Surface Modification of WE43 Magnesium Alloys with Dopamine Hydrochloride Modified GelMA Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
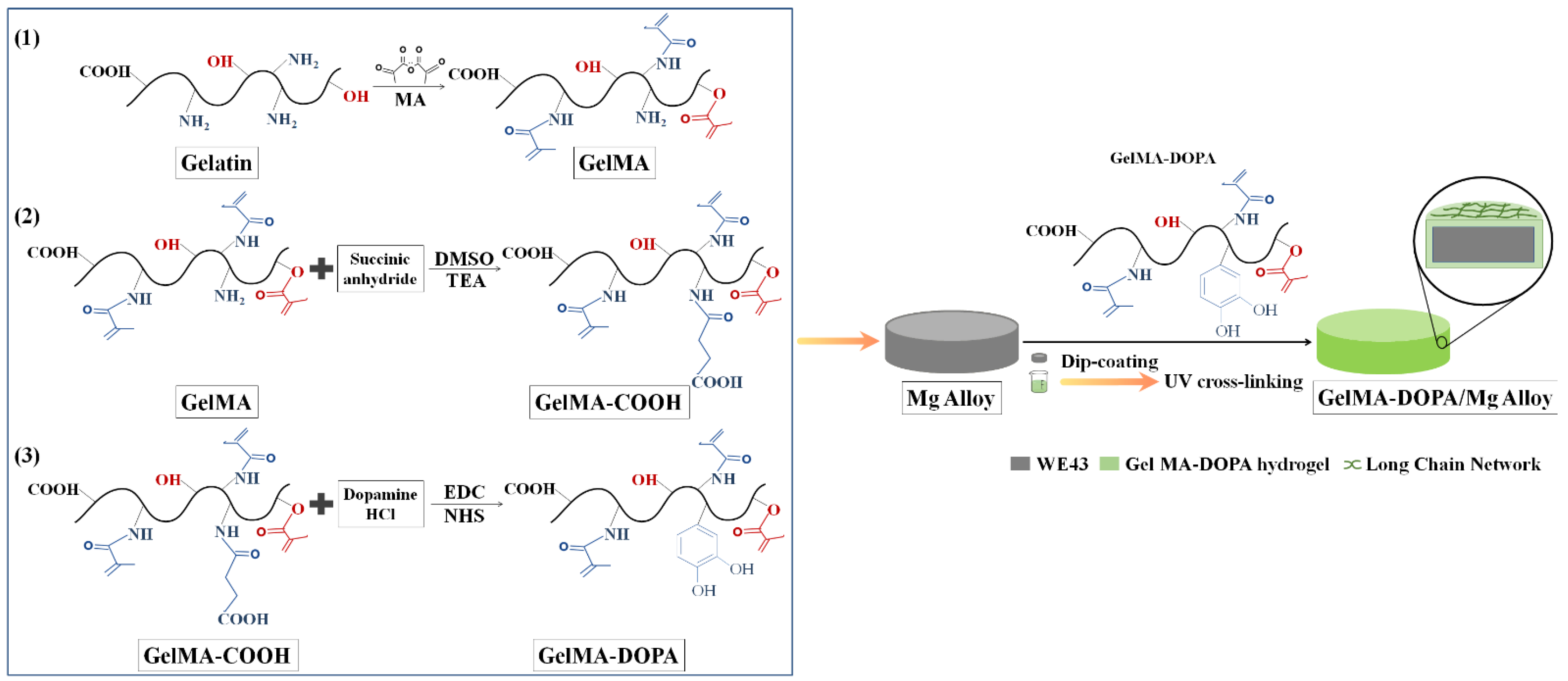
2.2.1. Synthesis of GelMA Prepolymer
2.2.2. Synthesis of GelMA-COOH Prepolymer
2.2.3. Synthesis of GelMA-DOPA Prepolymer
2.2.4. Preparation of GelMA-DOPA/Mg Composites
2.2.5. Characterizations
3. Results
3.1. Chemical groups of samples
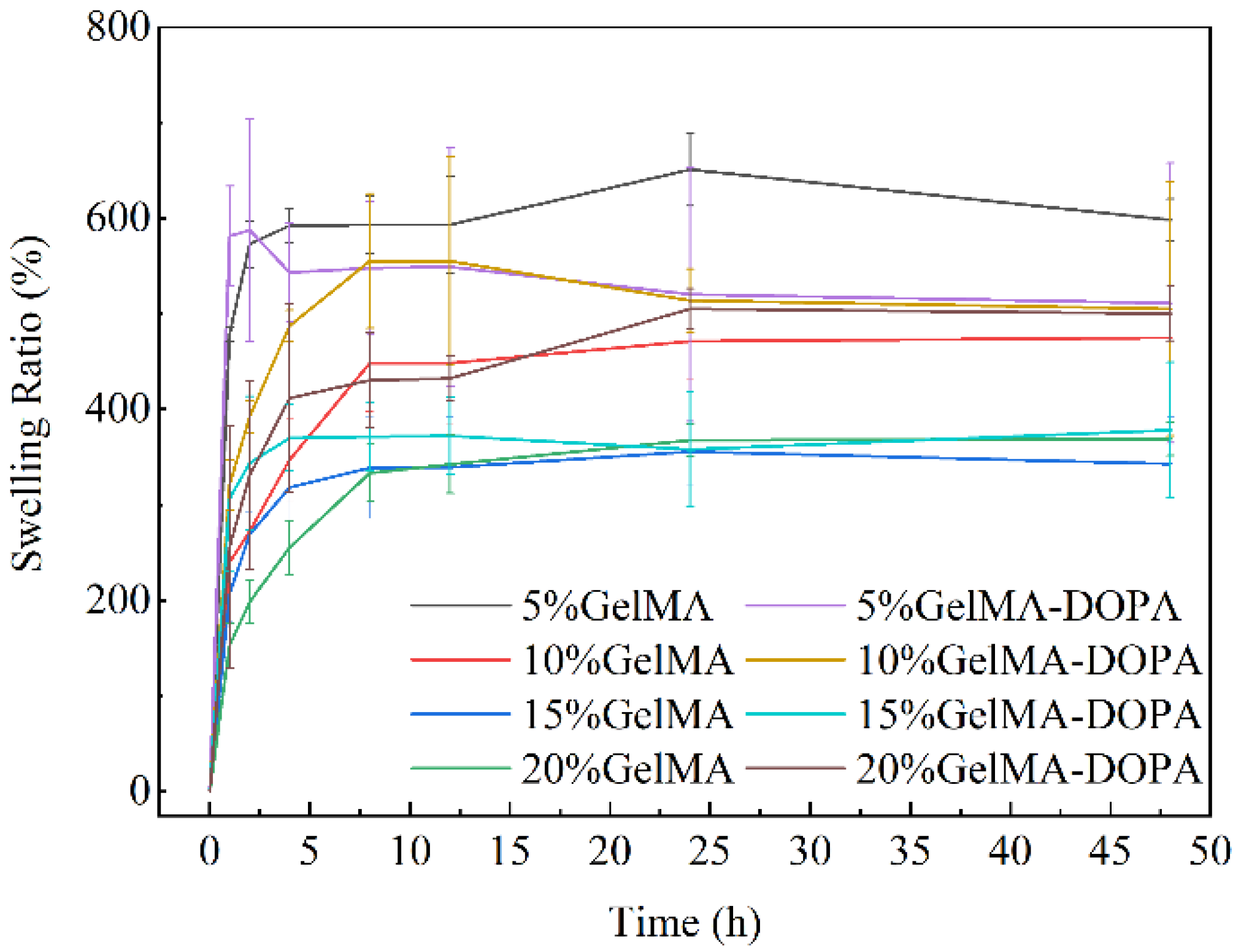
3.2. Swelling Performances of GelMA and GelMA-DOPA Hydrogels
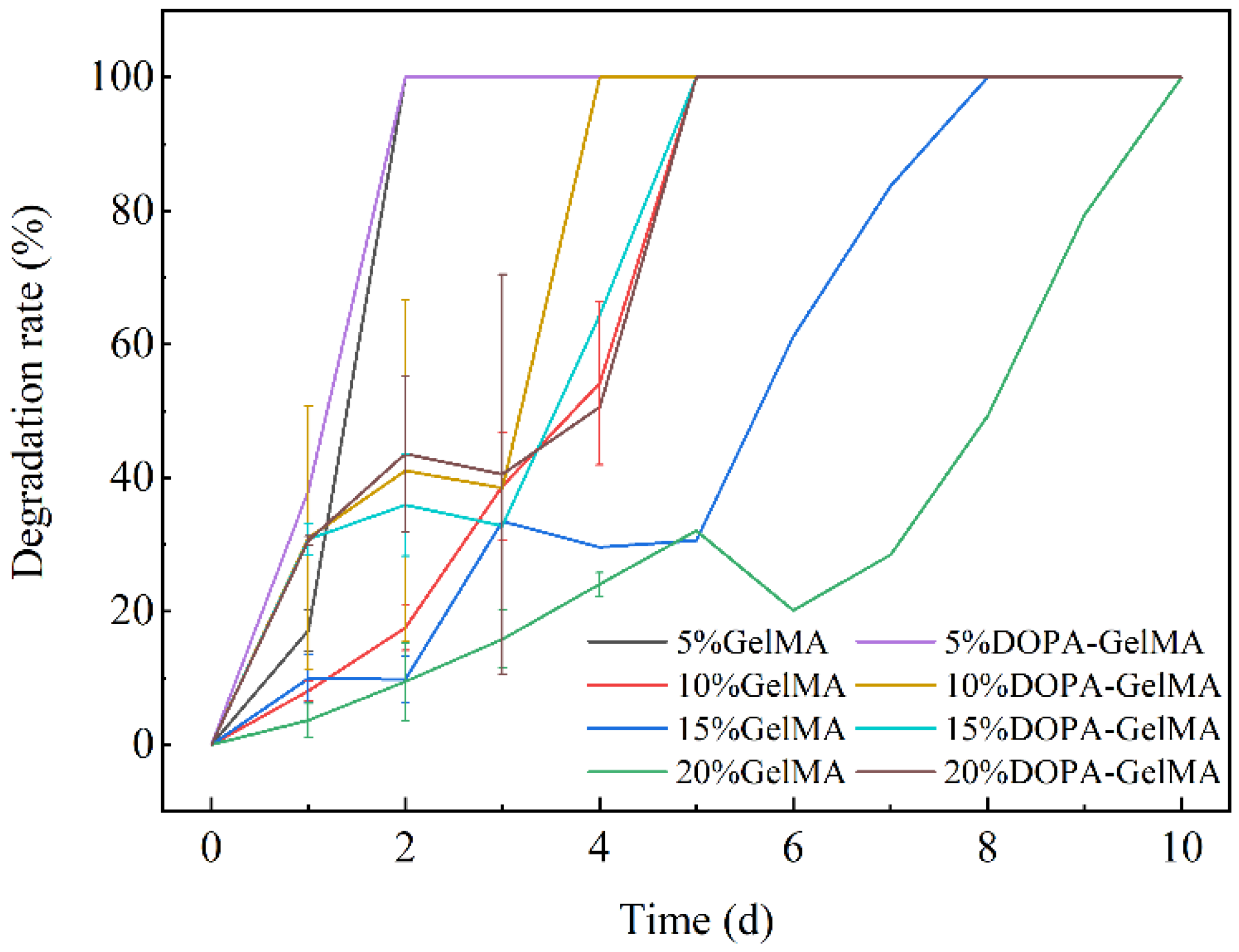
3.3. Degradation Performances of GelMA and GelMA-DOPA Hydrogels
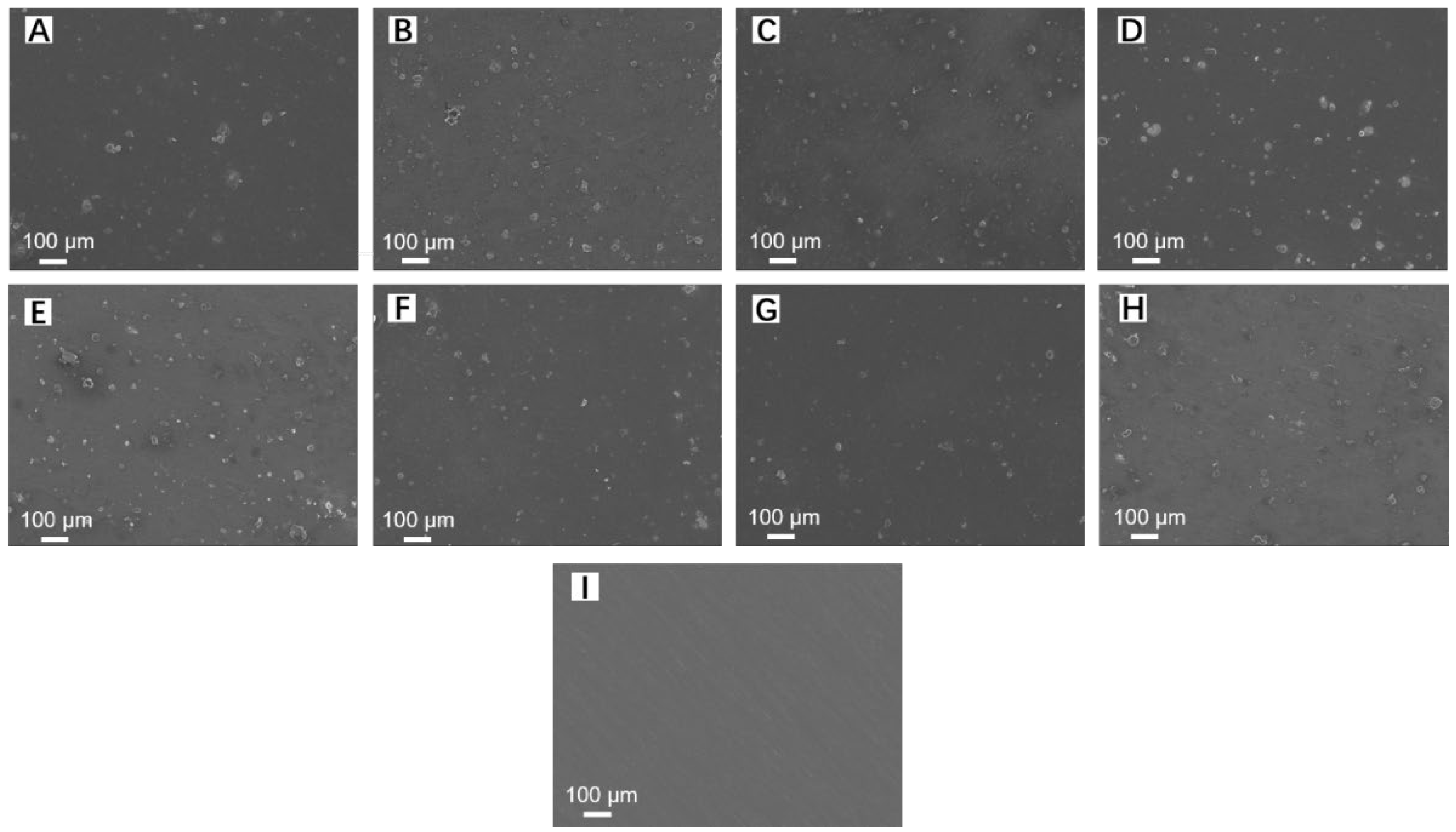
3.4. Morphologies of GelMA and GelMA-DOPA Hydrogel Coated Mg Alloys
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Z.; Sun, X.; Yang, H. The Role of Antibacterial Metallic Elements in Simultaneously Improving the Corrosion Resistance and Antibacterial Activity of Magnesium Alloys. Mater. Des. 2021, 198, 109350. [Google Scholar] [CrossRef]
- Li, L.; Cui, L.; Zeng, R.; Li, S.; Chen, X.; Zheng, Y.; Kannan, M. Advances in functionalized polymer coatings on biodegradable magnesium alloys—A review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Yu, X.; Huang, W.; Zhao, D.; Yang, K.; Tan, L.; Zhang, X.; Li, J.; Zhang, M.; Zhang, S.; Liu, T.; et al. Study of engineered low-modulus Mg/PLLA composites as potential orthopaedic implants: An in vitro and in vivo study. Colloids Surf. B-Biointerfaces 2019, 174, 280–290. [Google Scholar] [CrossRef]
