Design and Analysis of Biomedical Scaffolds Using TPMS-Based Porous Structures Inspired from Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mathematical Description and Generation of TPMS Unit Cells
- P surface (Schwarz P);
- G surface (Schoen’s G);
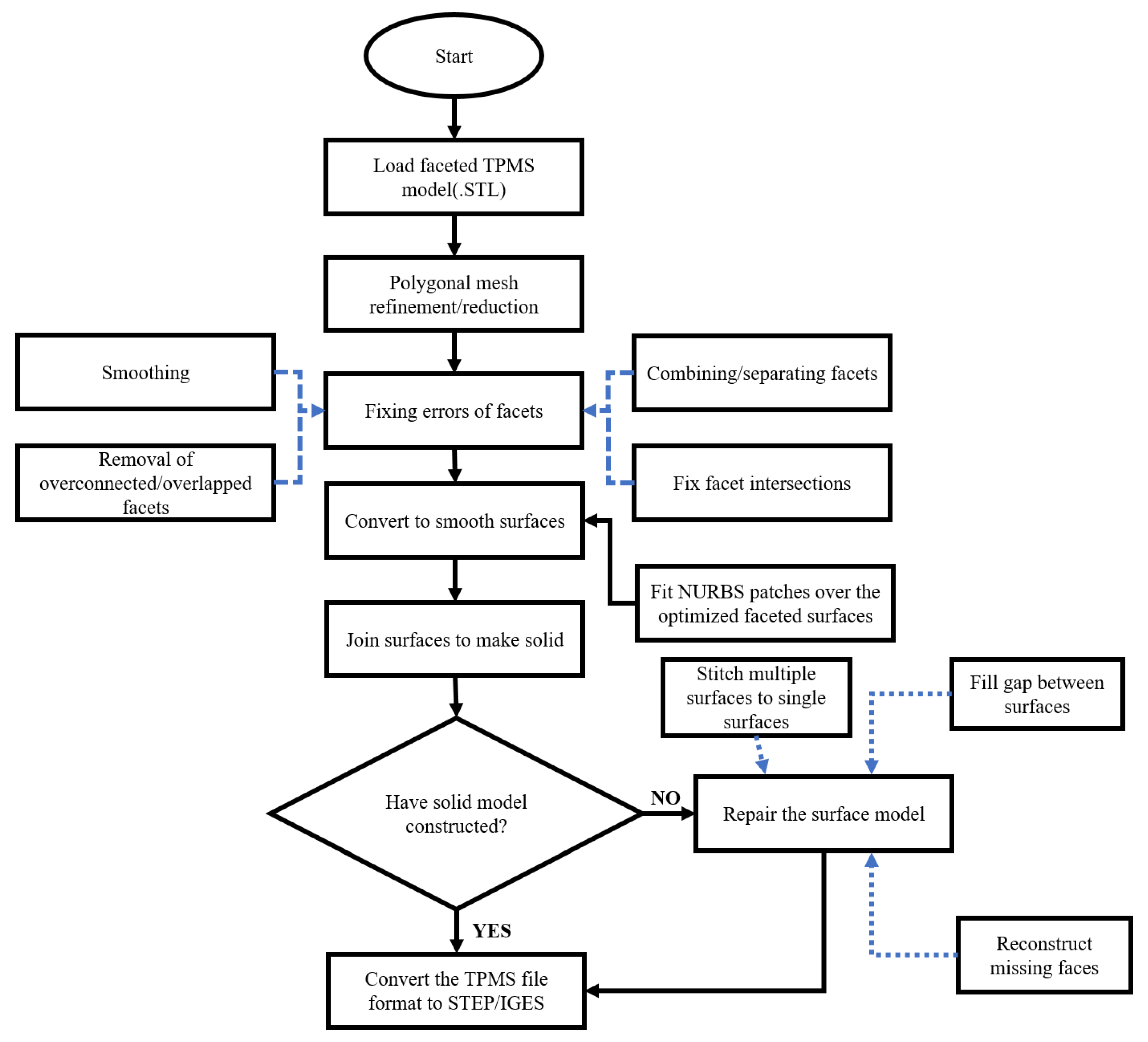
2.2. STL to Solid Multiscale Design Conversion Protocol of TPMS
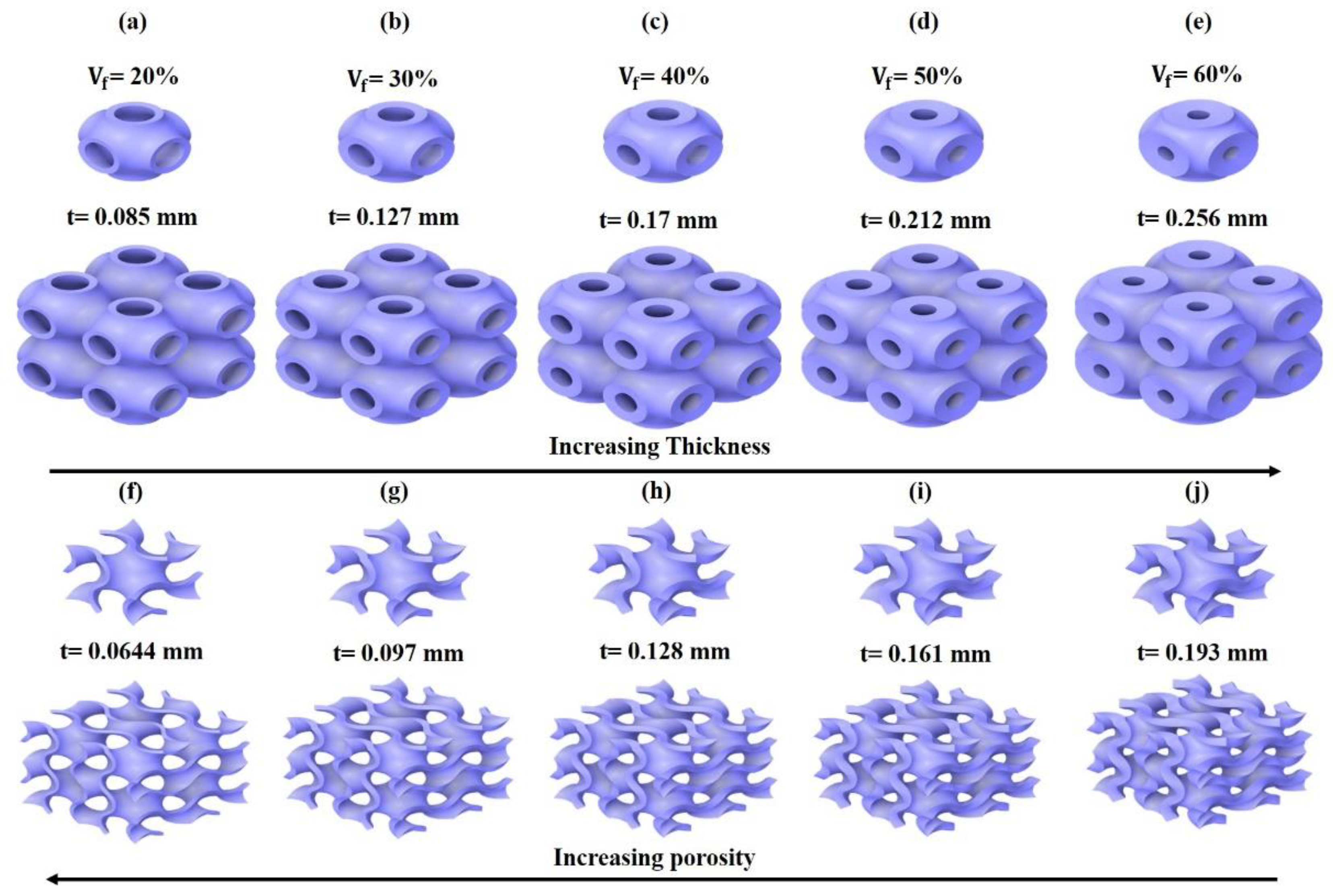
2.3. Generating Large TPMS Structures as Lattices
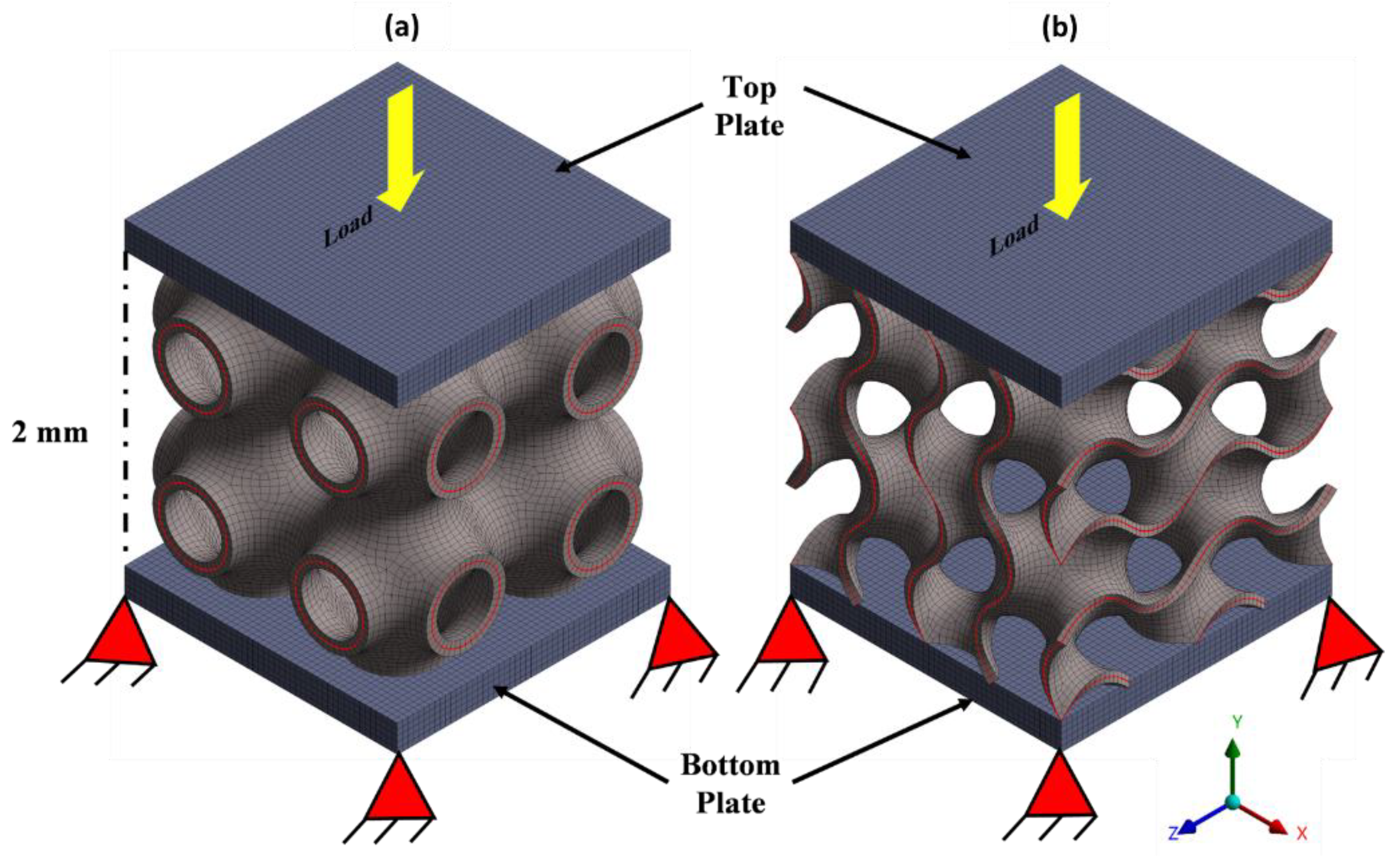
2.4. Simulation Procedure
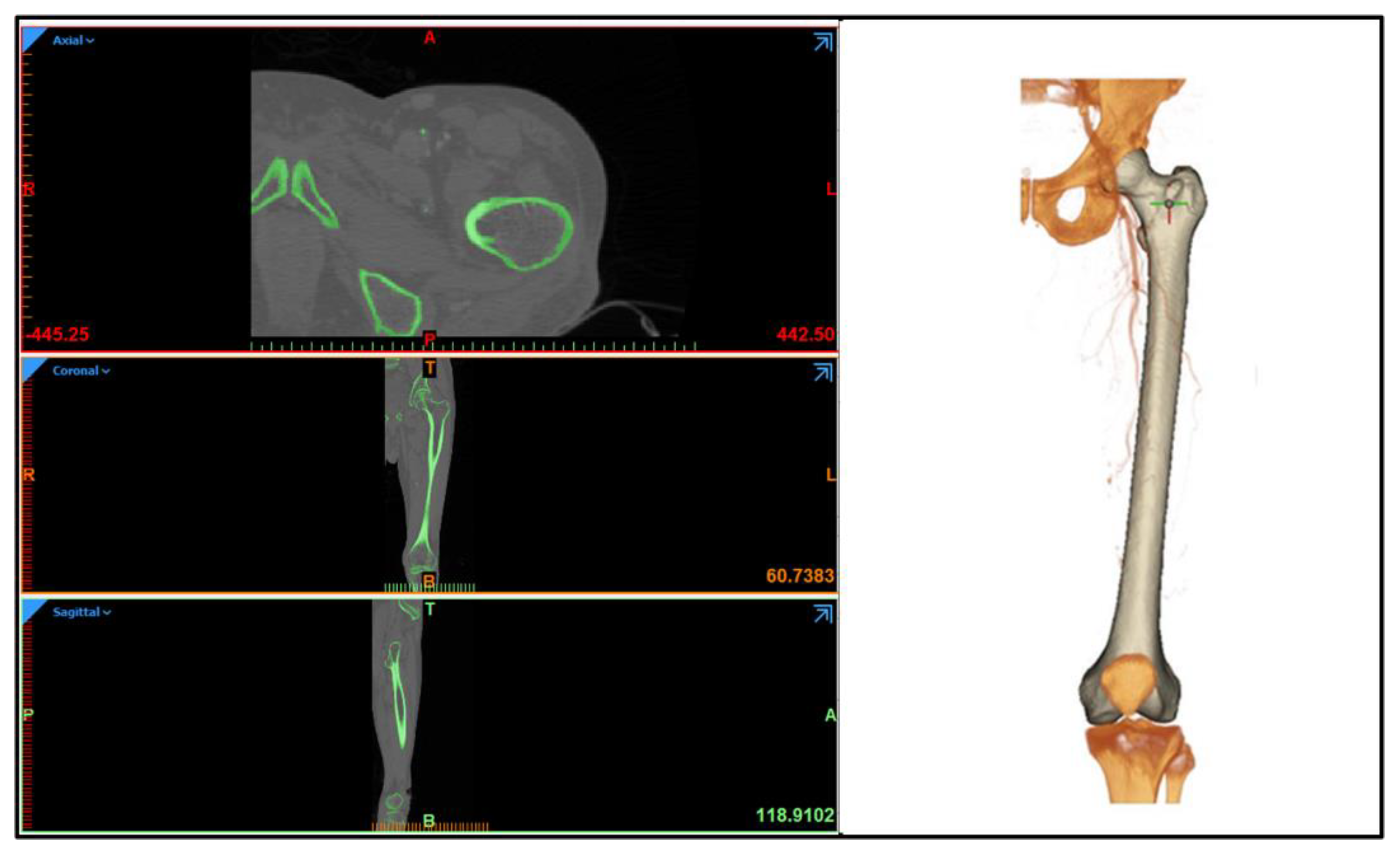
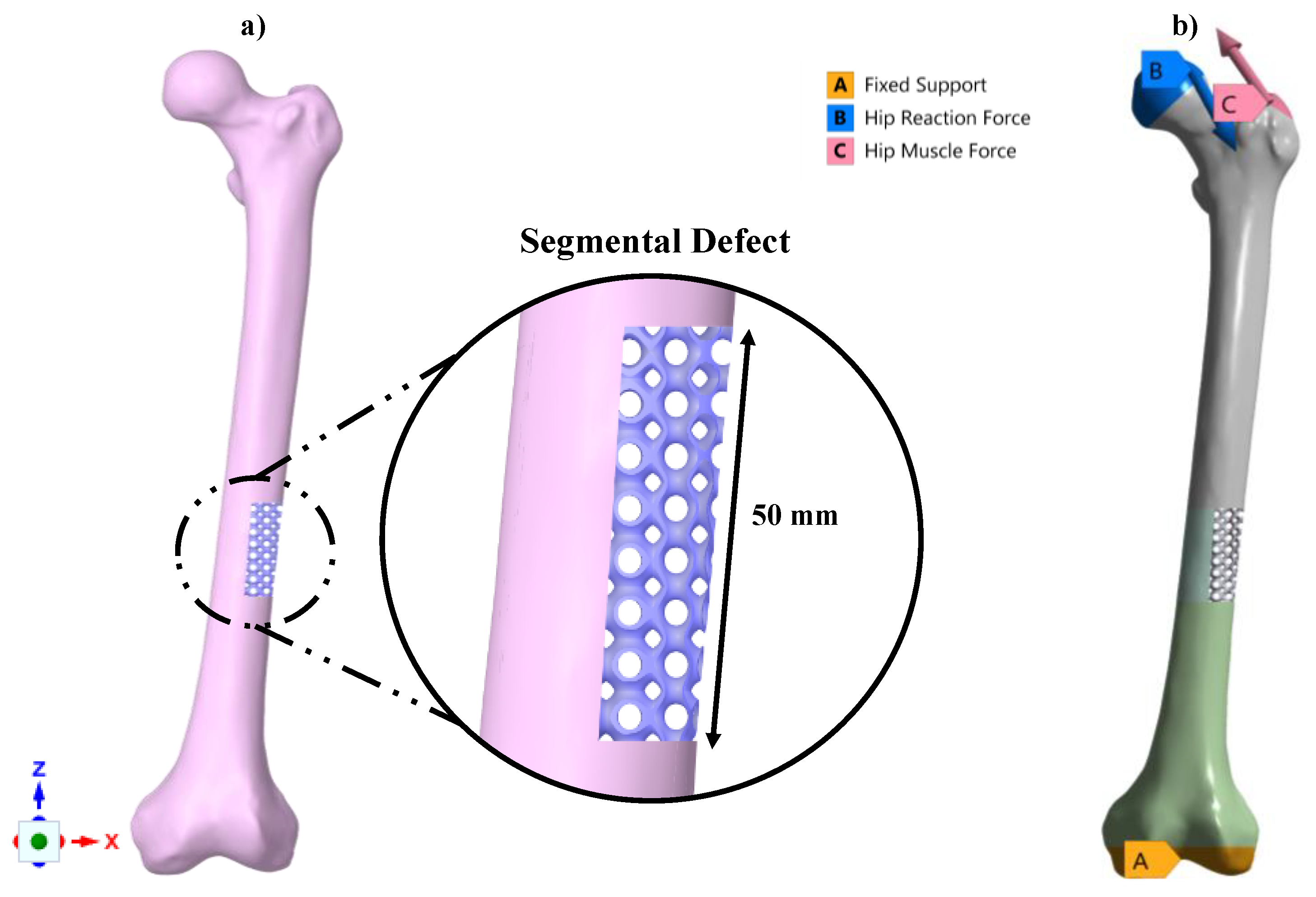
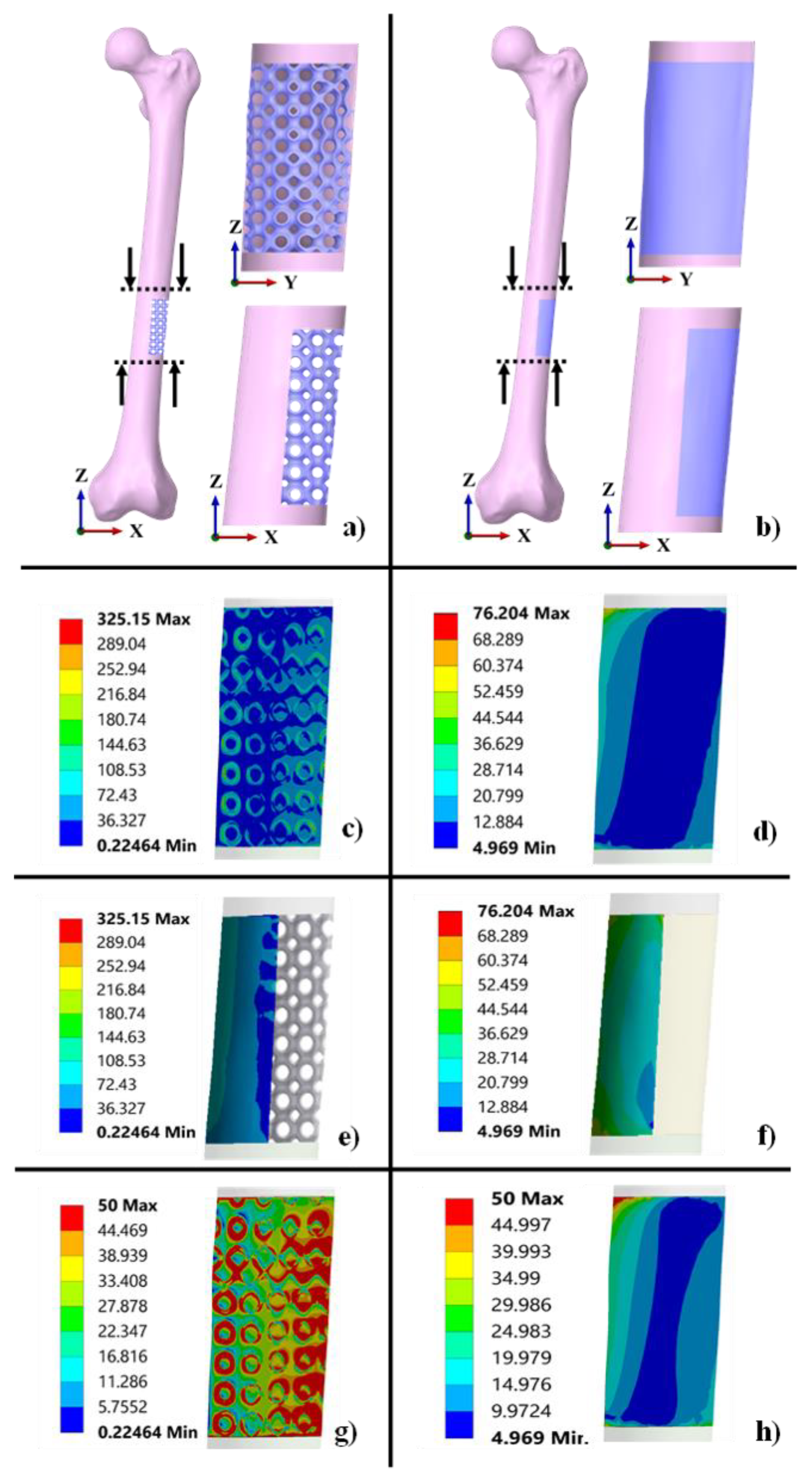
2.5. Functionality Assessment of Scaffold under Biomechanical Loading
3. Results and Discussion
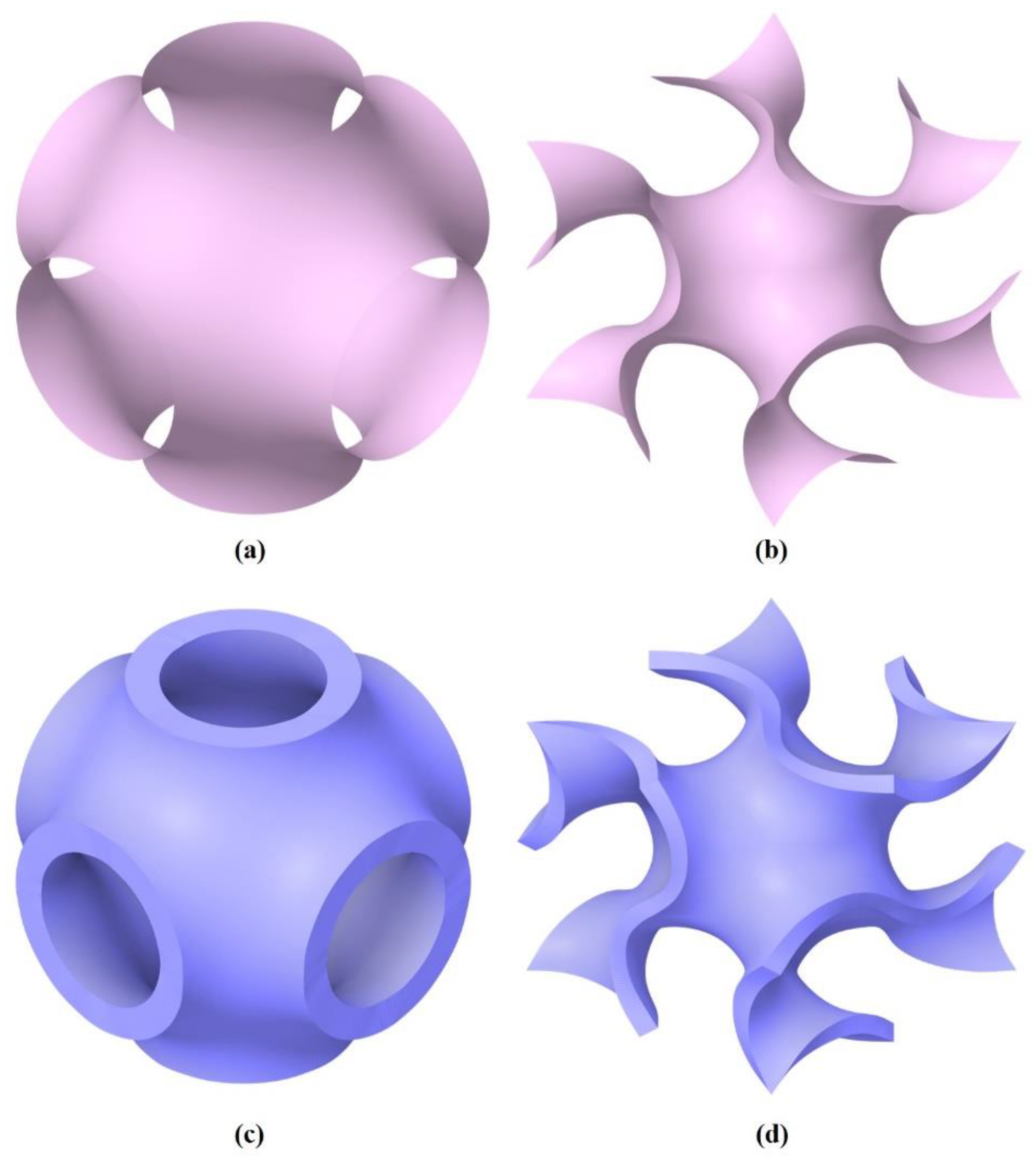
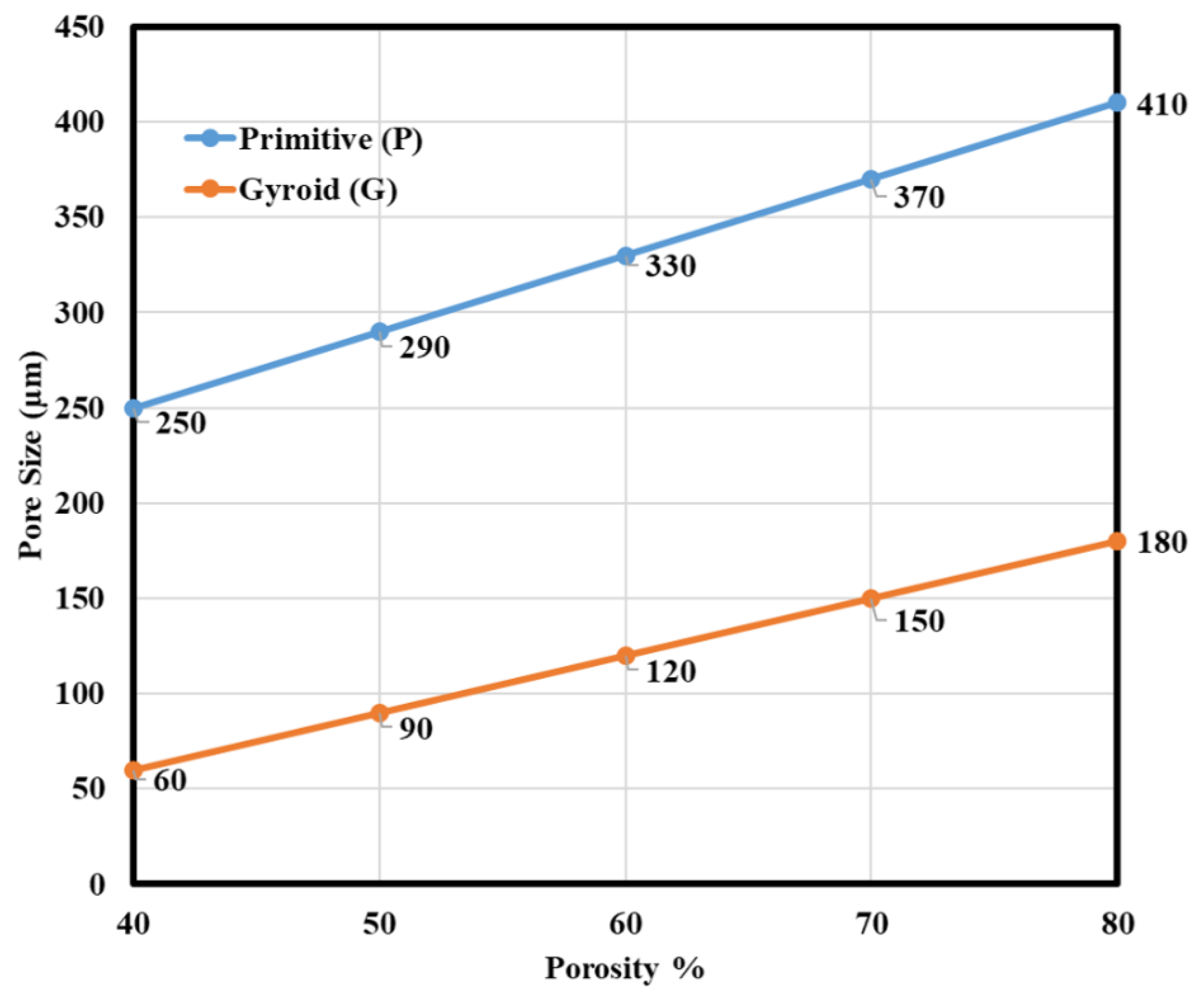
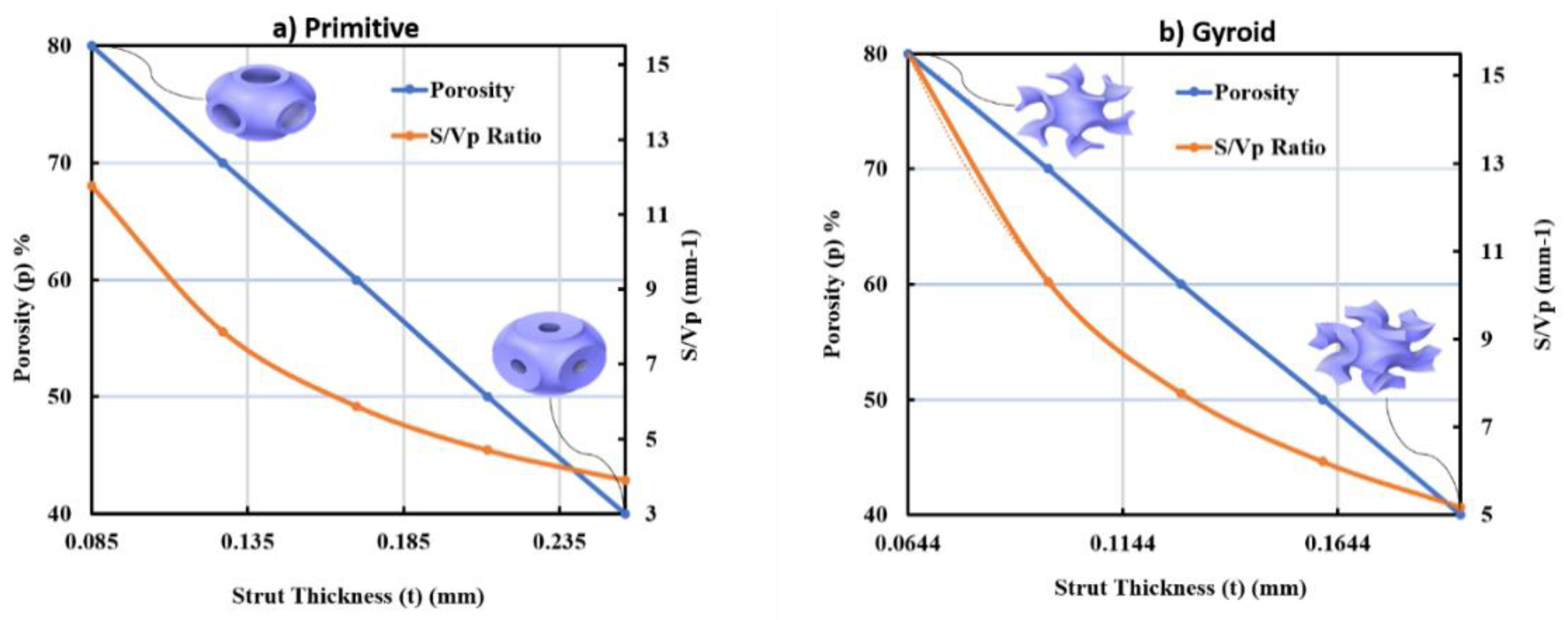
3.1. Morphological Results Obtained from the Developed TPMS Cells
- For the P unit cell:
- For the G unit cell:
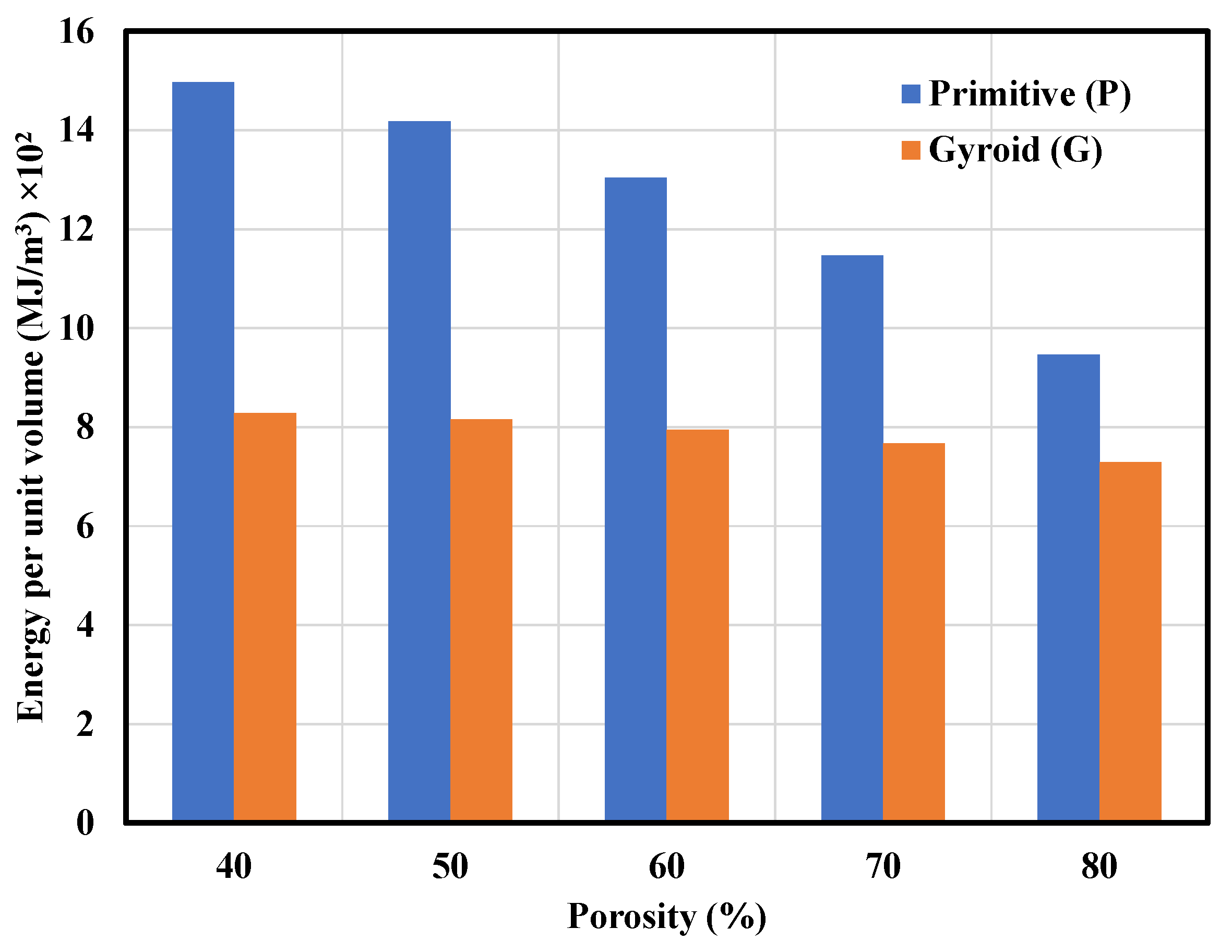
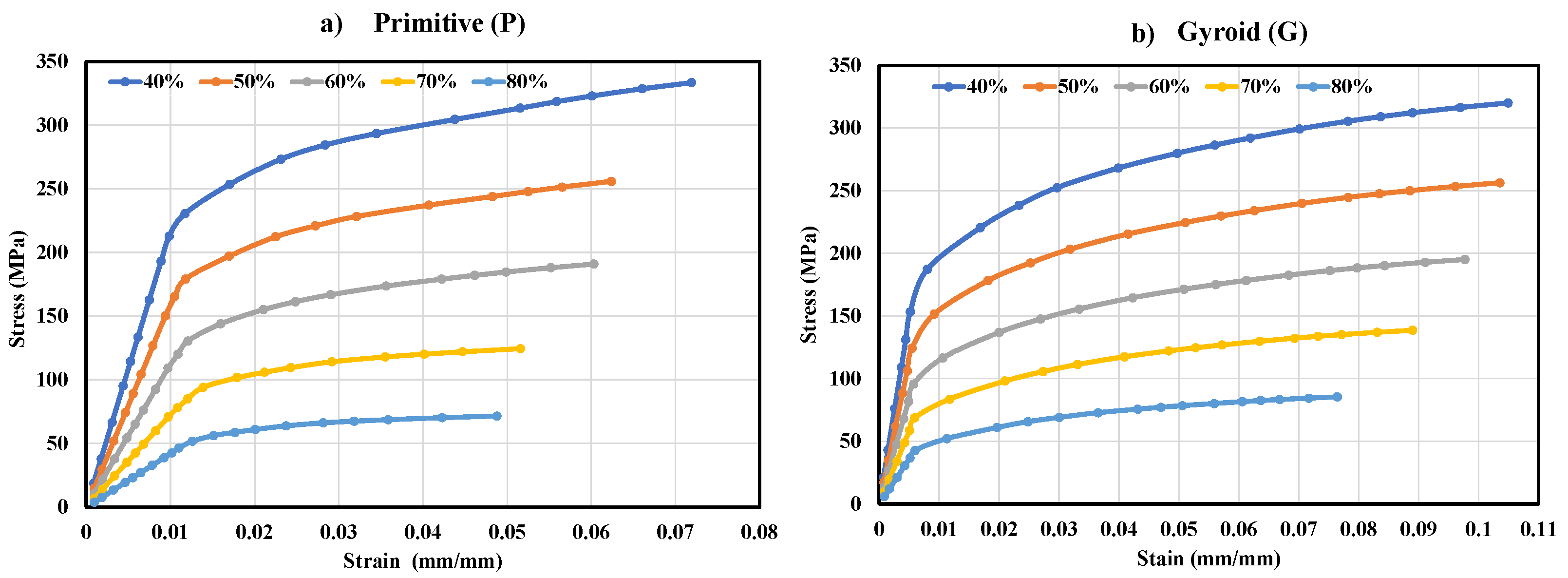
3.2. Mechanical Properties of the P and G TPMS Lattice
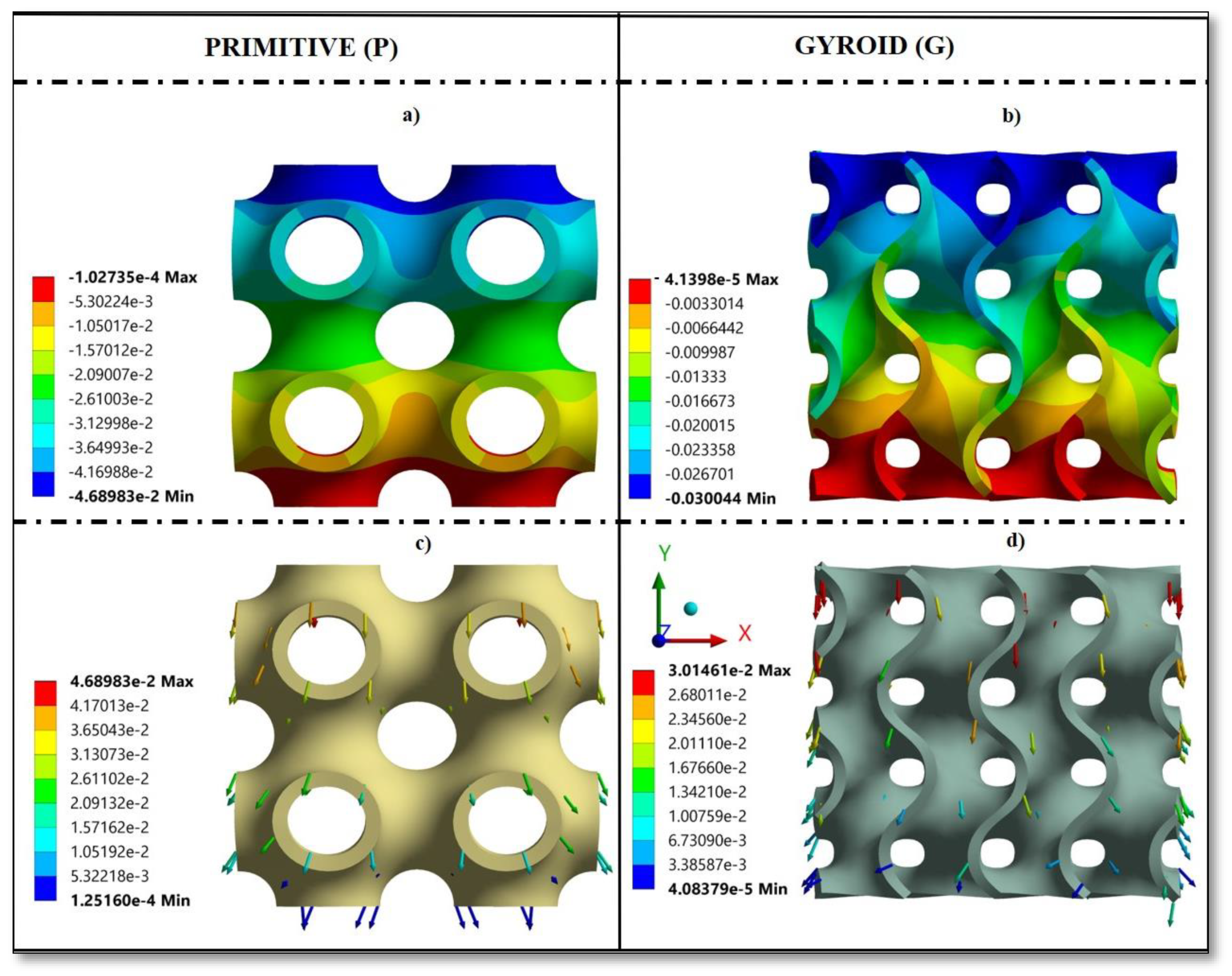
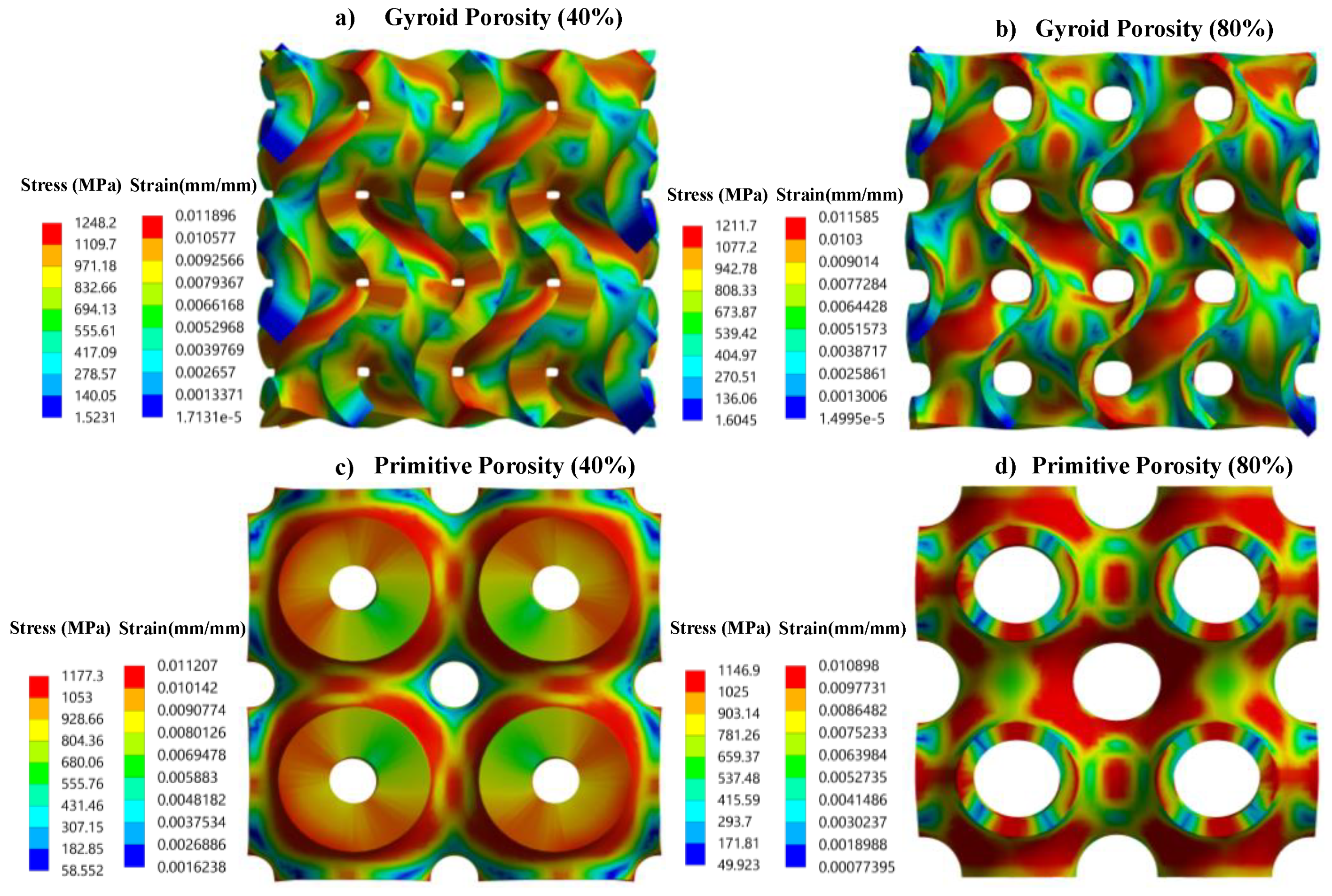
3.3. Stress and Strain Pattern
3.4. Functionality Assessment of Scaffold under Biomechanical Loading
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Component | Element Size | Total No. of Elements | Total No. of Nodes |
|---|---|---|---|
| Femur without scaffold | 2 mm | 1,074,830 | 1,612,424 |
| P Scaffold | 0.3 mm | 662,658 | 1,164,054 |
| Solid Scaffold | 2 mm | 2752 | 14,790 |
| Property | Value |
|---|---|
| Density | 1.8 × 10−6 kg·mm−3 |
| Engineering Constants | |
| E1 | 11.5 GPa |
| E2 | 11.5 GPa |
| E3 | 17.0 GPa |
| G12 | 3.6 GPa |
| G13 | 3.28 GPa |
| G23 | 3.28 GPa |
| ν12 | 0.58 |
| ν13 | 0.31 |
| ν23 | 0.31 |
| ν21 | 0.58 |
| ν31 | 0.46 |
| ν32 | 0.46 |
| Location | Directional Coordinates | ||
|---|---|---|---|
| X | Y | Z | |
| Hip Reaction Force (N) | 1492 | −915 | −2925 |
| Hip Muscle Force (N) | −1342 | 832 | 2055 |
References
- Reichert, J.C.; Saifzadeh, S.; Wullschleger, M.E.; Epari, D.R.; Schütz, M.A.; Duda, G.N.; Schell, H.; Van, G.M.; Redl, H.; Hutmacher, D.W. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 2009, 30, 2149–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekker, T.J.; Steele, J.R.; Federer, A.E.; Hamid, K.S.; Adams, S.B., Jr. Use of patient-specific 3D-printed titanium implants for complex foot and ankle limb salvage, deformity correction, and arthrodesis procedures. Foot Ankle Int. 2018, 39, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Kumta, S.M.; Geel, N.V.; Demol, J. One-step reconstruction with a 3D-printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Comput. Aided Surg. 2015, 20, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Kumta, S.M. Joint-preserving tumor resection and reconstruction using image-guided computer navigation. Clin. Orthop. Relat. Res. 2013, 471, 762–773. [Google Scholar] [CrossRef] [Green Version]
