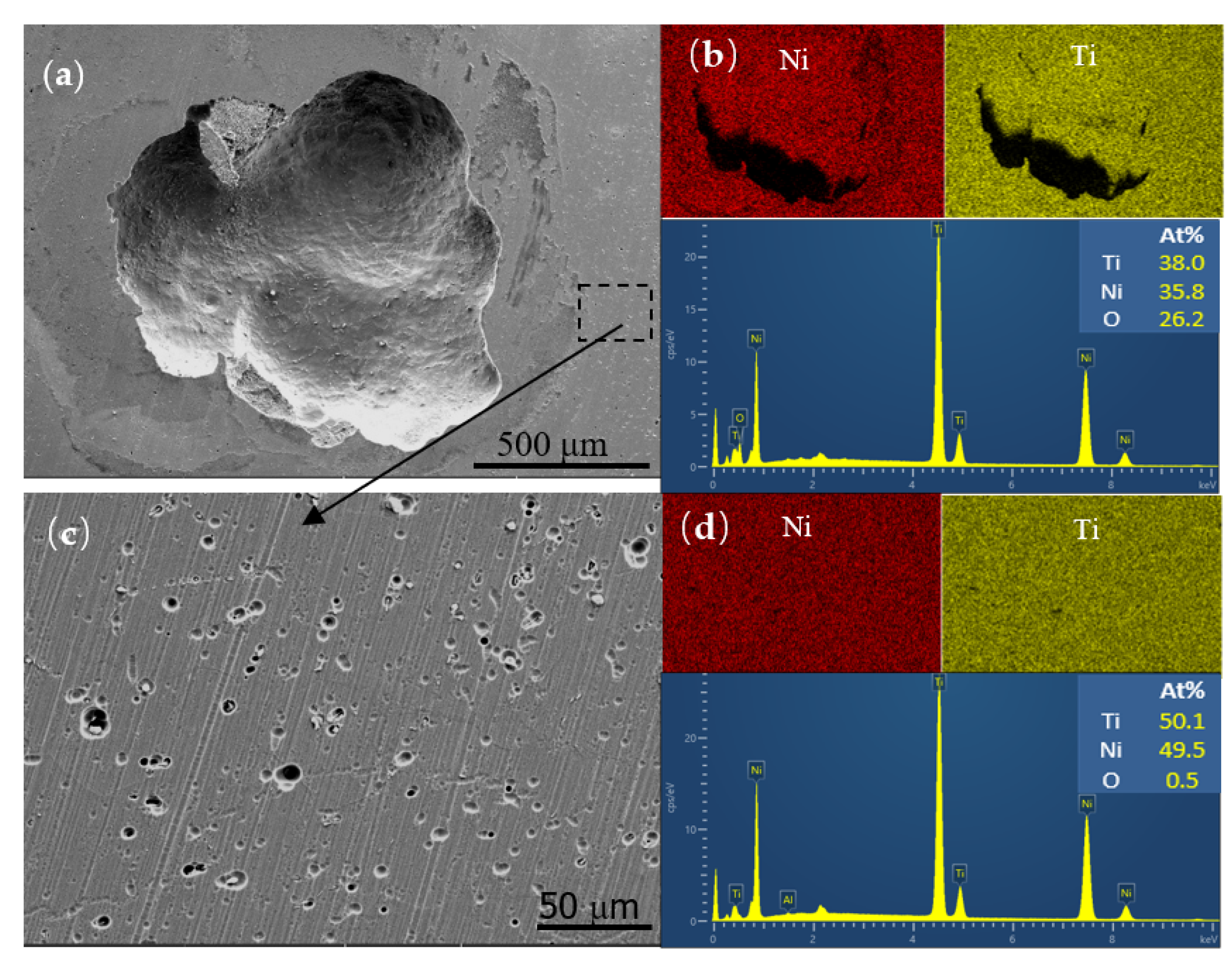
3.1. Microstructure and Phase Composition
3.2. OCP of TiNi Alloy in the ASS
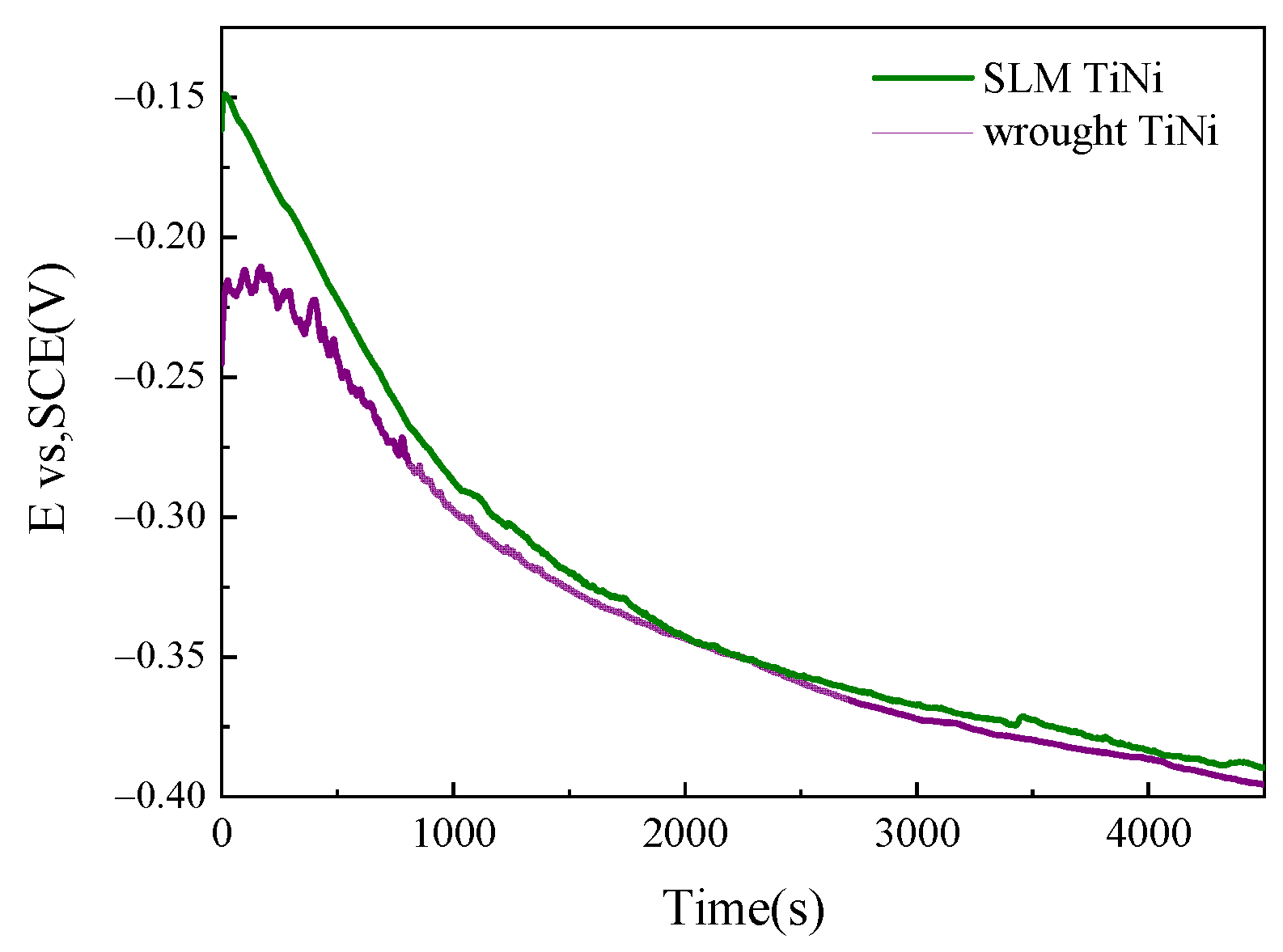
3.2.1. OCP of SLM and Wrought TiNi Sample
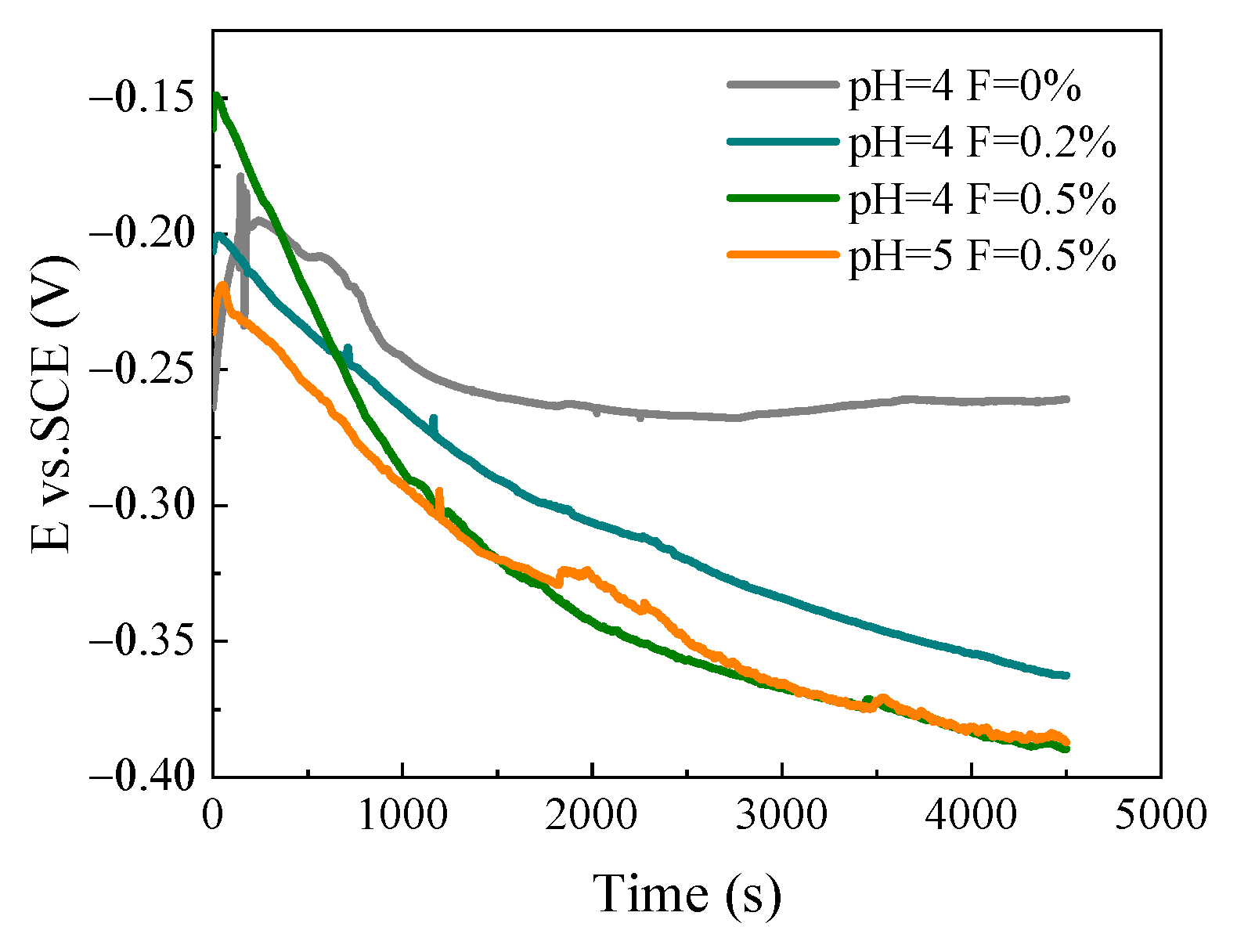
3.2.2. OCP of SLM TiNi Sample in ASS with Different pH and F− Concentration
3.3. EIS of TiNi Alloy in ASS
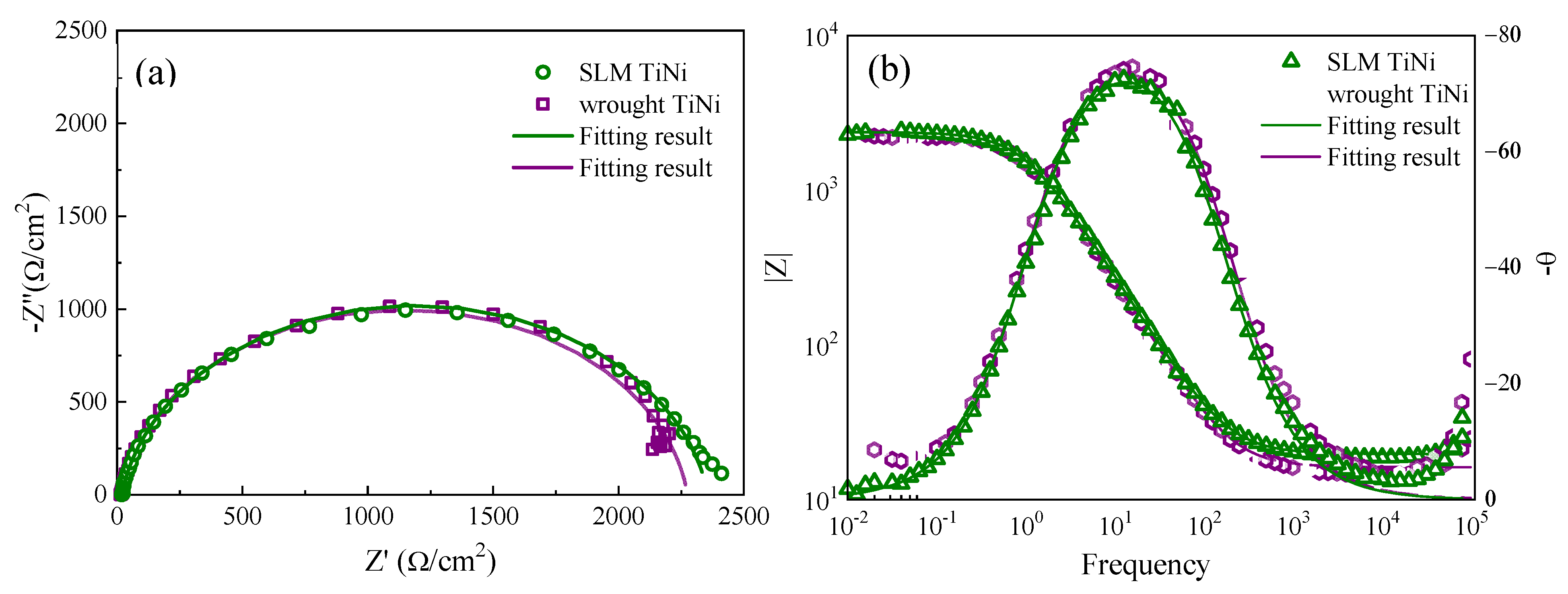
3.3.1. EIS of SLM and Wrought TiNi Samples
3.3.2. EIS of SLM TiNi Sample in ASS with Different pH and F− Concentrations
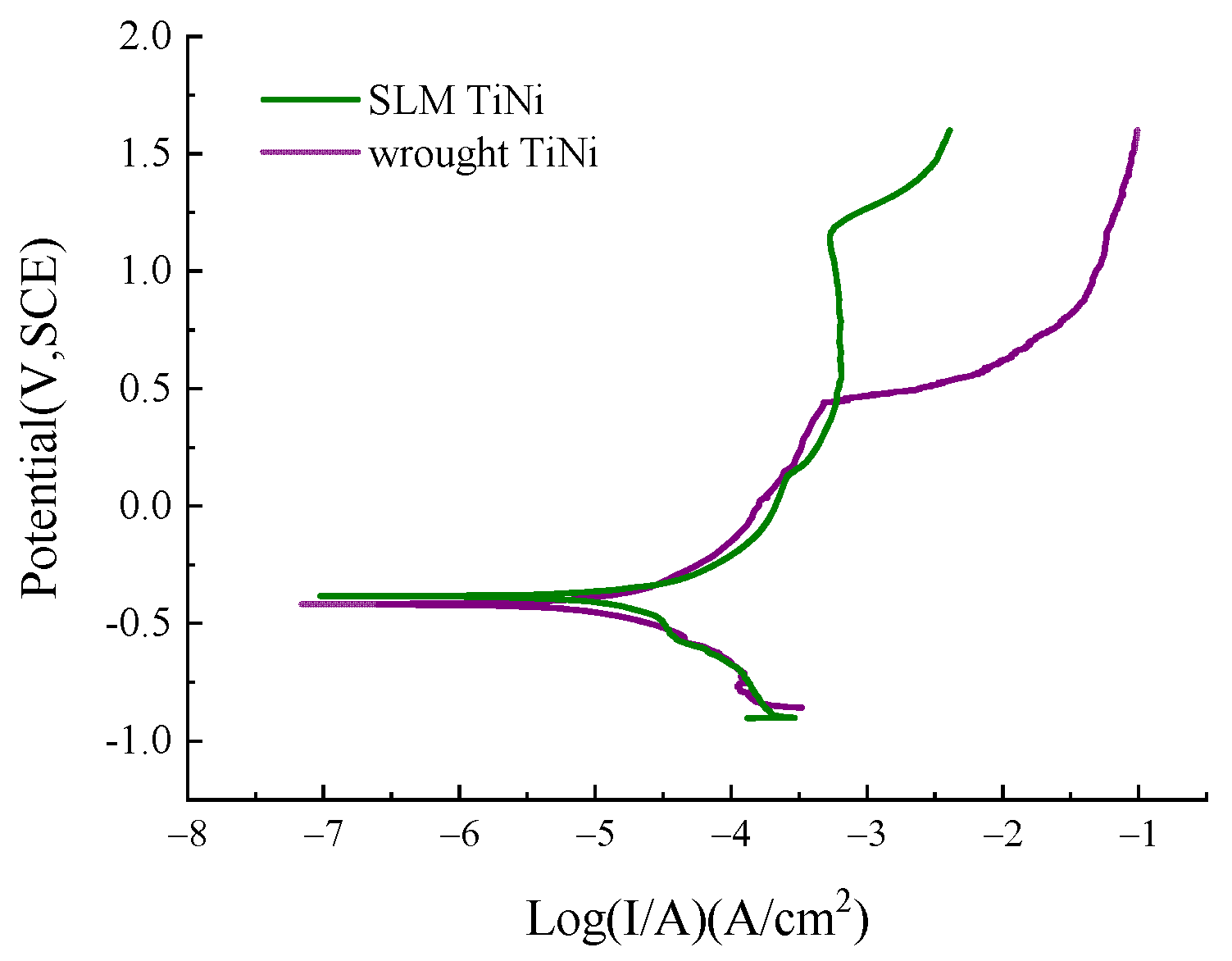
3.4. Potentiodynamic Polarization Curve of TiNi Alloy in ASS
3.4.1. Polarization Curves of SLM Alloy and Wrought TiNi
3.4.2. Polarization Curves of SLM TiNi Samples in ASS with Different pH and F− Concentrations
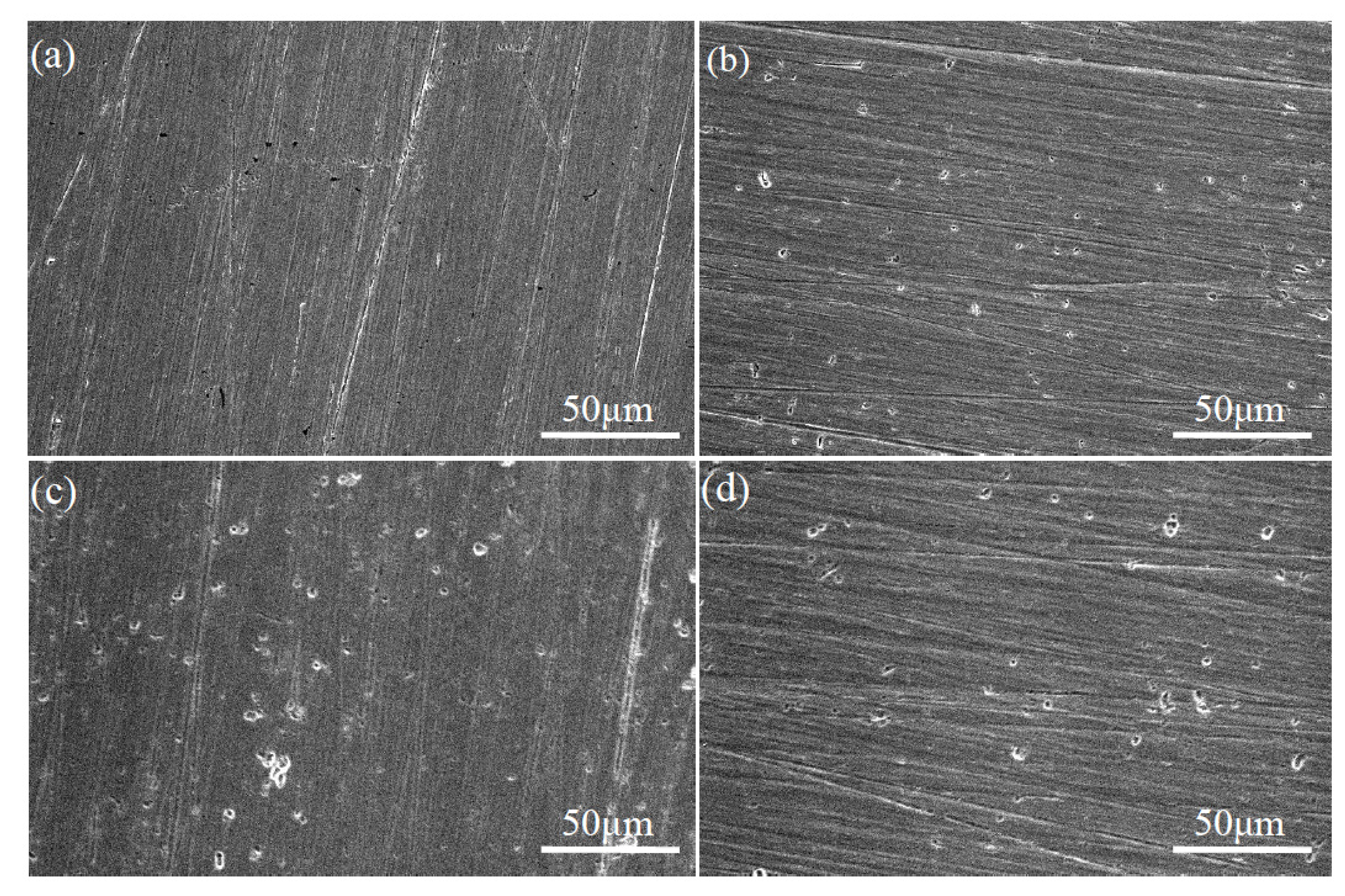
3.5. Corrosion Morphology of TiNi Samples in ASS
3.5.1. Corrosion Morphology of SLM and Wrought TiNi Samples
3.5.2. Corrosion Morphology of SLM TiNi Samples in ASS with Different pH and F− Concentrations
4. Discussion
4.1. Effect of F− on Corrosion Resistance of SLM TiNi Alloy
4.2. Corrosion Comparison between SLM and Wrought TiNi Samples
5. Conclusions
- The SLM sample shows slightly better corrosion resistance than that of the wrought sample due to the more uniform and dense passivation film formed on the surface of the SLM sample. The difference in corrosion potential and current density between the two is relatively small. However, the Epit of the former (1.13 V) is significantly higher than that of the latter (0.42 V);
