Bubble-Patterned Films by Inkjet Printing and Gas Foaming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Ink Preparation
2.2. Characterization of Inks and Substrates
2.3. Inkjet-Printing Process and Printed-PVA-Layer Characterization
2.4. Gas Foaming Process and PLA Substrate Characterization
2.5. Cell-Culture Experiments
3. Results and Discussion
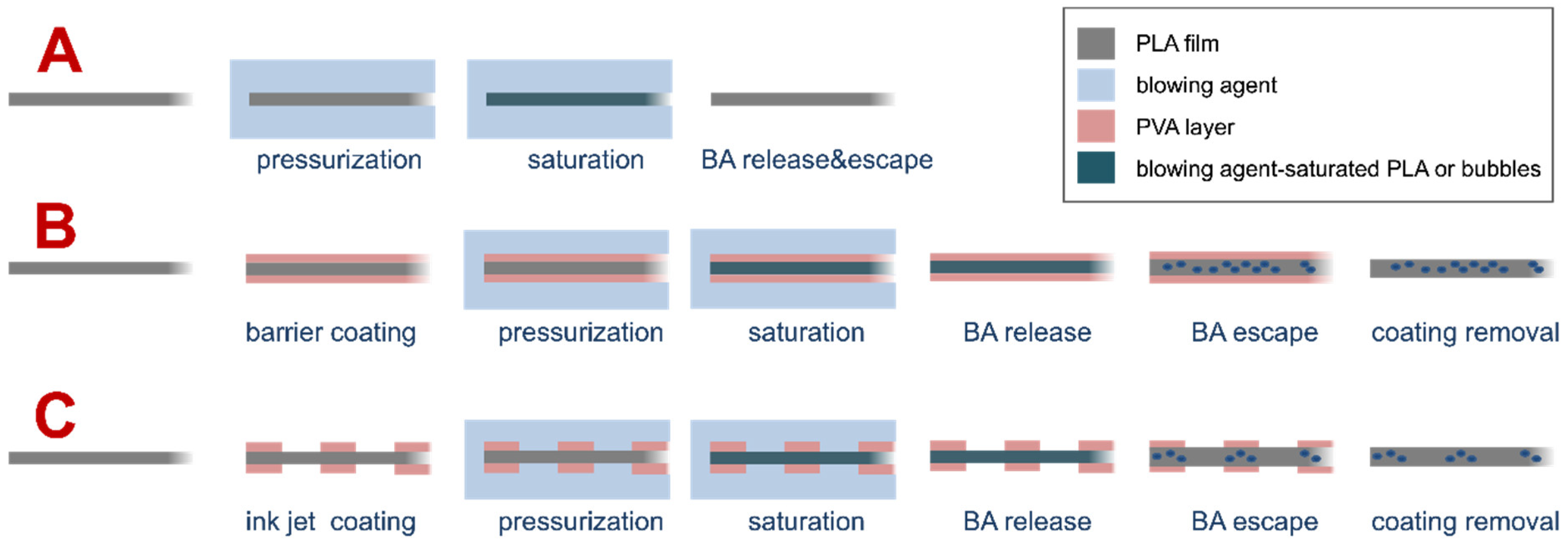
3.1. Description of the Investigated Process
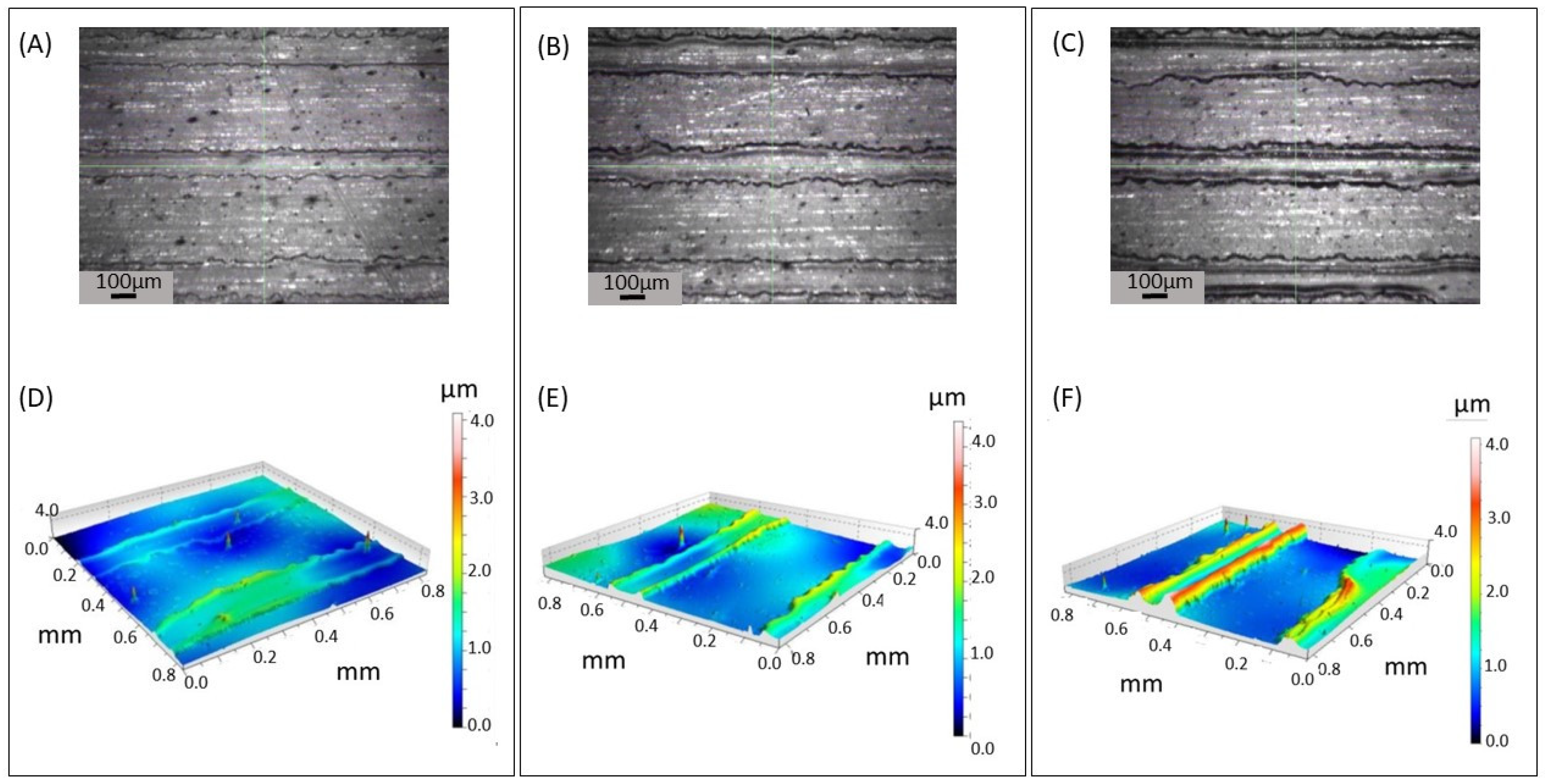
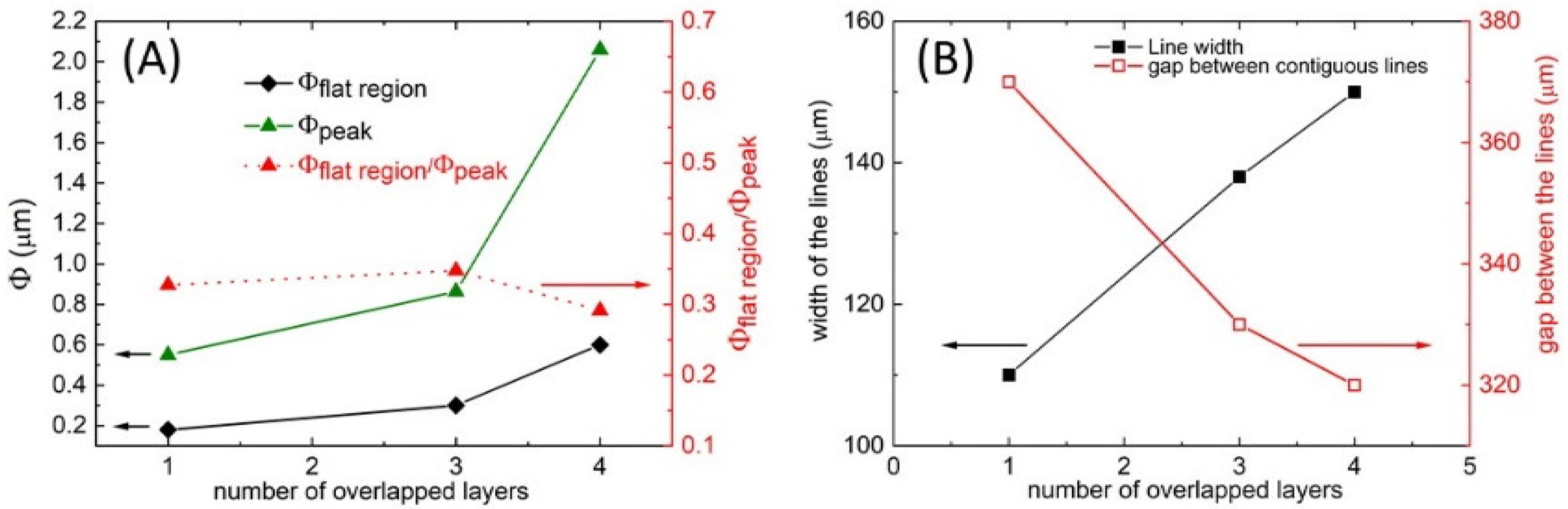
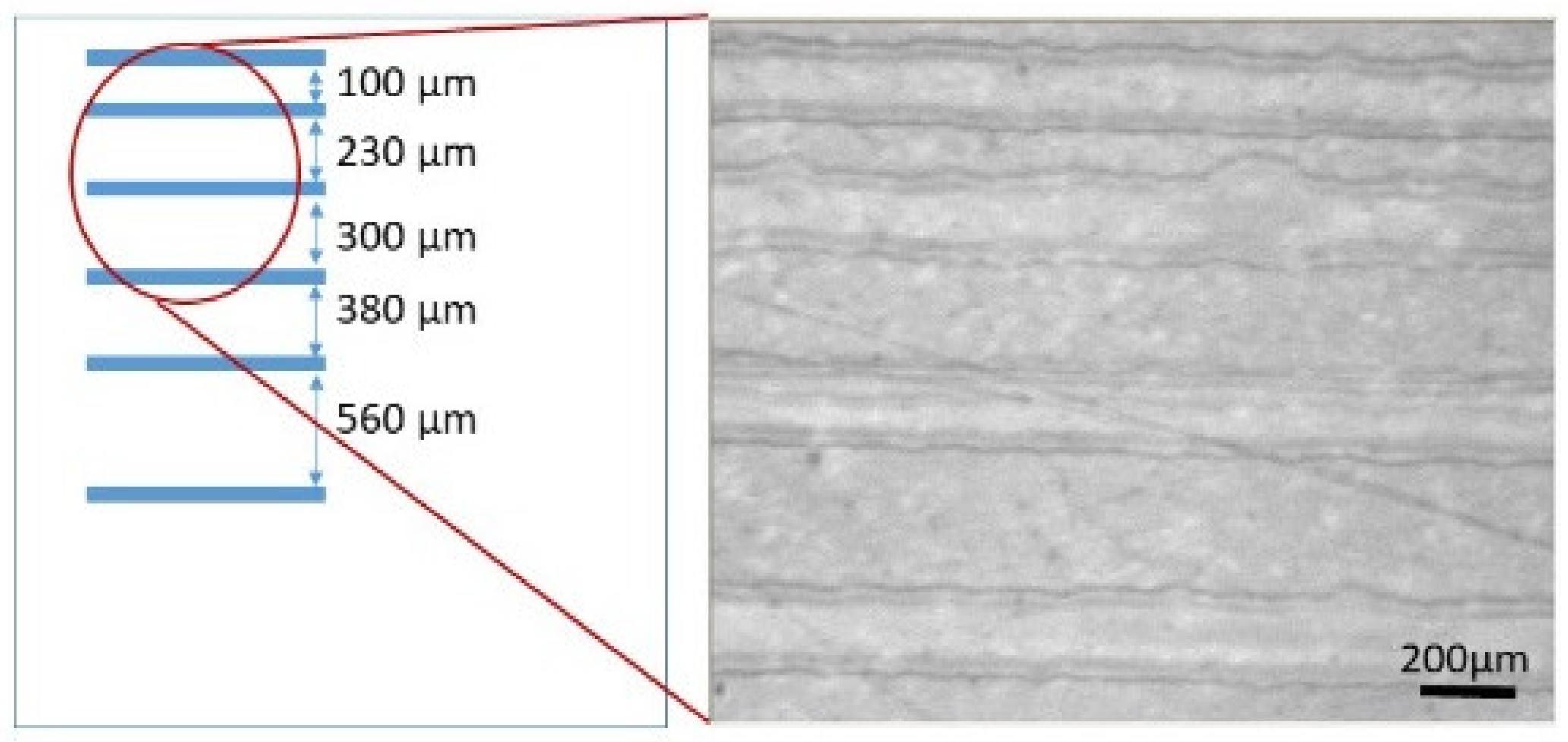
3.2. Patterning of PLA Substrate by Inkjet Printing Deposition of PVA Layer
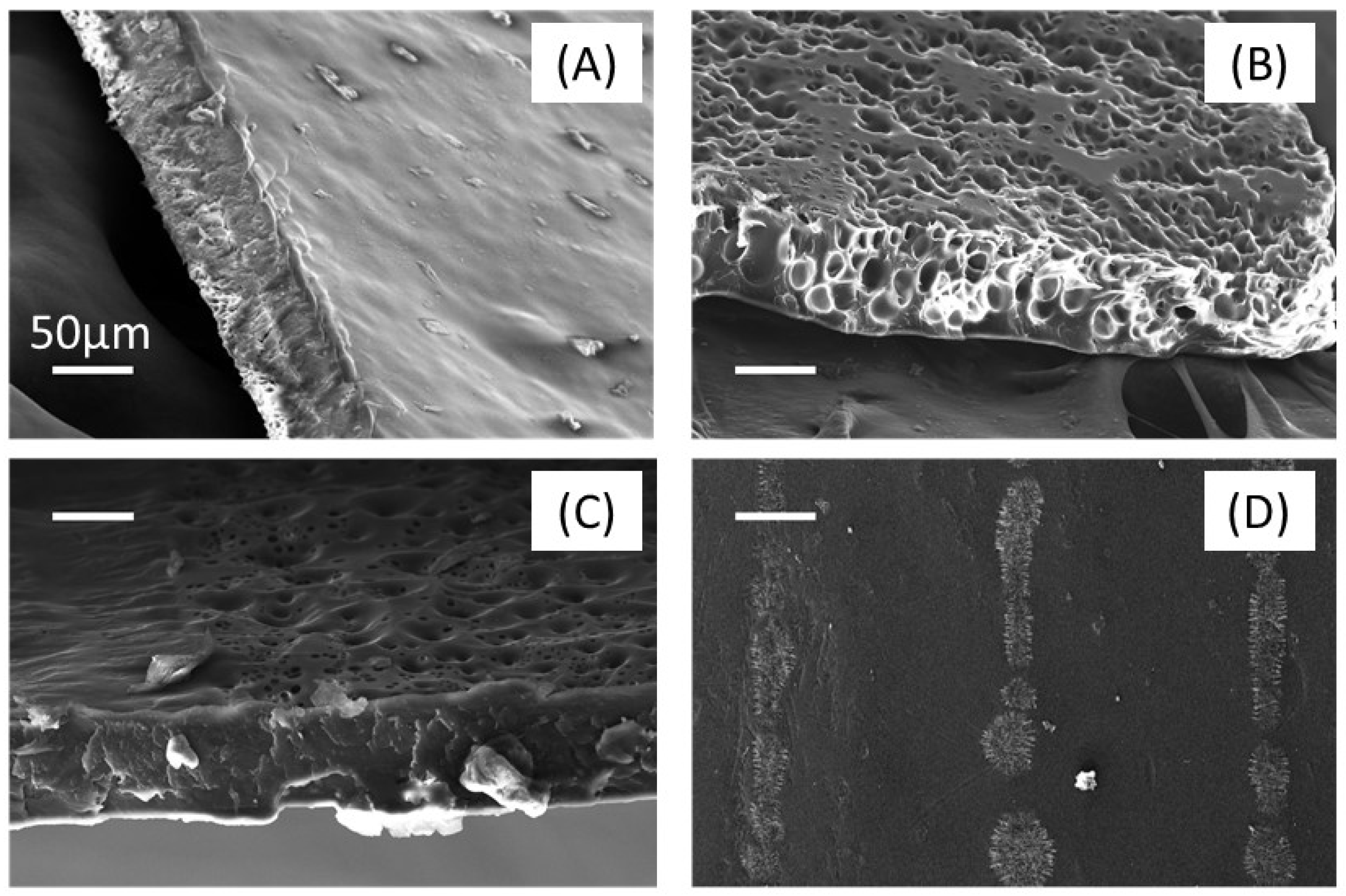
3.3. Gas Foaming of PLA Substrate Patterned with PVA Coating
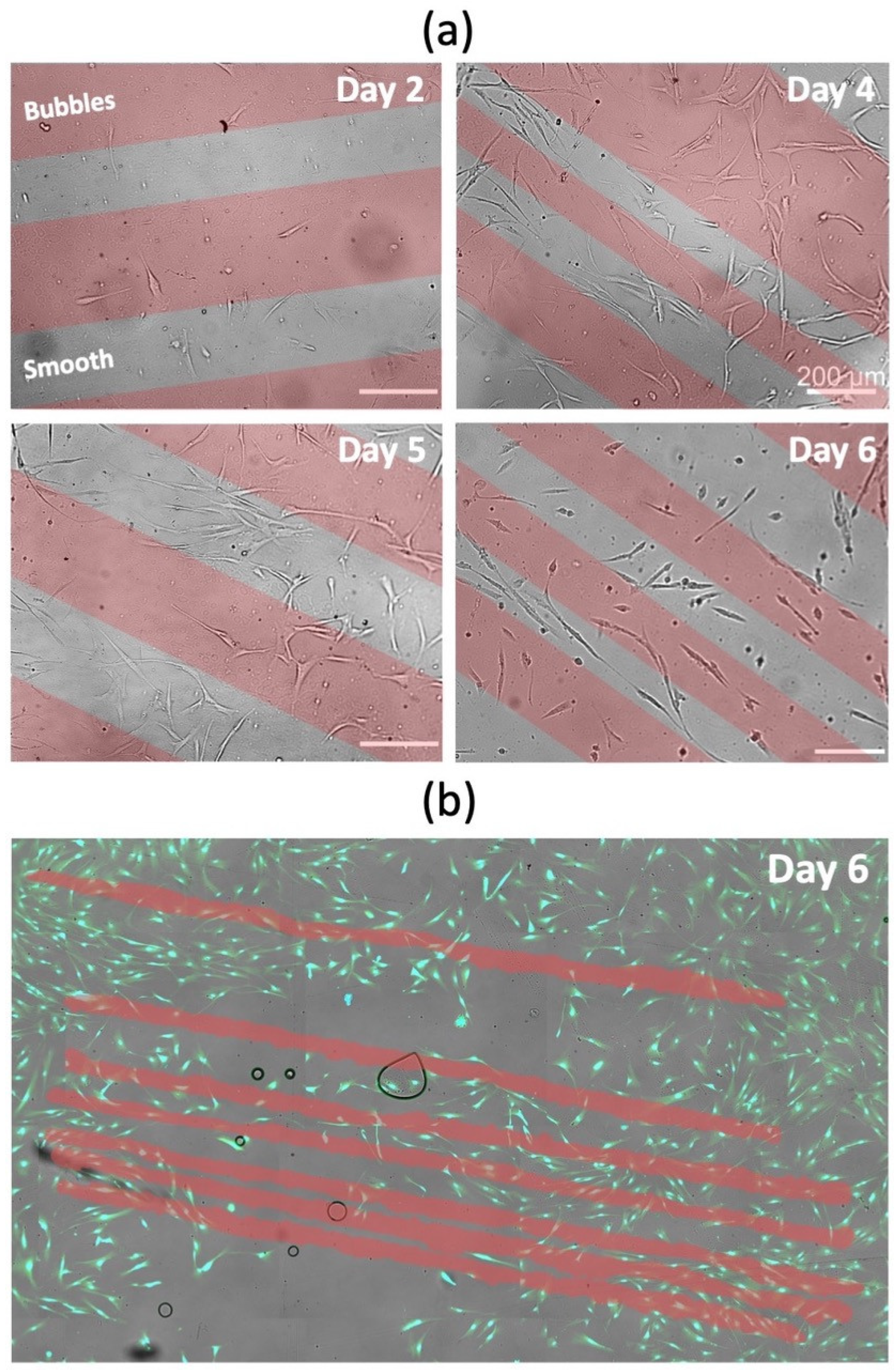
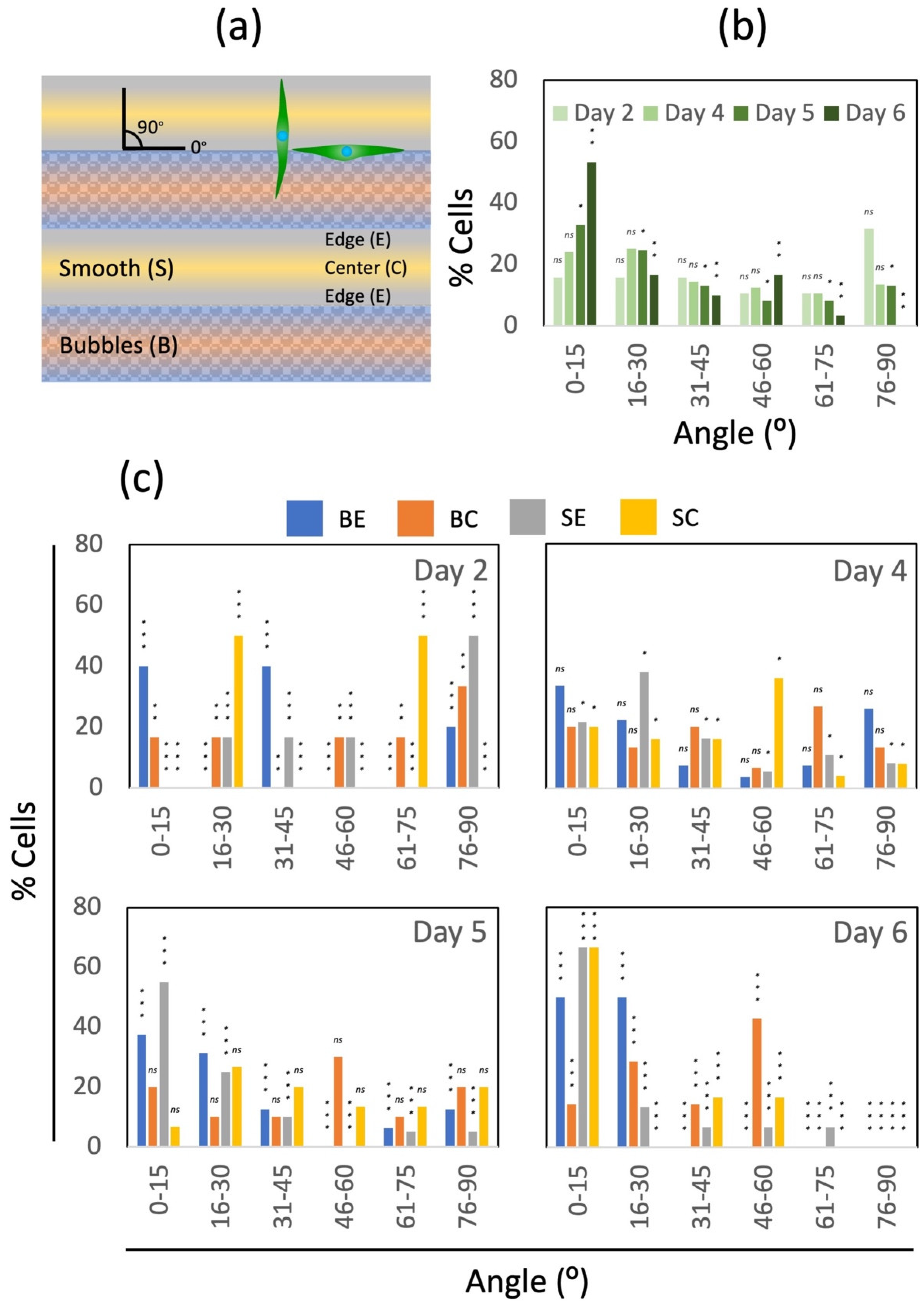
3.4. Cell-Culture Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman-Kim, D.; Mitchel, J.A.; Bellamkonda, R.V. Topography, cell response, and nerve regeneration. Annu. Rev. Biomed. Eng. 2010, 12, 203–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garland, S.P.; McKee, C.T.; Chang, Y.R.; Raghunathan, V.K.; Russell, P.; Murphy, C. A cell culture substrate with biologically relevant size-scale topography and compliance of the basement membrane. Langmuir 2014, 30, 2104–2108. [Google Scholar] [CrossRef] [PubMed]
- Mun, K.S.; Kumar, G.; Co, C.C.; Ho, C.C. Micropatterning different cell types with microarray amplification of natural directional persistence. Adv. Healthc. Mater. 2013, 2, 334–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voskuhl, J.; Brinkmann, J.; Jonkheijm, P. Advances in contact printing technologies of carbohydrate, peptide and protein arrays. Curr. Opin. Chem. Biol. 2014, 18, 1–7. [Google Scholar] [CrossRef]
- Jeon, H.; Simon, C.G., Jr.; Kim, G. A mini-review: Cell response to microscale, nanoscale, and hierarchical patterning of surface structure. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1580–1594. [Google Scholar] [CrossRef]