- Zekry, K.M.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Alkhooly, A.Z.; Abd-Elfattah, A.S.; Elsaid, A.N.; Ahmed, A.R.; Tsuchiya, H. Reconstruction of intercalary bone defect after resection of malignant bone tumor. J. Orthop. Surg. Res. 2019, 27, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kumta, S.M.; Leung, P.C.; Yip, K.; Hung, L.K.; Panozzo, A.; Kew, J. Vascularized Bone Grafts in the Treatment of Juxta-Articular Giant-Cell Tumors of the Bone. J. Reconstr. Microsurg. 1998, 14, 185–190. [Google Scholar] [CrossRef]
- Kumta, S.M.; Leung, P.C.; Griffith, J.F.; Roebuck, D.J.; Chow, L.T.; Li, C.K. A technique for enhancing union of allograft to host bone. J. Bone Jt. Surg. Br. Vol. 1998, 80, 994–998. [Google Scholar] [CrossRef]
- Mauffrey, C.; Barlow, B.T.; Smith, W. Management of segmental bone defects. J. Am. Acad. Orthop. Surg. 2015, 23, 143–153. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Sakellariou, V.I.; Tsibidakis, H.; Papagelopoulos, P.J. Adamantinoma of the tibia treated with a new intramedullary diaphyseal segmental defect implant. J. Int. Med. Res. 2009, 37, 1238–1245. [Google Scholar] [CrossRef]
- Healey, J.H.; Morris, C.D.; Athanasian, E.A.; Boland, P.J. Compress® knee arthroplasty has 80% 10-year survivorship and novel forms of bone failure. Clin. Orthop. Relat. Res. 2013, 471, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Ruggieri, P.; Mavrogenis, A.F.; Bianchi, G.; Sakellariou, V.I.; Mercuri, M.; Papagelopoulos, P.J. Outcome of the intramedullary diaphyseal segmental defect fixation system for bone tumors. Journal of Surgical Oncology. J. Surg. Oncol. 2011, 104, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.A.; Sewell, M.D.; Aston, W.J.; Pollock, R.C.; Skinner, J.A.; Cannon, S.R.; Briggs, T.W. Femoral diaphyseal endoprosthetic reconstruction after segmental resection of primary bone tumours. J. Bone Jt. Surg. Br. Vol. 2010, 92, 867–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Z.; Jung, G.S.; Kang, M.J.; Buehler, M.J. The mechanics and design of a lightweight three-dimensional graphene assembly. Sci. Adv. 2017, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Matassi, F.; Botti, A.; Sirleo, L.; Carulli, C.; Innocenti, M. Porous metal for orthopedics implants. Clin. Cases Miner. Bone Metab. 2013, 10, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, B.; Swieszkowski, W.; Godlinski, D.; Kurzydlowski, K.J. Highly porous titanium scaffolds for orthopaedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Pałka, K.; Pokrowiecki, R. Porous titanium implants: A review. Adv. Eng. Mater. 2018, 20, 1700648. [Google Scholar] [CrossRef]
- Rati, V.; Singh, N.; Rai, S.; Kumta, S. Triply periodic minimal surface-based porous scaffold design and analysis subjected to hard tissue reconstruction. In Advances in Computational Methods in Manufacturing; Narayanan, R.G., Joshi, S.N., Dixit, U.S., Eds.; Springer: Singapore, 2019; pp. 955–966. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Abu Al-Rub, R.K. Multifunctional mechanical metamaterials based on triply periodic minimal surface lattices. Adv. Eng. Mater. 2019, 21, 1900524. [Google Scholar] [CrossRef]
- Afshar, M.; Anaraki, A.P.; Montazerian, H.; Kadkhodapour, J. Additive manufacturing and mechanical characterization of graded porosity scaffolds designed based on triply periodic minimal surface architectures. J. Mech. Behav. Biomed. Mater. 2016, 62, 481–494. [Google Scholar] [CrossRef]