- The existence of F− (0.2%) in ASS could deteriorate the corrosion resistance of the SLM sample. The Ecorr of the sample decreases from −0.3 V to −0.36 V, and the Icorr increases from 9.85 × 10−2 to 13.9 μA/cm2 due to the addition of fluoride ions. With an increase in F− (0.5%) concentration, the corrosion rate of the SLM sample increased. F− could combine with titanium oxide on the alloy surface to form a titanium–fluorine complex, leading to the passivation film’s dissolution;
- The adjustment of pH value in the fluorine-containing solution may influence the corrosion behavior of SLM samples. The lower the solution’s pH value, the worse the alloy’s corrosion resistance. As the pH value changes from 5 to 4, the measured Eocp remains the same while Icorr (20.8–22.0 μA/cm2) and Ipp (80.1–181 μA/cm2) increase.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassab, E.J.; Gomes, J.P. Assessment of nickel titanium and beta titanium corrosion resistance behavior in fluoride and chloride environments. Angle Orthod. 2013, 83, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.; Espinar, E.; Llamas, J.; Manero, J.; Ginebra, M. Variation of the superelastic properties and nickel release from original and reused NiTi orthodontic archwires. J. Mech. Behav. Biomed. Mater. 2012, 6, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Hamada, K.; Moriyama, K.; Asaoka, K. Degradation and fracture of Ni–Ti superelastic wire in an oral cavity. Biomaterials 2001, 22, 2257–2262. [Google Scholar] [CrossRef]
- Burstone, C.J.; Qin, B.; Morton, J.Y. Chinese NiTi wire—A new orthodontic alloy. Am. J. Orthod. 1985, 87, 445–452. [Google Scholar] [CrossRef]
- Elahinia, M.H.; Hashemi, M.; Tabesh, M.; Bhaduri, S.B. Manufacturing and processing of NiTi implants: A review. Prog. Mater. Sci. 2012, 57, 911–946. [Google Scholar] [CrossRef]