- Liu, C.; Yang, H.; Wan, P.; Wang, K.; Tan, L.; Yang, K. Study on biodegradation of the second phase Mg17Al12 in Mg-Al-Zn Alloys: In vitro experiment and thermodynamic calculation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 35, 1–7. [Google Scholar] [CrossRef]
- Ji, Y.; Yu, X.; Zhu, H. Fabrication of Mg Coating on PEEK and Antibacterial Evaluation for Bone Application. Coatings 2021, 11, 1010. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Z.; Zang, S.; Yu, X.; Yang, H.; Chang, S. Research Progress on Surface Treatments of Biodegradable Mg Alloys: A Review. Acs Omega 2020, 5, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Wieja, F.; Jacobs, G.; Stein, S.; Kopp, A.; van Gaalen, K.; Kroger, N.; Zinser, M. Development and validation of a parametric human mandible model to determine internal stresses for the future design optimization of maxillofacial implants. J. Mech. Behav. Biomed. Mater. 2022, 125, 104893. [Google Scholar] [CrossRef]
- Bommala, V.; Krishna, M.; Rao, C. Magnesium matrix composites for biomedical applications. J. Magnes. Alloys 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Wu, W.; Yu, X.; Zhao, Y.; Jiang, X.; Yang, H. Characterization and Biocompatibility of Insoluble Corrosion Products of AZ91 Mg Alloys. Acs Omega 2019, 4, 15139–15148. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Bi, Y.; He, D.; Yu, H.; Li, Y. Research Progress on Surface Modification of Biodegradable Magnesium and Magnesium Alloys. Surf. Technol. 2019, 48, 11–19. [Google Scholar]
- Rahim, S.; Joseph, M.; Sampath Kumar, T.; Hanas, H. Recent Progress in Surface Modification of Mg Alloys for Biodegradable Orthopedic Applications. Front. Mater. 2022, 9, 848980. [Google Scholar] [CrossRef]