- Yánez, A.; Cuadrado, A.; Martel, O.; Afonso, H.; Monopoli, D. Gyroid porous titanium structures: A versatile solution to be used as scaffolds in bone defect reconstruction. Mater. Des. 2018, 140, 21–29. [Google Scholar] [CrossRef]
- Yoo, D.J. Recent trends and challenges in computer-aided design of additive manufacturing-based biomimetic scaffolds and bioartificial organs. Int. J. Precis. Eng. Manuf. 2014, 15, 2205–2217. [Google Scholar] [CrossRef]
- Abueidda, D.W.; Elhebeary, M.; Shiang, C.S.; Pang, S.; Al-Rub, R.K.; Jasiuk, I.M. Mechanical properties of 3D printed polymeric Gyroid cellular structures: Experimental and finite element study. Mater. Des. 2019, 165, 107597. [Google Scholar] [CrossRef]
- Herrera, A.; Yánez, A.; Martel, O.; Afonso, H.; Monopoli, D. Computational study and experimental validation of porous structures fabricated by electron beam melting: A challenge to avoid stress shielding. Mater. Sci. Eng. C 2014, 45, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Wieding, J.; Wolf, A.; Bader, R. Numerical optimization of open-porous bone scaffold structures to match the elastic properties of human cortical bone. J. Mech. Behav. Biomed. Mater. 2014, 37, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Maskery, I.; Sturm, L.; Aremu, A.O.; Panesar, A.; Williams, C.B.; Tuck, C.J.; Wildman, R.D.; Ashcroft, I.A.; Hague, R.J. Insights into the mechanical properties of several triply periodic minimal surface lattice structures made by polymer additive manufacturing. Polymer 2018, 152, 62–71. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- An, Y.H.; Draughn, R.A. Mechanical Testing of Bone and the Bone-Implant Interface, 1st ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Kapat, K.; Srivas, P.K.; Rameshbabu, A.P.; Maity, P.P.; Jana, S.; Dutta, J.; Majumdar, P.; Chakrabarti, D.; Dhara, S. Influence of porosity and pore-size distribution in Ti6Al4 V foam on physicomechanical properties, osteogenesis, and quantitative validation of bone ingrowth by micro-computed tomography. ACS Appl. Mater. Interfaces 2017, 9, 39235–39248. [Google Scholar] [CrossRef]
- Jinnai, H.; Watashiba, H.; Kajihara, T.; Nishikawa, Y.; Takahashi, M.; Ito, M. Surface curvatures of trabecular bone microarchitecture. Bone 2002, 30, 191–194. [Google Scholar] [CrossRef]
- Rammohan, A.V.; Lee, T.; Tan, V.B. A novel morphological model of trabecular bone based on the gyroid. Int. J. Appl. Mech. 2015, 7, 321–331. [Google Scholar] [CrossRef]
- Karcher, H. The triply periodic minimal surfaces of Alan Schoen and their constant mean curvature companions. Manuscr. Math. 1989, 64, 291–357. [Google Scholar] [CrossRef]
- Góźdź, W.T.; Hołyst, R. Triply periodic surfaces and multiply continuous structures from the Landau model of microemulsions. Phys. Rev. E 1996, 54, 5012. [Google Scholar] [CrossRef] [Green Version]
- Bobbert, F.S.; Lietaert, K.; Eftekhari, A.A.; Pouran, B.; Ahmadi, S.M.; Weinans, H.; Zadpoor, A.A. Additively manufactured metallic porous biomaterials based on minimal surfaces: A unique combination of topological, mechanical, and mass transport properties. Acta Biomater. 2017, 53, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D. New paradigms in hierarchical porous scaffold design for tissue engineering. Mater. Sci. Eng. C 2013, 33, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Schoen, A.H. Infinite Periodic Minimal Surfaces without Self-Intersections; National Aeronautics and Space Administration: Washington, DC, USA, 1970. [Google Scholar]