- Shishkovsky, I.V.; Volova, L.T.; Kuznetsov, M.V.; Morozov, Y.G.; Parkin, I.P. Porous biocompatible implants and tissue scaffolds synthesized by selective laser sintering from Ti and NiTi. J. Mater. Chem. 2008, 18, 1309–1317. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Wang, H.; Ruan, J. Low elastic modulus titanium–nickel scaffolds for bone implants. Mater. Sci. Eng. C 2014, 34, 110–114. [Google Scholar] [CrossRef]
- Mohd-Jani, J.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Des. 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- Farber, E.; Zhu, J.-N.; Popovich, A.; Popovich, V. A review of NiTi shape memory alloy as a smart material produced by additive manufacturing. Mater. Today Proc. 2020, 30, 761–767. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, Z.; Sun, Z.; Hao, S.; Yang, Y.; Li, M.; Song, C.; Qiu, P.; Cui, L. Selective laser melting of NiTi alloy with superior tensile property and shape memory effect. J. Mater. Sci. Technol. 2019, 35, 2238–2242. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Hao, S.J.; Liu, Y.T.; Xiong, Z.W.; Guo, W.Q.; Yang, Y.; Ren, Y.; Cui, L.S.; Ren, L.Q.; Zhang, Z.H. The microstructure of a selective laser melting (SLM)-fabricated NiTi shape memory alloy with superior tensile property and shape memory recoverability. Appl. Mater. Today 2020, 19, 100547. [Google Scholar] [CrossRef]
- Saedi, S.; Saghaian, S.E.; Jahadakbar, A.; Moghaddam, N.S.; Andani, M.T.; Lu, Y.C.; Elahinia, M.; Karaca, H.E. Shape memory response of porous NiTi shape memory alloys fabricated by selective laser melting. J. Mater. Sci. Mater. Med. 2018, 29, 40. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, B.; Kruth, J.-P. Selective laser melting of biocompatible metals for rapid manufacturing of medical parts. Rapid Prototyp. J. 2007, 13, 196–203. [Google Scholar] [CrossRef]
