Preparation of Ti-Al-Si Gradient Coating Based on Silicon Concentration Gradient and Added-Ce
Abstract
:1. Introduction
2. Coating Preparation Method and Experiment Method
2.1. Coating Preparation Method
2.2. Experiment Method
- (1)
- Degrease the surface of the TC4 alloy with a metal cleaning agent and dry it;
- (2)
- Melt the high-purity aluminum in the vertical pit furnace;
- (3)
- Pour the high-purity aluminum melt in step (2) into the high-purity quartz tube, keep the melt state at a high-temperature of 800 °C, immerse the TC4 alloy into the high-purity aluminum melt, hold for a certain time, quickly extract from the melt, and then quench.
3. Results and Discussion
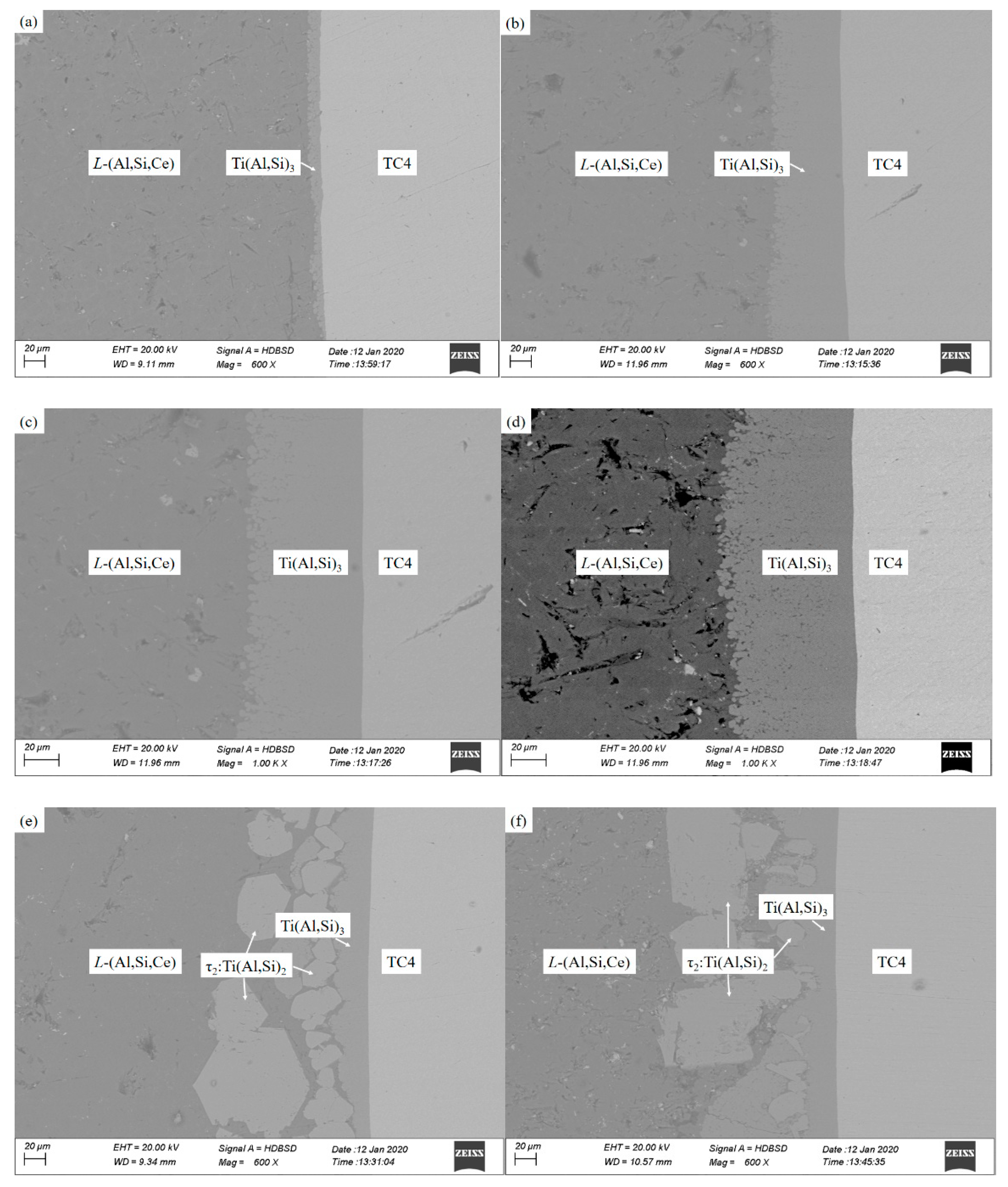
3.1. Microstructure Characterization of Unadded Cerium Coating
3.2. The Microstructure Characterization of Added Cerium Coating
3.3. The Microstructure Characterization of Added Cerium Coating
3.4. High-Temperature Oxidation Resistance