- Michielsen, K.; Stavenga, D.G. Gyroid cuticular structures in butterfly wing scales: Biological photonic crystals. J. R. Soc. Interface 2008, 5, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouya, C.; Overvelde, J.T.; Kolle, M.; Aizenberg, J.; Bertoldi, K.; Weaver, J.C.; Vukusic, P. Characterization of a mechanically tunable gyroid photonic crystal inspired by the butterfly parides sesostris. Adv. Opt. Mater. 2016, 4, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Corkery, R.W.; Tyrode, E.C. On the colour of wing scales in butterflies: Iridescence and preferred orientation of single gyroid photonic crystals. Interface Focus 2017, 7, 20160154. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, S.M.; Yavari, S.A.; Wauthle, R.; Pouran, B.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Additively manufactured open-cell porous biomaterials made from six different space-filling unit cells: The mechanical and morphological properties. Materials 2015, 8, 1871–1896. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Hao, L.; Hussein, A.; Young, P.; Raymont, D. Advanced lightweight 316L stainless steel cellular lattice structures fabricated via selective laser melting. Mater. Des. 2014, 55, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, S.; Robb, R.A. Schwarz meets Schwann: Design and fabrication of biomorphic and durataxic tissue engineering scaffolds. Med. Image Anal. 2006, 10, 693–712. [Google Scholar] [CrossRef]
- Kapfer, S.C.; Hyde, S.T.; Mecke, K.; Arns, C.H.; Schröder-Turk, G.E. Minimal surface scaffold designs for tissue engineering. Biomaterials 2011, 32, 6875–6882. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Badwal, P.S.; Gibson, I. Design and fabrication considerations for three-dimensional scaffold structures. KnE Eng. 2017, 2, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Montazerian, H.; Davoodi, E.; Asadi-Eydivand, M.; Kadkhodapour, J.; Solati-Hashjin, M. Porous scaffold internal architecture design based on minimal surfaces: A compromise between permeability and elastic properties. Mater. Des. 2017, 126, 98–114. [Google Scholar] [CrossRef]
- Shin, J.; Kim, S.; Jeong, D.; Lee, H.G.; Lee, D.; Lim, J.Y.; Kim, J. Finite element analysis of Schwarz P surface pore geometries for tissue-engineered scaffolds. Math. Probl. Eng. 2012, 2012, 694194. [Google Scholar] [CrossRef] [Green Version]
- Ambu, R.; Morabito, A.E. Design and analysis of tissue engineering scaffolds based on open porous non-stochastic cells. In Advances on Mechanics, Design Engineering and Manufacturing; Eynard, B., Nigrelli, V., Oliveri, S.M., Peris-Fajarnes, G., Rizzuti, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 777–787. [Google Scholar] [CrossRef]
- Jung, Y.; Torquato, S. Fluid permeabilities of triply periodic minimal surfaces. Phys. Rev. E 2005, 72, 056319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soro, N.; Attar, H.; Wu, X.; Dargusch, M.S. Investigation of the structure and mechanical properties of additively manufactured Ti-6Al-4V biomedical scaffolds designed with a Schwartz primitive unit-cell. Mater. Sci. Eng. A 2019, 745, 195–202. [Google Scholar] [CrossRef]