- Marandi, L.; Sen, I. In-vitro mechanical behavior and high cycle fatigue characteristics of NiTi-based shape memory alloy wire. Int. J. Fatigue 2021, 148, 106226. [Google Scholar] [CrossRef]
- Eliades, T.; Eliades, G.; Athanasiou, A.E.; Bradley, T.G. Surface characterization of retrieved NiTi orthodontic archwires. Eur. J. Orthod. 2000, 22, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prymak, O.; Klocke, A.; Kahl-Nieke, B.; Epple, M. Fatigue of orthodontic nickel–titanium (NiTi) wires in different fluids under constant mechanical stress. Mater. Sci. Eng. A 2004, 378, 110–114. [Google Scholar] [CrossRef]
- Chitra, P.; Prashantha, G.; Rao, A. Effect of fluoride agents on surface characteristics of NiTi wires. An ex vivo investigation. J. Oral Biol. Craniofac. Res. 2020, 10, 435–440. [Google Scholar] [CrossRef]
- Kassab, E.; Gomes, J.A.D.C.P. Corrosion induced fracture of NiTi wires in simulated oral environments. J. Mech. Behav. Biomed. Mater. 2021, 116, 104323. [Google Scholar] [CrossRef]
- Wang, S.; Feng, Y.; Lin, X. Study on Microstructure and Properties of Ni50.8Ti49.2 Shape Memory Alloy by Selective Laser Melting. J. Mech. Eng. 2020, 56, 46–52. [Google Scholar]
- Gilbert, J.L.; Mali, S.A. Medical Implant Corrosion: Electrochemistry at Metallic Biomaterial Surfaces. In Degradation of Implant Materials, 1st ed.; Eliaz, N., Ed.; Springer: New York, NY, USA, 2012; pp. 1–28. [Google Scholar]