- Angelova, L.; Daskalova, A.; Mincheva, R.; Carette, X.; Trifonov, A.; Filipov, E.; Aceti, D.; Buchvarov, I. Ultra-fast laser modification of poly-lactic acid (PLA)—Towards enhanced biocompatibility. J. Phys. Conf. Ser. 2022, 2240, 012042. [Google Scholar] [CrossRef]
- Li, P.; Dou, X.; Schönherr, H. Micropatterning and nanopatterning with polymeric materials for advanced biointerface-controlled systems. Polym. Int. 2019, 68, 1015–1032. [Google Scholar] [CrossRef]
- Tilkin, R.G.; Régibeau, N.; Lambert, S.D.; Grandfils, C. Correlation between Surface Properties of Polystyrene and Polylactide Materials and Fibroblast and Osteoblast Cell Line Behavior: A Critical Overview of the Literature. Biomacromolecules 2020, 21, 1995–2013. [Google Scholar] [CrossRef]
- Bu, Y.; Ma, J.; Bei, J.; Wang, S. Surface Modification of Aliphatic Polyester to Enhance Biocompatibility. Front. Bioeng. Biotechnol. 2019, 7, 98. [Google Scholar] [CrossRef]
- Lednev, I.; Salomatina, E.; Ilyina, S.; Zaitsev, S.; Kovylin, R.; Smirnova, L. Development of Biodegradable Polymer Blends Based on Chitosan and Polylactide and Study of Their Properties. Materials 2021, 14, 4900. [Google Scholar] [CrossRef]
- Wu, F.; Zheng, J.; Li, Z.; Liu, M. Halloysite nanotubes coated 3D printed PLA pattern for guiding human mesenchymal stem cells (hMSCs) orientation. Chem. Eng. J. 2019, 359, 672–683. [Google Scholar] [CrossRef]
- Gasparotto, M.; Bellet, P.; Scapin, G.; Busetto, R.; Rampazzo, C.; Vitiello, L.; Shah, D.I.; Filippini, F. 3D Printed Graphene-PLA Scaffolds Promote Cell Alignment and Differentiation. Int. J. Mol. Sci. 2022, 23, 1736. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwak, S.; Lee, S.; Cho, W.K.; Leec, J.K.; Kang, S.M. One-step functionalization of zwitterionic poly[(3-(methacryloylamino)propyl)dimethyl(3-sulfopropyl)ammonium hydroxide] surfaces by metal–polyphenol coating. Chem. Commun. 2015, 51, 5340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Han, L.; Jia, L. A novel platelet-repellent polyphenolic surface and its micropattern for platelet adhesion detection. ACS Appl. Mater. Interfaces 2016, 8, 26570–26577. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Yavuz, H.; Ünal, S.; Denizli, A. Molecularly imprinted polymer based sensors for medical applications. Sensors 2019, 19, 1279. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Yang, X.; Wang, N.; Mu, L.; Pan, J.; Lan, X.; Li, H.; Deng, F. Anti-Biofouling Polymers with Special Surface Wettability for Biomedical Applications. Front. Bioeng. Biotechnol. 2021, 9, 807357. [Google Scholar] [CrossRef]
- Hasirci, V.; Kenar, H. Novel surface patterning approaches for tissue engineering and their effect on cell behavior. Nanomedicine 2006, 1, 73–90. [Google Scholar] [CrossRef]
- Hasirci, V.; Pepe-Mooney, B.J. Understanding the cell behavior on nano-/micro-patterned surfaces. Nanomedicine 2012, 7, 1375–1389. [Google Scholar] [CrossRef] [Green Version]
- Bettinger, C.J.; Langer, R.; Borenstein, J.T. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew. Chem. Int. Ed. 2009, 48, 5406–5415. [Google Scholar] [CrossRef] [Green Version]
- Ermis, M.; Antmen, E.; Hasirci, V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior. Bioact. Mater. 2018, 3, 355–369. [Google Scholar] [CrossRef]
- Fujita, S.; Ono, D.; Ohshima, M.; Iwata, H. Supercritical CO2-assisted embossing for studying cell behaviour on microtextured surfaces. Biomaterials 2008, 29, 4494–4500. [Google Scholar] [CrossRef] [PubMed]
- Arzt, E.; Quan, H.; McMeeking, R.M.; Hensel, R. Functional surface microstructures inspired by nature—From adhesion and wetting principles to sustainable new devices. Prog. Mater. Sci. 2021, 120, 100823. [Google Scholar] [CrossRef]
- Kingsley, D.M.; Dias, A.D.; Roberge, C.L.; Corr, D.T. Chapter 5—Laser Direct-Write Bioprinting: A Powerful Tool for Engineering Cellular Microenvironments. In 3D Bioprinting and Nanotechnology in Tissue Engineering and Regenerative Medicine, 2nd ed.; Zhang, L.G., Fisher, J.P., Leong, K.W., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 123–151. ISBN 9780128245521. [Google Scholar] [CrossRef]