- Yánez, A.; Herrera, A.; Martel, O.; Monopoli, D.; Afonso, H. Compressive behaviour of gyroid lattice structures for human cancellous bone implant applications. Mater. Sci. Eng. C 2016, 68, 445–448. [Google Scholar] [CrossRef]
- Yang, L.; Yan, C.; Fan, H.; Li, Z.; Cai, C.; Chen, P.; Shi, Y.; Yang, S. Investigation on the orientation dependence of elastic response in Gyroid cellular structures. J. Mech. Behav. Biomed. Mater. 2019, 90, 73–85. [Google Scholar] [CrossRef]
- Khaderi, S.N.; Deshpande, V.S.; Fleck, N.A. The stiffness and strength of the gyroid lattice. Int. J. Solids Struct. 2014, 51, 3866–3877. [Google Scholar] [CrossRef] [Green Version]
- Ataee, A.; Li, Y.; Fraser, D.; Song, G.; Wen, C. Anisotropic Ti-6Al-4V gyroid scaffolds manufactured by electron beam melting (EBM) for bone implant applications. Mater. Des. 2018, 137, 345–354. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Z.; Hu, X.; Kuang, H.; Zhai, J. The effect of porosity on the mechanical properties of 3D-printed triply periodic minimal surface (TPMS) bio scaffold. Bio-Des. Manuf. 2019, 2, 242–255. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Waller, M.A.; Yannas, I.V.; Gibson, L.J.; Prendergast, P.J. The effect of pore size on permeability and cell attachment in collagen scaffolds for tissue engineering. Technol. Health Care 2007, 15, 3–17. [Google Scholar] [CrossRef]
- Dias, M.R.; Guedes, J.M.; Flanagan, C.L.; Hollister, S.J.; Fernandes, P.R. Optimization of scaffold design for bone tissue engineering: A computational and experimental study. Med. Eng. Phys. 2014, 36, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Yavari, S.A.; Ahmadi, S.M.; Wauthle, R.; Pouran, B.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Relationship between unit cell type and porosity and the fatigue behavior of selective laser melted meta-biomaterials. J. Mech. Behav. Biomed. Mater. 2015, 43, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.P.; Baran, G.R.; Wei, Y.; Davis, B.L. Biomechanics-Part II. In Bone Pathology, 2nd ed.; Khurana, J.S., Ed.; Humana: Totowa, NJ, USA, 2009; pp. 69–77. [Google Scholar]
- Wall, A.; Board, T. The compressive behaviour of bone as a two-phase porous structure. J. Bone Jt. Surg. 1977, 59, 954–962. [Google Scholar]
- Maskery, I.; Aremu, A.O.; Parry, L.; Wildman, R.D.; Tuck, C.J.; Ashcroft, I.A. Effective design and simulation of surface-based lattice structures featuring volume fraction and cell type grading. Mater. Des. 2018, 155, 220–232. [Google Scholar] [CrossRef]
- Yang, L.; Mertens, R.; Ferrucci, M.; Yan, C.; Shi, Y.; Yang, S. Continuous graded Gyroid cellular structures fabricated by selective laser melting: Design, manufacturing and mechanical properties. Mater. Des. 2019, 162, 394–404. [Google Scholar] [CrossRef]
- Günther, F.; Wagner, M.; Pilz, S.; Gebert, A.; Zimmermann, M. Design procedure for triply periodic minimal surface based biomimetic scaffolds. J. Mech. Behav. Biomed. Mater. 2022, 126, 104871. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Rowshan, R.; Al-Rub, R.K. Topology-mechanical property relationship of 3D printed strut, skeletal, and sheet based periodic metallic cellular materials. Addit. Manuf. 2018, 19, 167–183. [Google Scholar] [CrossRef]