- Hu, T.; Chu, C.; Xin, Y.; Wu, S.; Yeung, K.; Chu, P.K. Corrosion products and mechanism on NiTi shape memory alloy in physiological environment. J. Mater. Res. 2010, 25, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Qiu, P.; Gao, P.P.; Wang, S.Y.; Li, Z.H.; Yang, Y.; Zhang, Q.Q.; Xiong, Z.W.; Hao, S.J. Study on corrosion behavior of the selective laser melted NiTi alloy with superior tensile property and shape memory effect. Corros. Sci. 2020, 175, 108891. [Google Scholar] [CrossRef]
- Yu, Z.L.; Xu, Z.Z.; Guo, Y.T.; Sha, P.W.; Liu, R.L.; Xin, R.L.; Li, L.X.; Chen, L.X.; Wang, X.B.; Zhang, Z.H.; et al. Analysis of microstructure, mechanical properties, wear characteristics and corrosion behavior of SLM-NiTi under different process parameters. J. Manuf. Process. 2022, 75, 637–650. [Google Scholar] [CrossRef]
- Fadlallah, S.A.; El-Bagoury, N.; El-Rab, S.M.F.G.; Ahmed, R.A.; El-Ousamii, G. An overview of NiTi shape memory alloy: Corrosion resistance and antibacterial inhibition for dental application. J. Alloys Compd. 2014, 583, 455–464. [Google Scholar] [CrossRef]
- Matono, Y.; Nakagawa, M.; Matsuya, S.; Ishikawa, K.; Terada, Y. Corrosion Behavior of Pure Titanium and Titanium Alloys in Various Concentrations of Acidulated Phosphate Fluoride (APF) Solutions. Dent. Mater. J. 2006, 25, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Schiff, N.; Dalard, F.; Lissac, M.; Morgon, L.; Grosgogeat, B. Corrosion resistance of three orthodontic brackets: A comparative study of three fluoride mouthwashes. Eur. J. Orthod. 2005, 27, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Reclaru, L.; Meyer, J.-M. Effects of fluorides on titanium and other dental alloys in dentistry. Biomaterials 1998, 19, 85–92. [Google Scholar] [CrossRef]