- Schiele, N.R.; Corr, D.T.; Huang, Y.; Raof, N.A.; Xie, Y.; Chrisey, D.B. Laser-based direct-write techniques for cell printing. Biofabrication 2010, 2, 032001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.; Kim, M.; Kim, G.H. 3D-printed biomimetic scaffold simulating microfibril muscle structure. Adv. Funct. Mater. 2018, 28, 1800405. [Google Scholar] [CrossRef]
- Bolívar-Monsalve, E.J.; Ceballos-González, C.F.; Borrayo-Montaño, K.I.; Quevedo-Moreno, D.A.; Yee-de León, J.F.; Khademhosseini, A.; Weissdefgh, P.S.; Alvarez, M.M.; Trujillo-de Santiago, G. Continuous chaotic bioprinting of skeletal muscle-like constructs. Bioprinting 2021, 21, e00125. [Google Scholar] [CrossRef]
- Jungst, T.; Pennings, I.; Schmitz, M.; Rosenberg, A.J.; Groll, J.; Gawlitta, D. Heterotypic scaffold design orchestrates primary cell organization and phenotypes in cocultured small diameter vascular grafts. Adv. Funct. Mater. 2019, 29, 1905987. [Google Scholar] [CrossRef] [Green Version]
- Alamán, J.; Alicante, R.; Ignacio, J.; Sánchez-Somolinos, P.; Sánchez-Somolinos, C. Inkjet Printing of Functional Materials for Optical and Photonic Applications. Materials 2016, 9, 910. [Google Scholar] [CrossRef] [Green Version]
- Beedasy, V.; Smith, P.J. Printed Electronics as Prepared by Inkjet Printing. Materials 2020, 13, 704. [Google Scholar] [CrossRef] [Green Version]
- De Girolamo Del Mauro, A.; Grimaldi, I.A.; Loffredo, F.; Massera, E.; Polichetti, T.; Villani, F.; Di Francia, G. Geometry of the inkjet printed sensing layer for a better Volatile Organic Compound sensor response. J. Appl. Polym. Sci. 2011, 122, 3644–3650. [Google Scholar] [CrossRef]
- Grimaldi, I.A.; De Girolamo Del Mauro, A.; Nenna, G.; Loffredo, F.; Minarini, C.; Villani, F. Microstructuring of Polymer Films by Inkjet Etching. J. Appl. Polym. Sci. 2011, 122, 3637–3643. [Google Scholar] [CrossRef]
- Grimaldi, I.A.; De Girolamo Del Mauro, A.; Nenna, G.; Loffredo, F.; Minarini, C.; Villani, F. Inkjet Etching of Polymer Surfaces to Manufacture Microstructures for OLED Applications. AIP Conf. Proc. 2010, 1255, 104–106. [Google Scholar]
- Vafaei, S.; Tuck, C.; Ashcroft, I.; Wildman, R. Surface microstructuring to modify wettability for 3D printing of nano-filled inks. Chem. Eng. Res. Des. 2016, 109, 414–420. [Google Scholar] [CrossRef]