- Robertson, D.M.; St. Pierre, L.; Chahal, R. Preliminary observations of bone ingrowth into porous materials. J. Biomed. Mater. Res. 1976, 10, 335–344. [Google Scholar] [CrossRef]
- Gómez, S.; Vlad, M.D.; López, J.; Fernández, E. Design and properties of 3D scaffolds for bone tissue engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef]
- Heinl, P.; Müller, L.; Körner, C.; Singer, R.F.; Müller, F.A. Cellular Ti–6Al–4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008, 4, 1536–1544. [Google Scholar] [CrossRef]
- Lee, D.W.; Khan, K.A.; Al-Rub, R.K. Stiffness and yield strength of architectured foams based on the Schwarz Primitive triply periodic minimal surface. Int. J. Plast. 2017, 95, 1–20. [Google Scholar] [CrossRef]
- Ghouse, S.; Reznikov, N.; Boughton, O.R.; Babu, S.; Ng, K.G.; Blunn, G.; Cobb, J.P.; Stevens, M.M.; Jeffers, J.R. The design and in vivo testing of a locally stiffness-matched porous scaffold. Appl. Mater. Today 2019, 15, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Hao, L.; Hussein, A.; Young, P. Ti–6Al–4V triply periodic minimal surface structures for bone implants fabricated via selective laser melting. J. Mech. Behav. Biomed. Mater. 2015, 51, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, B.V.; Bose, S.; Bandyopadhyay, A. Low stiffness porous Ti structures for load-bearing implants. Acta Biomater. 2007, 3, 997–1006. [Google Scholar] [CrossRef]
- Abd Malek, N.M.; Mohamed, S.R.; Ghani, S.C.; Harun, W.W. Critical evaluation on structural stiffness of porous cellular structure of cobalt chromium alloy. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Kuantan, Pahang, Malaysia, 2015. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Ding, S.; Wen, C. Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioact. Mater. 2019, 4, 56–70. [Google Scholar] [CrossRef]
- Al-Tamimi, A.A.; Peach, C.; Fernandes, P.R.; Cseke, A.; Bartolo, P.J. Topology optimization to reduce the stress shielding effect for orthopedic applications. Procedia CIRP 2017, 65, 202–206. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, W.; Li, Y. Fabrication and characterization of porous Ti6Al4V parts for biomedical applications using electron beam melting process. Mater. Lett. 2009, 63, 403–405. [Google Scholar] [CrossRef]
- Ma, S.; Song, K.; Lan, J.; Ma, L. Biological and mechanical property analysis for designed heterogeneous porous scaffolds based on the refined TPMS. J. Mech. Behav Biomed. Mater. 2020, 107, 103727. [Google Scholar] [CrossRef]
- Papazetis, G.; Vosniakos, G.C. Direct porous structure generation of tissue engineering scaffolds for layer-based additive manufacturing. J. Adv. Manuf. Technol. 2016, 86, 871–883. [Google Scholar] [CrossRef]
- Sapkal, P.S.; Kuthe, A.M.; Kashyap, R.S.; Nayak, A.R.; Kuthe, S.A.; Kawle, A.P. Indirect casting of patient-specific tricalcium phosphate zirconia scaffolds for bone tissue regeneration using rapid prototyping methodology. J. Porous Mater. 2017, 24, 1013–1023. [Google Scholar] [CrossRef]
- Xia, Y.; Feng, C.; Xiong, Y.; Luo, Y.; Li, X. Mechanical properties of porous titanium alloy scaffold fabricated using additive manufacturing technology. Int. J. Appl. Electromagn. 2019, 59, 1087–1095. [Google Scholar] [CrossRef]