- Nakagawa, M.; Matsuya, S.; Shiraishi, T.; Ohta, M. Effect of fluoride concentration and pH on corrosion behavior of titanium for dental use. J. Dent. Res. 1999, 78, 1568–1572. [Google Scholar] [CrossRef]
- Al-Mayouf, A.; Al-Swayih, A.; Al-Mobarak, N.; Al-Jabab, A. Corrosion behavior of a new titanium alloy for dental implant applications in fluoride media. Mater. Chem. Phys. 2004, 86, 320–329. [Google Scholar] [CrossRef]
- Walker, M.P.; White, R.J.; Kula, K.S. Effect of fluoride prophylactic agents on the mechanical properties of nickel-titanium-based orthodontic wires. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 662–669. [Google Scholar] [CrossRef]
- Basame, S.B.; White, H.S. Pitting corrosion of titanium the relationship between pitting potential and competitive anion adsorption at the oxide film/electrolyte interface. J. Electrochem. Soc. 2000, 147, 1376. [Google Scholar] [CrossRef]
- de Castro, S.M.; Ponces, M.J.; Lopes, J.D.; Vasconcelos, M.; Pollmann, M.C. Orthodontic wires and its corrosion—The specific case of stainless steel and beta-titanium. J. Dent. Sci. 2015, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Toumelin-Chemla, F.; Rouelle, F.; Burdairon, G. Corrosive properties of fluoride-containing odontologic gels against titanium. J. Dent. 1996, 24, 109–115. [Google Scholar] [CrossRef]
- Mirjalili, M.; Momeni, M.; Ebrahimi, N.; Moayed, M.H. Comparative study on corrosion behaviour of Nitinol and stainless steel orthodontic wires in simulated saliva solution in presence of fluoride ions. Mater. Sci. Eng. C 2013, 33, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Fovet, Y.; Gal, J.Y.; Toumelin-Chemla, F. Influence of pH and fluoride concentration on titanium passivating layer: Stability of titanium dioxide. Talanta 2001, 53, 1053–1063. [Google Scholar] [CrossRef]