- Narayanan, T.; Park, I.; Lee, M. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Li, C.; Feng, X.; Fan, X.; Yu, X.; Yin, Z.; Konnan, M.; Chen, X.; Guan, S.; Zhang, J.; Zeng, R. Corrosion and Wear Resistance of Micro-Arc Oxidation Composite Coatings on Magnesium Alloy AZ31-The Influence of Inclusions of Carbon Spheres. Adv. Eng. Mater. 2019, 21, 1900446. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, Y.; Wang, Q.; Tan, L.; Yang, K.; Li, Y.; Li, W. Study on Biodegradable Property of Surface Modified AZ31B Magnesium Alloy in Different Simulated Body Fluid. Mater. Rev. 2011, 25, 66–69, 86. [Google Scholar]
- Liu, G.; Hu, J.; Ding, Z.; Wang, C. Bioactive calcium phosphate coating formed on micro-arc oxidized magnesium by chemical deposition. Appl. Surf. Sci. 2011, 257, 2051–2057. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, F.; Liu, X. Protection of magnesium alloys: From physical barrier coating to smart self-healing coating. J. Alloys Compd. 2021, 853, 157010. [Google Scholar] [CrossRef]
- Fan, X.; Huo, Y.; Li, C.; Kannan, M.; Chen, X.; Guan, S.; Zeng, R.; Ma, Q. Corrosion resistance of nanostructured magnesium hydroxide coating on magnesium alloy AZ31: Influence of EDTA. Rare Met. 2019, 38, 520–531. [Google Scholar] [CrossRef]
- Guo, L.; Gu, C.; Feng, J.; Guo, Y.; Jin, Y.; Tu, J. Hydrophobic epoxy resin coating with ionic liquid conversion pretreatment on magnesium alloy for promoting corrosion resistance. J. Mater. Sci. Technol. 2020, 37, 9–18. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, T.; Yu, X.; Sun, X.; Yang, H. Functionalization treatment of micro-arc oxidation coatings on magnesium alloys: A review. J. Alloys Compd. 2021, 879, 160453. [Google Scholar] [CrossRef]