- Bao, B.; Jiang, J.; Li, F.; Zhang, P.; Chen, S.; Yang, Q.; Wang, S.; Su, B.; Jiang, L.; Song, Y. Fabrication of patterned concave microstructures by Inkjet Imprinting. Adv. Funct. Mater. 2015, 25, 3286–3294. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Liu, G.; Chu, F.; Chen, C.; Zhang, Y.; Tian, H.; Song, Y. Patterning a Superhydrophobic Area on a Facile Fabricated Superhydrophilic Layer Based on an Inkjet-Printed Water-Soluble, Polymer Template. Langmuir 2020, 36, 9952–9959. [Google Scholar] [CrossRef]
- Gomes, H.L.; Medeiros, M.C.R.; Villani, F.; Canudo, J.; Loffredo, F.; Miscioscia, R.; Martinez-Domingo, C.; Ramon, E.; Sowade, E.; Mitra, K.Y.; et al. All-inkjet printed organic transistors: Dielectric surface passivation techniques for improved operational stability and lifetime. Microelectron. Reliab. 2015, 55, 1192–1195. [Google Scholar] [CrossRef]
- Lee, K.B.; Kelbauskas, L.; Brunner, A.; Meldrum, D.R. A versatile method for dynamically controlled patterning of small populations of epithelial cells on substrates via non-contact piezoelectric inkjet printing. PLoS ONE 2017, 12, e0176079. [Google Scholar] [CrossRef] [Green Version]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef]
- Villani, F.; Grimaldi, I.A.; Nenna, G.; De Girolamo Del Mauro, A.; Loffredo, F.; Minarini, C. Study of the interference effects in an optical cavity for organic light-emitting diode applications. Opt. Lett. 2010, 35, 3333–3335. [Google Scholar] [CrossRef]
- Chen, S.; Su, M.; Zhang, C.; Gao, M.; Bao, B.; Yang, Q.; Su, B.; Song, Y. Fabrication of Nanoscale Circuits on Inkjet-Printing Patterned Substrates. Adv. Mater. 2015, 27, 3928–3933. [Google Scholar] [CrossRef]
- Kim, J.D.; Choi, J.S.; Kim, B.S.; Choi, Y.C.; Cho, Y.W. Piezoelectric inkjet printing of polymers: Stem cell patterningon polymer substrate. Polymer 2010, 51, 2147–2154. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, E.; Iannace, S.; Mensitieri, G. Mass transport of low molecular weight compounds in polymers. In Supercritical Fluid Science and Technology, 1st ed.; Foaming with Supercritical Fluids Series; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 6; pp. 179–230. [Google Scholar]