- Velten, D.; Biehl, V.; Aubertin, F.; Valeske, B.; Possart, W.; Breme, J. Preparation of TiO2 layers on cp-Ti and Ti6Al4V by thermal and anodic oxidation and by sol-gel coating techniques and their characterization. J. Biomed. Mater. Res. 2002, 59, 18–28. [Google Scholar] [CrossRef]
- Vojtěch, D.; Voděrová, M.; Fojt, J.; Novák, P.; Kubásek, T. Surface structure and corrosion resistance of short-time heat-treated NiTi shape memory alloy. Appl. Surf. Sci. 2010, 257, 1573–1582. [Google Scholar] [CrossRef]
- Man, H.; Cui, Z.; Yue, T. Corrosion properties of laser surface melted NiTi shape memory alloy. Scr. Mater. 2001, 45, 1447–1453. [Google Scholar]

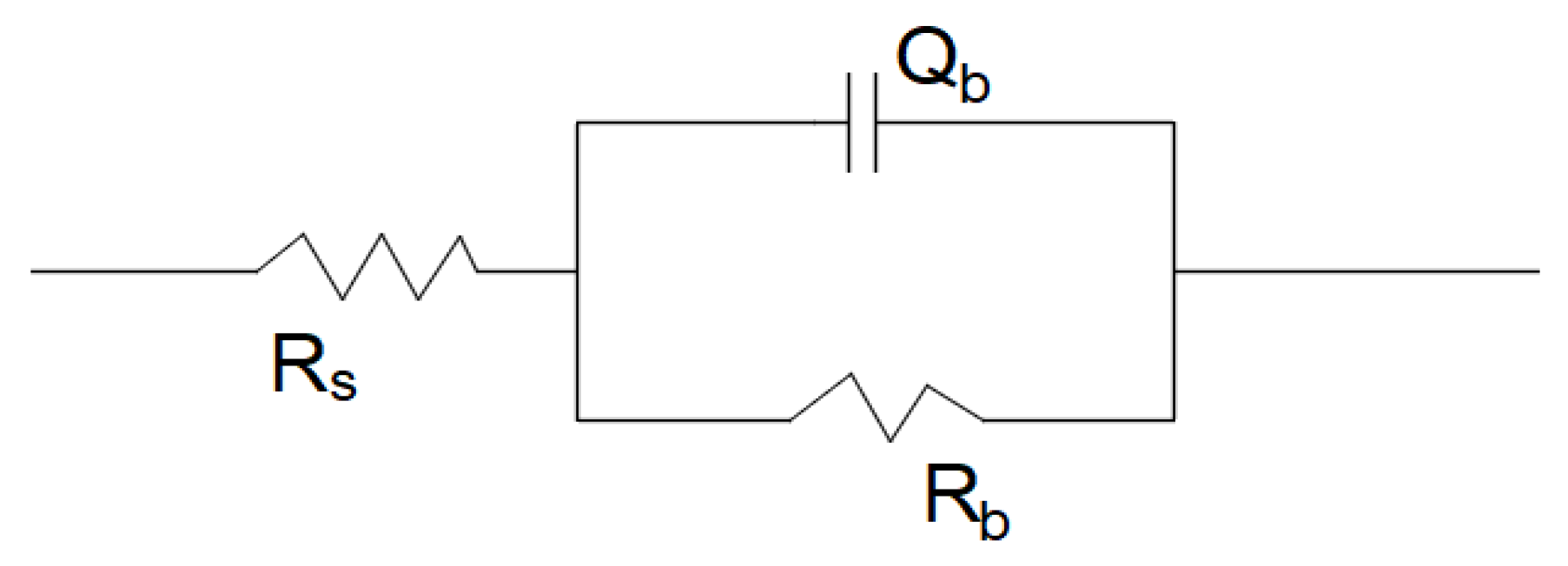
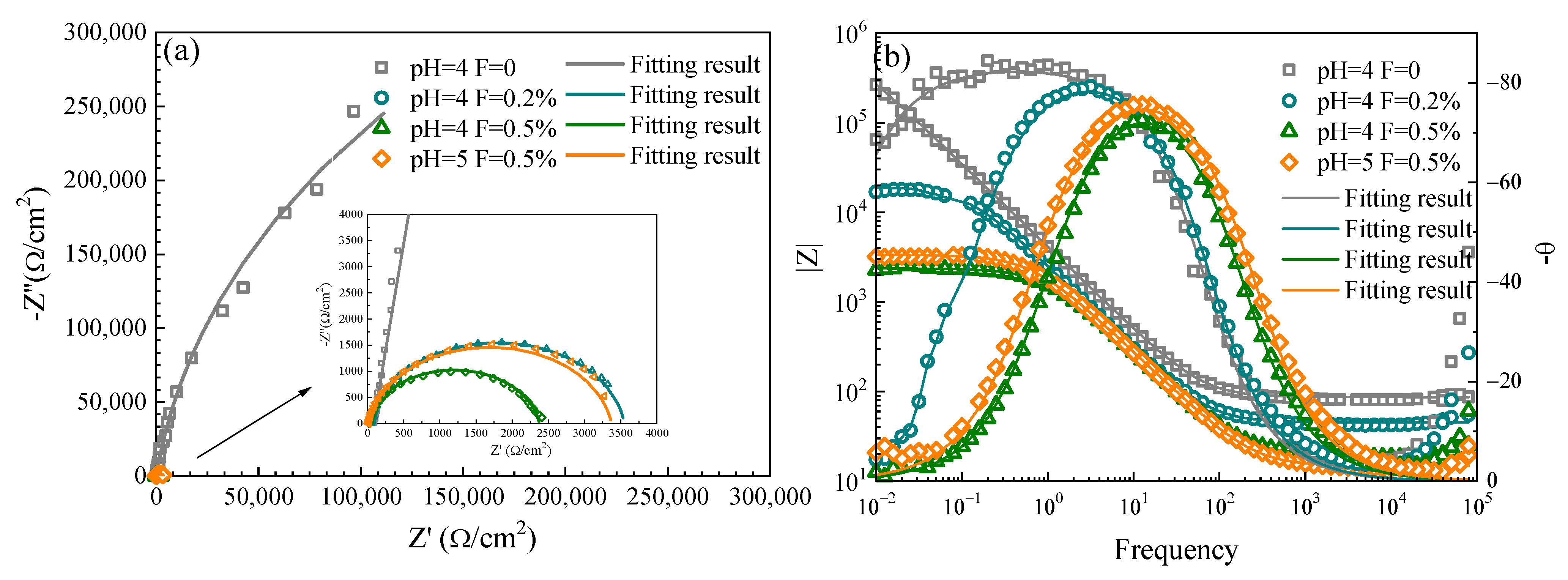
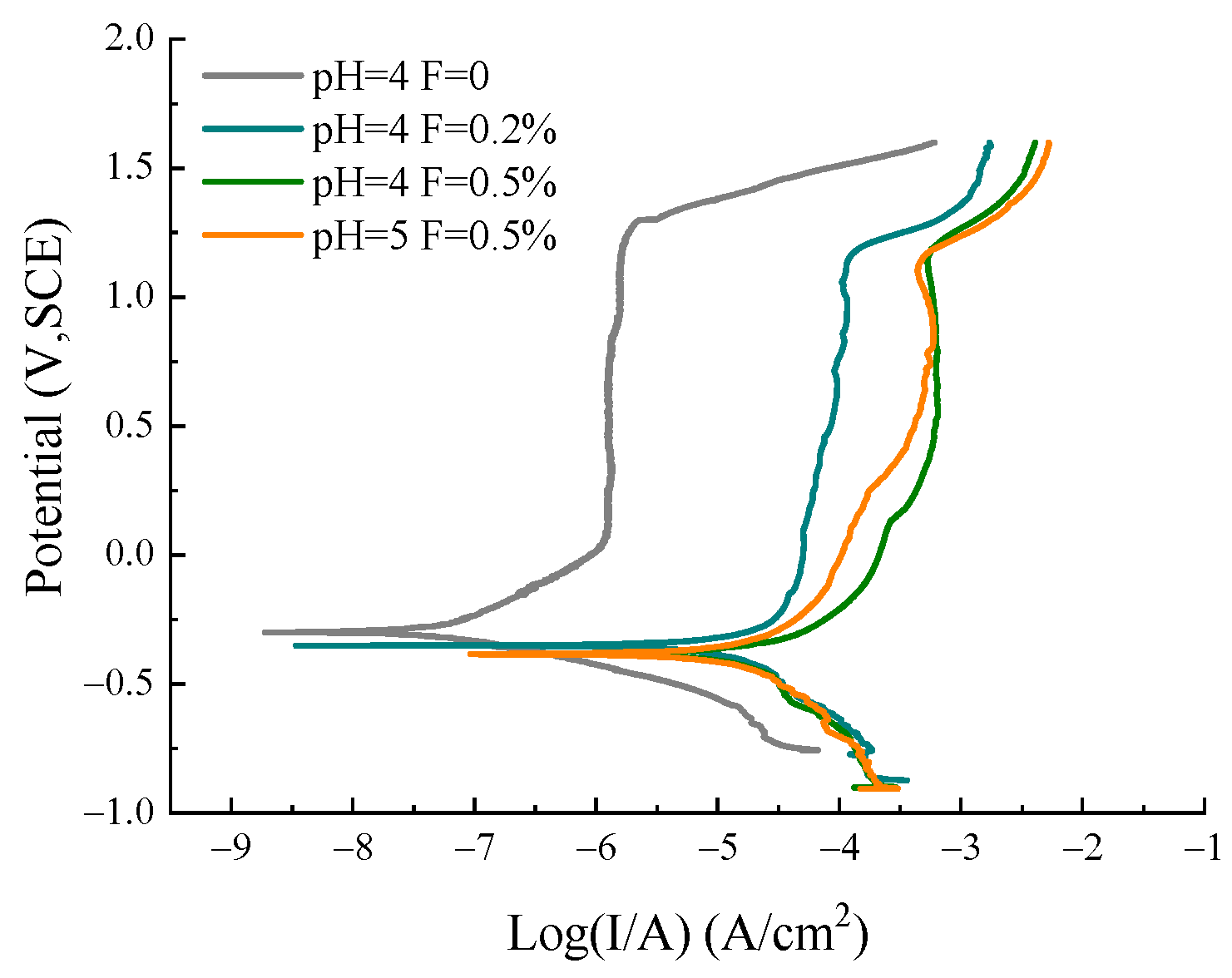

| Samples | Rs/Ω·cm2 | Rb/K·Ωcm2 | Qb/μFcm−2 | nb | Chi-Square |
|---|---|---|---|---|---|
| SLM TiNi | 22.24 ± 1.98 | 2.585 ± 0.594 | 82.49 ± 0.67 | 0.9189 ± 0.0052 | (5.89 ± 0.75) × 10−3 |
| Wrought TiNi | 18.75 ± 2.07 | 2.484 ± 0.223 | 74.36 ± 7.71 | 0.9205 ± 0.0066 | (4.81 ± 0.45) × 10−3 |
| Samples | Rs/Ω·cm2 | Rb/KΩ·cm2 | Qb/μFcm−2 | nb | Chi-Square |
|---|---|---|---|---|---|
| pH 4 without F− | 90.09 ± 2.40 | 542.967 ± 72.206 | 45.31 ± 7.25 | 0.9267 ± 0.0011 | (2.85 ± 0.43) × 10−2 |
| pH 4 0.2% F− | 47.51 ± 4.30 | 4.237 ± 0.653 | 74.31 ± 2.16 | 0.9246 ± 0.0013 | (1.44 ± 0.12) × 10−2 |
| pH 4 0.5% F− | 22.24 ± 1.98 | 2.585 ± 0.594 | 82.49 ± 0.67 | 0.9189 ± 0.0052 | (5.89 ± 0.75) × 10−3 |
| pH 5 0.5% F− | 18.12 ± 2.60 | 3.674 ± 0.231 | 80.42 ± 4.52 | 0.9203 ± 0.0104 | (6.31 ± 1.15) × 10−3 |