- Liu, W.; Li, T.; Yang, C.; Wang, D.; He, G.; Cheng, M.; Wang, Q.; Zhang, X. Lithium-Incorporated Nanoporous Coating Formed by Micro Arc Oxidation (MAO) on Magnesium Alloy with Improved Corrosion Resistance, Angiogenesis and Osseointegration. J. Biomed. Nanotechnol. 2019, 15, 1172–1184. [Google Scholar] [CrossRef]
- Lin, X.; Tan, L.; Zhang, Q.; Yang, K.; Hu, Z.; Qiu, J.; Cai, Y. The in vitro degradation process and biocompatibility of a ZK60 magnesium alloy with a forsterite-containing micro-arc oxidation coating. Acta Biomater. 2013, 9, 8631–8642. [Google Scholar] [CrossRef]
- Elmensouri, G.; Kara, I.; Ahlatci, H.; Turen, Y. Wear Resistance of Sheet Magnesium Alloy AZ31 with Micro Arc Oxidation Coatings after Shot Peening. Met. Sci. Heat Treat. 2021, 63, 426–429. [Google Scholar] [CrossRef]
- Fischerauer, S.; Kraus, T.; Wu, X.; Tangl, S.; Sorantin, E.; Hanzi, A.; Loffler, J.; Uggowitzer, P.; Weinberg, A. In vivo degradation performance of micro-arc-oxidized magnesium implants: A micro-CT study in rats. Acta Biomater. 2013, 9, 5411–5420. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Tian, S.; Li, H.; Zhao, Y.; Jia, D.; Zhou, Y. Formation mechanism, degradation behavior, and cytocompatibility of a double-layered structural MAO/rGO-CaP coating on AZ31 Mg. Colloids Surf. B-Biointerfaces 2020, 190, 110901. [Google Scholar] [CrossRef]