- Wang, L.; Kang, J.; Sun, C.; Li, D.; Cao, Y.; Jin, Z. Mapping porous microstructures to yield desired mechanical properties for application in 3D printed bone scaffolds and orthopaedic implants. Mater. Des. 2017, 133, 62–68. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, K.; Yu, Z. Tetrahedron-based porous scaffold design for 3D printing. Designs 2019, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Mao, Z.; Zhang, P.; Zhang, D.Z.; Jiang, J.; Ma, Z. Functionally graded porous scaffolds in multiple patterns: New design method, physical and mechanical properties. Mater. Des. 2018, 160, 849–860. [Google Scholar] [CrossRef]
- Savio, G.; Meneghello, R.; Concheri, G. Design of variable thickness triply periodic surfaces for additive manufacturing. Prog. Addit. Manuf. 2019, 4, 281–290. [Google Scholar] [CrossRef]
- Yang, N.; Wang, S.; Gao, L.; Men, Y.; Zhang, C. Building implicit-surface-based composite porous architectures. Compos. Struct. 2017, 173, 35–43. [Google Scholar] [CrossRef]
- Abueidda, D.W.; Al-Rub, R.K.; Dalaq, A.S.; Lee, D.W.; Khan, K.A.; Jasiuk, I. Effective conductivities and elastic moduli of novel foams with triply periodic minimal surfaces. Mech. Mater. 2016, 95, 102–115. [Google Scholar] [CrossRef]
- Yoo, D.J. Computer-aided porous scaffold design for tissue engineering using triply periodic minimal surfaces. Int. J. Precis. Eng. Manuf. 2011, 12, 61–71. [Google Scholar] [CrossRef]
- Yoo, D.J. New paradigms in cellular material design and fabrication. Int. J. Precis. Eng. Manuf. 2015, 16, 2577–2589. [Google Scholar] [CrossRef]
- Yang, N.; Quan, Z.; Zhang, D.; Tian, Y. Multi-morphology transition hybridization CAD design of minimal surface porous structures for use in tissue engineering. Comput.-Aided Des. 2014, 56, 11–21. [Google Scholar] [CrossRef]
- Yang, N.; Zhou, K. Effective method for multi-scale gradient porous scaffold design and fabrication. Mater. Sci. Eng. C 2014, 43, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Gandy, P.J.; Bardhan, S.; Mackay, A.L.; Klinowski, J. Nodal surface approximations to the P, G, D and I-WP triply periodic minimal surfaces. Chem. Phys. Lett. 2001, 336, 187–195. [Google Scholar] [CrossRef]
- Shi, J.; Zhu, L.; Li, L.; Li, Z.; Yang, J.; Wang, X. A TPMS-based method for modeling porous scaffolds for bionic bone tissue engineering. Sci. Rep. 2018, 8, 7395. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D.J. Porous scaffold design using the distance field and triply periodic minimal surface models. Biomaterials 2011, 32, 7741–7754. [Google Scholar] [CrossRef]
- Smith, M.; Guan, Z.; Cantwell, W.J. Finite element modelling of the compressive response of lattice structures manufactured using the selective laser melting technique. Int. J. Mech. Sci. 2013, 67, 28–41. [Google Scholar] [CrossRef]
- Reilly, D.T.; Burstein, A.H. The elastic and ultimate properties of compact bone tissue. J. Biomech. 1975, 8, 393–405. [Google Scholar] [CrossRef]
- Mann, K.A.; Bartel, D.L.; Wright, T.M.; Burstein, A.H. Coulomb frictional interfaces in modeling cemented total hip replacements: A more realistic model. J. Biomech. 1995, 28, 1067–1078. [Google Scholar] [CrossRef]
- Schliephake, H.; Neukam, F.W.; Klosa, D. Influence of pore dimensions on bone ingrowth into porous hydroxylapatite blocks used as bone graft substitutes: A histometric study. Int. J. Oral Maxillofac. Surg. 1991, 20, 53–58. [Google Scholar] [CrossRef]