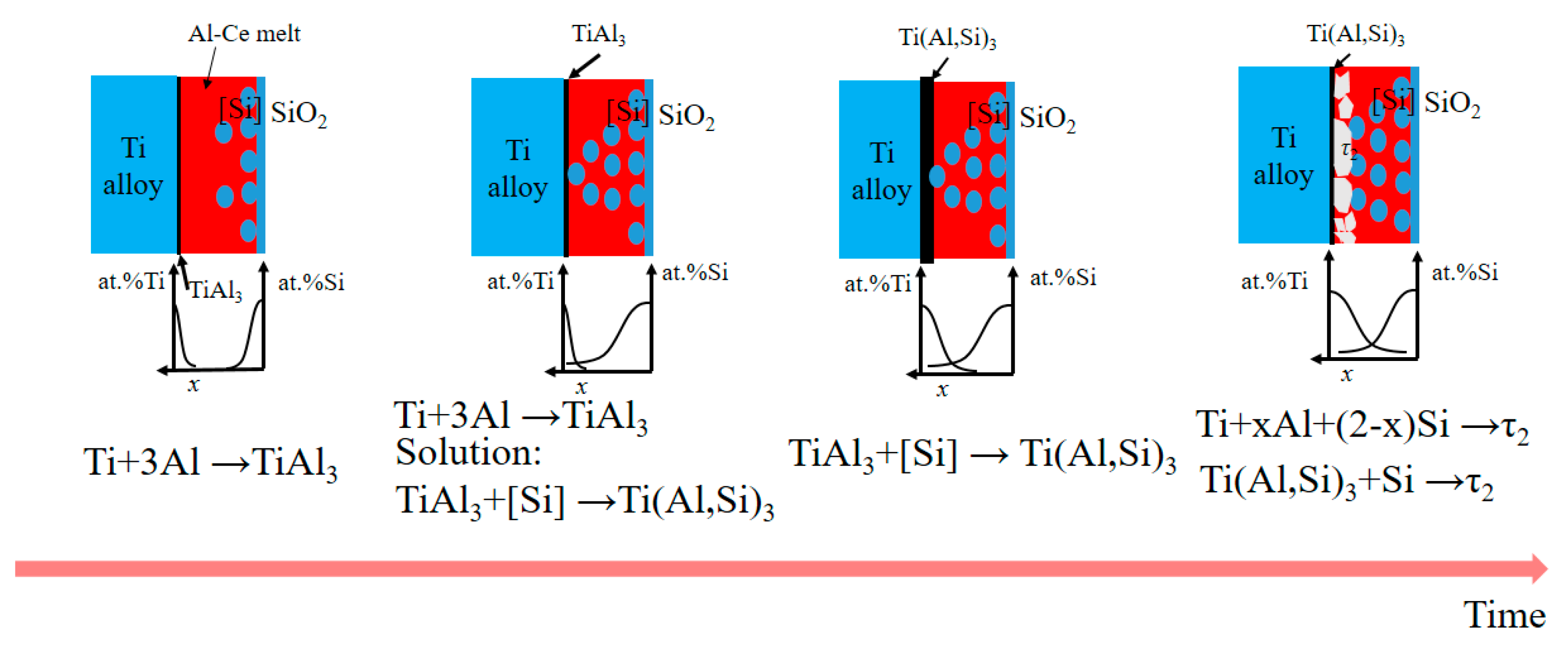
3.5. The Formation Mechanism of High-Temperature Oxidation Microstructure of Adding Ce Coatings
4. Conclusions
- (1)
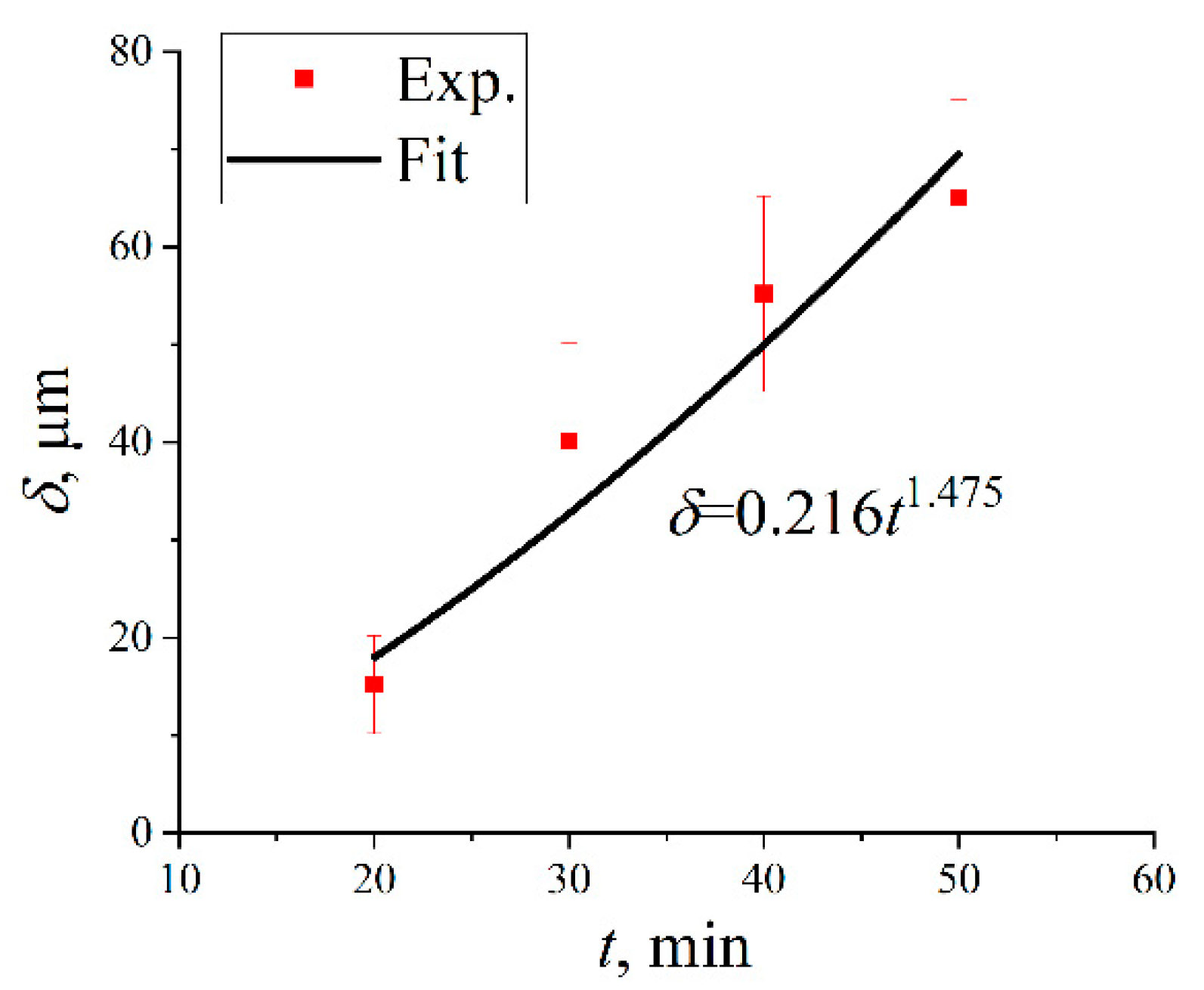
- The thickness of the Ti(Al,Si)3 phase with a Ce-added Ti-Al-Si gradient coating increases gradually with the increase in hot infiltration time, and the τ2: Ti(Al,Si)2 phase appears and grows when the hot infiltration time exceeds a certain point.