- Handa, Y.P.; Zhiyi, Z. A novel stress-induced nucleation and foaming process and its applications in making homogeneous foams, anisotropic foams, and multilayered foams. Cell. Polym. 2000, 19, 77–91. [Google Scholar]
- Ramachandran, K.; Shao, Z.; Di Luccio, T.; Shen, B.; Ruiz Bello, E.E.; Tammaro, L.; Villani, F.; Loffredo, F.; Borriello, C.; Di Benedetto, F.; et al. Tungsten disulfide (WS2) nanotubes (WSNT) enhance flow-induced crystallization and radio-opacity of polylactide (PLA) without adversely affecting in vitro toxicity. Acta Biomater. 2022, 138, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, A.; Avi Schroeder, J. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Prontera, C.T.; Villani, F.; Palamà, I.E.; Maglione, M.G.; Manini, P.; Maiorano, V.; Tammaro, L. Fabrication and biocompatibility analysis of flexible organic light emitting diodes on poly(lactic acid) substrates: Toward the development of greener bio-electronic devices. Polym. Adv. Technol. 2022, 33, 1523–1532. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Villani, F.; Rizzo, A.; Caputo, I.; Paolella, G.; Vigliotta, G. Antibacterial Al-doped ZnO coatings on PLA films. J. Mater. Sci. 2020, 55, 4830–4847. [Google Scholar] [CrossRef]
- Orsi, S.; Di Maio, E.; Iannace, S.; Netti, P.A. Hollow micro-and nano-particles by gas foaming. Nano Res. 2014, 7, 1018–1026. [Google Scholar] [CrossRef]
- Nuruddin, M.; Chowdhury, R.A.; Szeto, R.; Howarter, J.A.; Erk, K.A.; Szczepanski, C.R.; Youngblood, J.P. Structure–Property Relationship of Cellulose Nanocrystal–Polyvinyl Alcohol Thin Films for High Barrier Coating Application. ACS Appl. Mater. Interfaces 2021, 13, 12472–12482. [Google Scholar] [CrossRef]
- Allafchian, A.; Saeedi, S.; Jalali, S.A.H. Biocompatibility of electrospun cell culture scaffolds made from balangu seed mucilage/PVA composites. Nanotechnology 2022, 33, 075302. [Google Scholar] [CrossRef]