- Li, C.; Yu, C.; Zeng, R.; Zhang, B.; Cui, L.; Wan, J.; Xia, Y. In vitro corrosion resistance of a Ta2O5 nanofilm on MAO coated magnesium alloy AZ31 by atomic layer deposition. Bioact. Mater. 2020, 5, 34–43. [Google Scholar] [CrossRef]
- Shang, W.; Wu, F.; Wang, Y.; Baboukani, A.; Wen, Y.; Jiang, J. Corrosion Resistance of Micro-Arc Oxidation/Graphene Oxide Composite Coatings on Magnesium Alloys. Acs Omega 2020, 5, 7262–7270. [Google Scholar] [CrossRef] [Green Version]
- Shang, W.; Chen, B.; Shi, X.; Chen, Y.; Xiao, X. Electrochemical corrosion behavior of composite MAO/sol-gel coatings on magnesium alloy AZ91D using combined micro-arc oxidation and sol-gel technique. J. Alloys Compd. 2009, 474, 541–545. [Google Scholar] [CrossRef]
- Liao, S.; Chang, C.; Chen, C.; Lee, C.; Lin, W. Functionalization of pure titanium MAO coatings by surface modifications for biomedical applications. Surf. Coat. Technol. 2020, 394, 125812. [Google Scholar] [CrossRef]
- Liu, T.; Weng, W.; Zhang, Y.; Sun, X.; Yang, H. Applications of Gelatin Methacryloyl (GelMA) Hydrogels in Microfluidic Technique-Assisted Tissue Engineering. Molecules 2020, 25, 5305. [Google Scholar] [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Jin, M.; Zhang, Y.; Weng, W.; Wang, T.; Yang, H.; Zhou, L. K+/Sr2+/Na+ triple-doped hydroxyapatites/GelMA composite hydrogel scaffold for the repair of bone defects. Ceram. Int. 2021, 47, 30929–30937. [Google Scholar] [CrossRef]