| Corrosion Parameters | SLM TiNi | Wrought TiNi |
|---|---|---|
| Ecorr (V) | −0.38 ± 0.006 | −0.41 ± 0.009 |
| Icorr (μA/cm2) | 22.0 ± 0.13 | 11.8 ± 1.57 |
| Ipass (μA/cm2) | 181 ± 9.18 | 55.46 ± 7.11 |
| Epit (V) | 1.13 ± 0.02 | 0.42 ± 0.02 |
| Corrosion Parameters | pH 4 without F− | pH 4 0.2% F− | pH 4 0.5% F− | pH 5 0.5% F− |
|---|---|---|---|---|
| Ecorr (V) | −0.30 ± 0.005 | −0.36 ± 0.009 | −0.38 ± 0.006 | −0.38 ± 0.014 |
| Icorr (μA/cm2) | (9.85 ± 0.46) × 10−2 | 13.9 ± 3.05 | 22.0 ± 0.13 | 20.8 ± 5.85 |
| Ipass (μA/cm2) | 1.25 ± 0.16 | 34.8 ± 0.51 | 181 ± 9.18 | 80.1 ± 5.63 |
| Epit (V) | 1.27 ± 0.04 | 1.14 ± 0.00 | 1.13 ± 0.02 | 1.13 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, C.; Wang, X.; Hu, M.; Su, Y.; Li, S.; Gai, X.; Sheng, L. Corrosion Behavior of TiNi Alloy Fabricated by Selective Laser Melting in Simulated Saliva. Coatings 2022, 12, 840. https://doi.org/10.3390/coatings12060840
Jia C, Wang X, Hu M, Su Y, Li S, Gai X, Sheng L. Corrosion Behavior of TiNi Alloy Fabricated by Selective Laser Melting in Simulated Saliva. Coatings. 2022; 12(6):840. https://doi.org/10.3390/coatings12060840
Chicago/Turabian StyleJia, Chenfan, Xinyu Wang, Ming Hu, Yucheng Su, Shujun Li, Xin Gai, and Liyuan Sheng. 2022. "Corrosion Behavior of TiNi Alloy Fabricated by Selective Laser Melting in Simulated Saliva" Coatings 12, no. 6: 840. https://doi.org/10.3390/coatings12060840
APA StyleJia, C., Wang, X., Hu, M., Su, Y., Li, S., Gai, X., & Sheng, L. (2022). Corrosion Behavior of TiNi Alloy Fabricated by Selective Laser Melting in Simulated Saliva. Coatings, 12(6), 840. https://doi.org/10.3390/coatings12060840