- de Gans, B.J.; Duineveld, P.C.; Schubert, U.S. Inkjet Printing of Polymers: State of the Art and Future Developments. Adv. Mater. 2004, 16, 203–2013. [Google Scholar] [CrossRef]
- Taki, K.; Kitano, D.; Ohshima, M. Effect of Growing Crystalline Phase on Bubble Nucleation in Poly(L-Lactide)/CO2 Batch Foaming. Ind. Eng. Chem. Res. 2011, 50, 3247–3252. [Google Scholar] [CrossRef]
- Wong, A.; Park, C.B. A visualization system for observing plastic foaming processes under shear stress. Polym. Test. 2012, 31, 417–424. [Google Scholar] [CrossRef]
- Viswanathan, P.; Ondeck, M.G.; Chirasatitsin, S.; Ngamkham, K.; Reilly, G.C.; Engler, A.J.; Battaglia, G. 3D surface topology guides stem cell adhesion and differentiation. Biomaterials 2015, 52, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.Y.; Donahue, H.J. Cell Sensing and Response to Micro- and Nanostructured Surfaces Produced by Chemical and Topographic Patterning. Tissue Eng. 2007, 13, 1879–1891. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, J.C.; Deneke, C.; Lanzoni, E.M.; Costa, C.A.; Bae, Y.; Scott, J.L.; Sharma, R.I. Modulating cell response on cellulose surfaces; tunable attachment and scaffold mechanics. Cellulose 2018, 25, 925–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubillaga, V.; Salaberria, A.M.; Palomares, T.; Alonso-Varona, A.; Kootala, S.; Labidi, J.; Fernandes, S.C.M. Chitin Nanoforms Provide Mechanical and Topological Cues to Support Growth of Human Adipose Stem Cells in Chitosan Matrices. Biomacromolecules 2018, 19, 3000–3012. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, Q.; Zheng, Z.L.; Gao, Y.T.; Zhu, G.Y.; Wei, Q.; Xu, J.Z.; Li, Z.M.; Zhao, C.S. Topographic Cues Guiding Cell Polarization via Distinct Cellular Mechanosensing Pathways. Small 2022, 18, 2104328. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Y.; Zhang, L.; Jin, L.; Yuan, F.; Tan, J.; Yuan, G.; Pei, J. Nano-micrometer surface roughness gradients reveal topographical influences on differentiating responses of vascular cells on biodegradable magnesium. Bioact. Mater. 2021, 6, 262–272. [Google Scholar] [CrossRef]