- De Groot, K. Macropore tissue ingrowth-a quantitative and qualitative study on hydroxyapatite ceramic. Biomaterials 1986, 7, 137–143. [Google Scholar]
- Vijayavenkataraman, S.; Zhang, L.; Zhang, S.; His, F.J.Y.; Lu, W.F. Triply periodic minimal surfaces sheet scaffolds for tissue engineering applications: An optimization approach toward biomimetic scaffold design. ACS Appl. Bio Mater. 2018, 1, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Langton, D.J.; Sidaginamale, R.; Lord, J.K.; Nargol, A.V.; Joyce, T.J. Taper junction failure in large-diameter metal-on-metal bearings. Bone Jt. Res. 2012, 1, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feih, S.; Daynes, S.; Chang, S.; Wang, M.Y.; Wei, J.; Lu, W.F. Energy absorption characteristics of metallic triply periodic minimal surface sheet structures under compressive loading. Addit. Manuf. 2018, 23, 505–515. [Google Scholar] [CrossRef]
- Maskery, I.; Aboulkhair, N.T.; Aremu, A.O.; Tuck, C.J.; Ashcroft, I.A.; Wildman, R.D.; Hague, R.J. A mechanical property evaluation of graded density Al-Si10-Mg lattice structures manufactured by selective laser melting. Mater. Sci. Eng. A 2016, 670, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Cahill, S.; Lohfeld, P.E. McHugh, Finite element predictions compared to experimental results for the effective modulus of bone tissue engineering scaffolds fabri-cated by selective laser sintering. J. Mater. Sci. Mater. Med. 2009, 20, 1255–1262. [Google Scholar] [CrossRef]
- Solomin, L.; Slongo, T. Long bone defect classification: What it should be? J. Bone Rep. Recomm. 2016, 2, 1–2. [Google Scholar] [CrossRef]
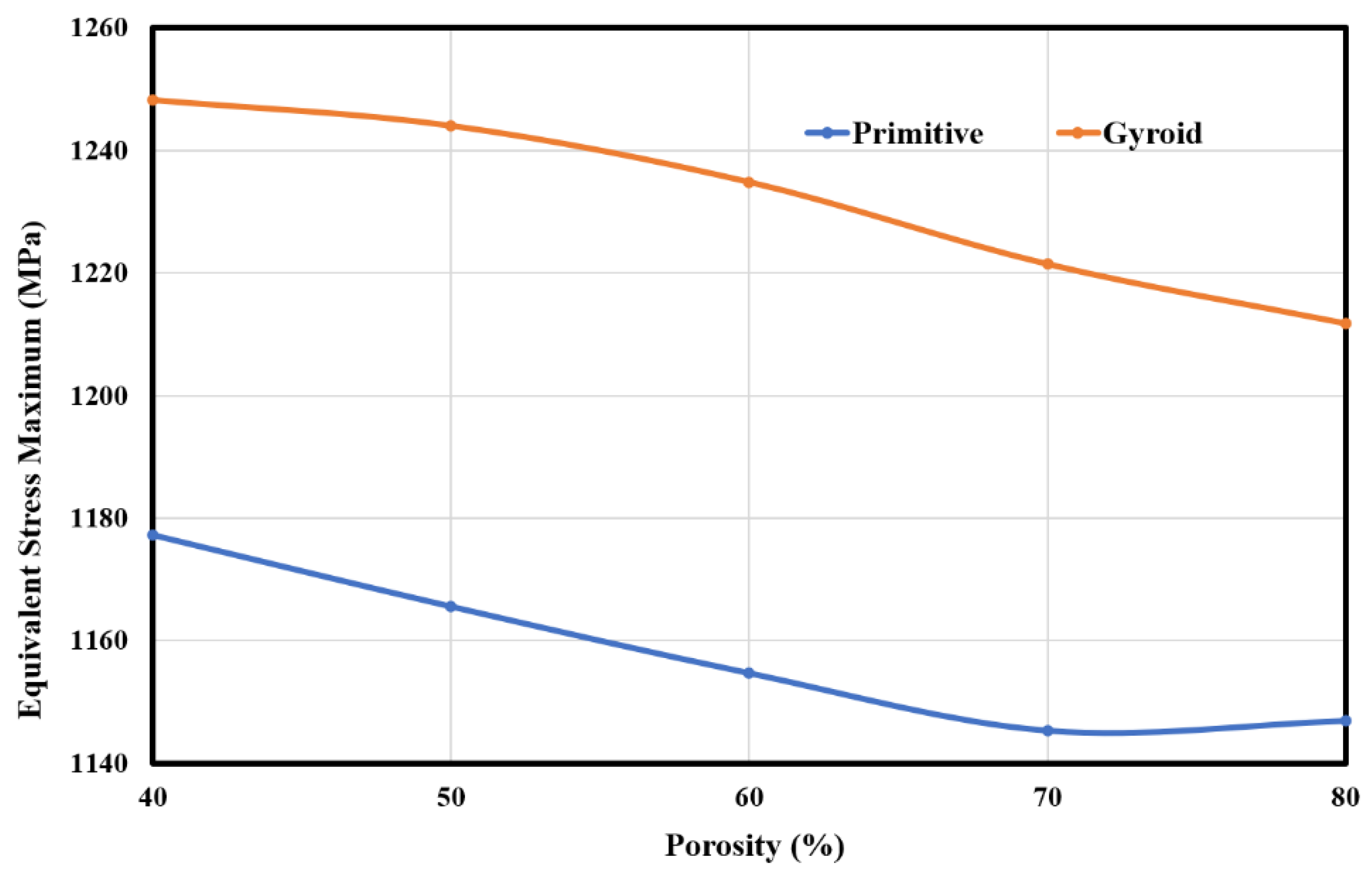
| Lattice | Mechanical Properties | Porosity | ||||
|---|---|---|---|---|---|---|
| 40% | 50% | 60% | 70% | 80% | ||
| P | Effective Young’s modulus (GPa) | 21.66 | 16.02 | 11.24 | 7.26 | 4.21 |
| Compressive Yield stress (MPa) | 237 | 185 | 139 | 97 | 56 | |
| G | Effective Young’s modulus (GPa) | 29.63 | 22.77 | 16.73 | 11.62 | 7.16 |
| Compressive Yield stress (MPa) | 187 | 148 | 109 | 77 | 48 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, R.; Kumar, J.; Singh, N.K.; Rai, S.K.; Saxena, K.K.; Xu, J. Design and Analysis of Biomedical Scaffolds Using TPMS-Based Porous Structures Inspired from Additive Manufacturing. Coatings 2022, 12, 839. https://doi.org/10.3390/coatings12060839
Verma R, Kumar J, Singh NK, Rai SK, Saxena KK, Xu J. Design and Analysis of Biomedical Scaffolds Using TPMS-Based Porous Structures Inspired from Additive Manufacturing. Coatings. 2022; 12(6):839. https://doi.org/10.3390/coatings12060839
Chicago/Turabian StyleVerma, Rati, Jitendra Kumar, Nishant Kumar Singh, Sanjay Kumar Rai, Kuldeep K. Saxena, and Jinyang Xu. 2022. "Design and Analysis of Biomedical Scaffolds Using TPMS-Based Porous Structures Inspired from Additive Manufacturing" Coatings 12, no. 6: 839. https://doi.org/10.3390/coatings12060839
APA StyleVerma, R., Kumar, J., Singh, N. K., Rai, S. K., Saxena, K. K., & Xu, J. (2022). Design and Analysis of Biomedical Scaffolds Using TPMS-Based Porous Structures Inspired from Additive Manufacturing. Coatings, 12(6), 839. https://doi.org/10.3390/coatings12060839