- Weng, W.; Wu, W.; Yu, X.; Sun, M.; Lin, Z.; Ibrahim, M.; Yang, H. Effect of GelMA Hydrogel Coatings on Corrosion Resistance and Biocompatibility of MAO-Coated Mg Alloys. Materials 2020, 13, 3834. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yue, K.; Kazemzadeh-Narbat, M.; Liu, Y.; Khalilpour, A.; Li, B.; Zhang, Y.; Annabi, N.; Khademhosseini, A. Mussel-Inspired Multifunctional Hydrogel Coating for Prevention of Infections and Enhanced Osteogenesis. Acs Appl. Mater. Interfaces 2017, 9, 11428–11439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, Y.; Sun, M.; Jin, M.; Xia, W.; Yang, H.; Wang, T. Effect of Freezing Process on the Microstructure of Gelatin Methacryloyl Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 810155. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Hou, M.; Zhang, J.; Wang, T.; Cao, C.; Yang, H.; Zhang, X. Surface Modification of WE43 Magnesium Alloys with Dopamine Hydrochloride Modified GelMA Coatings. Coatings 2022, 12, 1074. https://doi.org/10.3390/coatings12081074
Ji Y, Hou M, Zhang J, Wang T, Cao C, Yang H, Zhang X. Surface Modification of WE43 Magnesium Alloys with Dopamine Hydrochloride Modified GelMA Coatings. Coatings. 2022; 12(8):1074. https://doi.org/10.3390/coatings12081074
Chicago/Turabian StyleJi, Yang, Mengdie Hou, Jin Zhang, Tianlin Wang, Can Cao, Huazhe Yang, and Xiaodong Zhang. 2022. "Surface Modification of WE43 Magnesium Alloys with Dopamine Hydrochloride Modified GelMA Coatings" Coatings 12, no. 8: 1074. https://doi.org/10.3390/coatings12081074
APA StyleJi, Y., Hou, M., Zhang, J., Wang, T., Cao, C., Yang, H., & Zhang, X. (2022). Surface Modification of WE43 Magnesium Alloys with Dopamine Hydrochloride Modified GelMA Coatings. Coatings, 12(8), 1074. https://doi.org/10.3390/coatings12081074