- Shi, X.; Cui, L.; Sun, H.; Jiang, N.; Heng, L.; Zhuang, X.; Gan, Z.; Chen, X. Promoting cell growth on porous PLA microspheres through simple degradation methods. Polym. Degrad. Stab. 2019, 161, 319–325. [Google Scholar] [CrossRef]
- Saniei, H.; Mousavi, S. Surface modification of PLA 3D-printed implants by electrospinning with enhanced bioactivity and cell affinity. Polymer 2020, 196, 122467. [Google Scholar] [CrossRef]
- Jeon, H.; Koo, S.; Reese, W.M.; Loskill, P.; Grigoropoulos, C.P.; Healy, K.E. Directing cell migration and organization via nanocrater-patterned cell-repellent interfaces. Nat. Mater. 2015, 14, 918–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| PVA Ink | PLA Substrate | |||||
|---|---|---|---|---|---|---|
| Contact Angles | Surface Energy | |||||
| Viscosity (mPa·s) | Surface Tension (mN/m) | θH2O (°) | θCH2I2 (°) | Dispersive Component (mN/m) | Polar Component (mN/m) | Total Surface Energy (mN/m) |
| 2.07 | 55.3 ± 0.55 | 73.9 ± 1.2 | 50.3 ± 0.9 | 34.13 | 7.26 | 41.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loffredo, F.; Villani, F.; Choy Buentello, D.; Trujillo-de Santiago, G.; Alvarez, M.M.; Miscioscia, R.; Di Maio, E. Bubble-Patterned Films by Inkjet Printing and Gas Foaming. Coatings 2022, 12, 806. https://doi.org/10.3390/coatings12060806
Loffredo F, Villani F, Choy Buentello D, Trujillo-de Santiago G, Alvarez MM, Miscioscia R, Di Maio E. Bubble-Patterned Films by Inkjet Printing and Gas Foaming. Coatings. 2022; 12(6):806. https://doi.org/10.3390/coatings12060806
Chicago/Turabian StyleLoffredo, Fausta, Fulvia Villani, David Choy Buentello, Grissel Trujillo-de Santiago, Mario Moisés Alvarez, Riccardo Miscioscia, and Ernesto Di Maio. 2022. "Bubble-Patterned Films by Inkjet Printing and Gas Foaming" Coatings 12, no. 6: 806. https://doi.org/10.3390/coatings12060806
APA StyleLoffredo, F., Villani, F., Choy Buentello, D., Trujillo-de Santiago, G., Alvarez, M. M., Miscioscia, R., & Di Maio, E. (2022). Bubble-Patterned Films by Inkjet Printing and Gas Foaming. Coatings, 12(6), 806. https://doi.org/10.3390/coatings12060806