- (2)
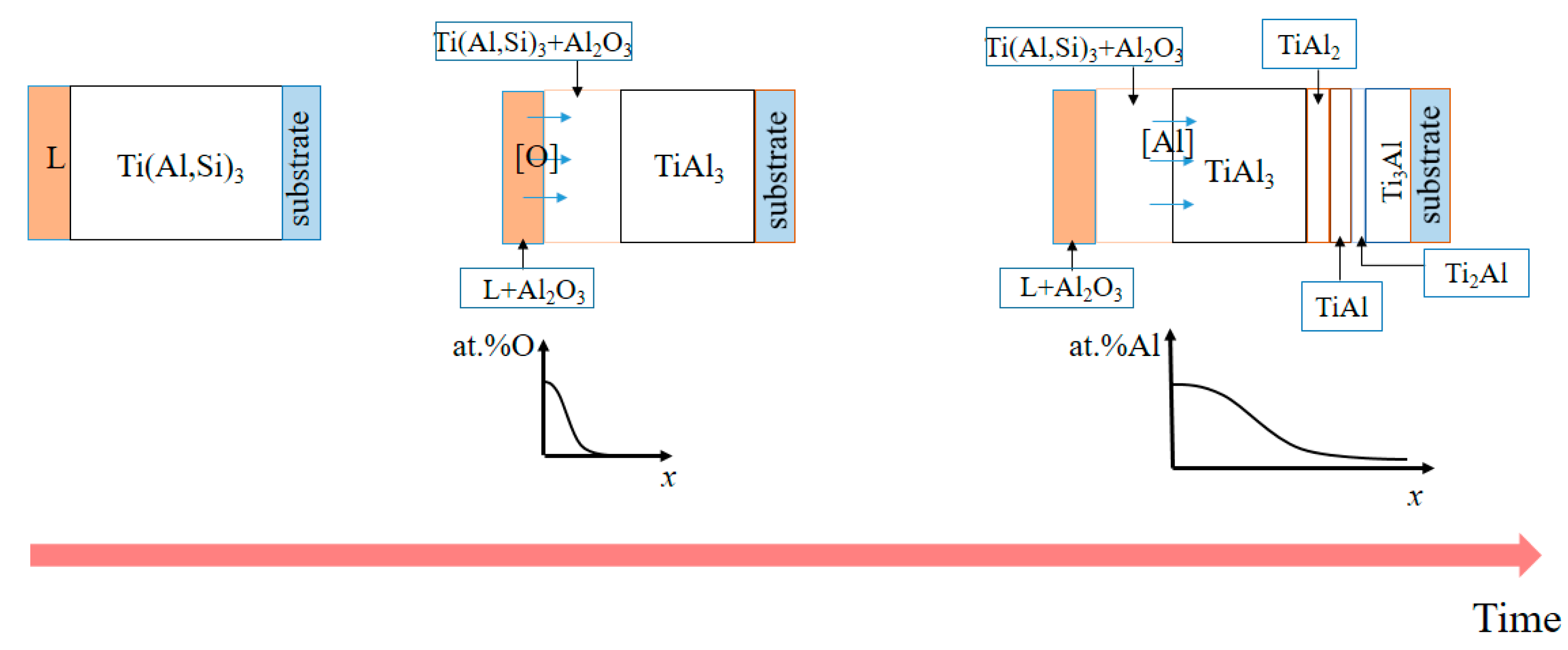
- Within 50 min of hot infiltration, the dense Ti(Al,Si)3 phase layer and L-(Al, Si, Ce) phase layer are formed outward from the substrate, respectively. When the hot infiltration time is extended, the dense Ti(Al,Si)3 phase, L-(Al, Si, Ce) phase + bulk τ2: Ti(Al,Si)2 phase and L-(Al, Si, Ce) phase are obtained from the substrate in sequence.
- (3)
- Several new Ti-Al system alloy phase layers (Ti3Al, TiAl and TiAl3, etc.) are formed between the substrate and Ti(Al,Si)3 during the high-temperature oxidation process, which can further prevent the diffusion of oxygen and cracks to the substrate.
- (4)
- Adding Ce can form Ce-rich quaternary phase (TiAl6Si2Ce2) in L-(Al, Si, Ce) alloy layer and suppress the formation of the Al-Si-Ti ternary phase and τ2: Ti(Al,Si)2 phase. The concentration of Ti, Si and Ce in the TiAl6Si2Ce2 phase is much higher than that of these elements in the surrounding L-(Al, Si, Ce) alloy, indicating that the element segregation effect occurs after the addition of Ce.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, J.M. A review of aerospace materials. Spacecr. Environ. Eng. 2013, 30, 115–121. [Google Scholar]
- Dai, J.J.; Zhu, J.Y.; Chen, C.Z.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloy. Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Liu, Y.Y.; Jin, Y.F.; Ma, X.Z.; Chai, L.H.; Cui, Y.P. Research on 650 °C high temperature titanium alloy technology for aero-engine. Aeronaut. Manuf. Technol. 2019, 62, 22–30. [Google Scholar]
- Li, F.G.; Yang, L.; Zhou, Y.C. Study Advances of high temperature coating for aeroengine to resist marine atmospheric corrosion. Therm. Spray Technol. 2019, 11, 1–9. [Google Scholar]
- Li, M.S. High Temperature Corrosion of Metals; Metallurgical Industry Press: Beijing, China, 2001. [Google Scholar]
- Yang, Y.; Kushima, A.; Han, W.Z.; Xin, H.L.; Li, J. Self-healing aluminum oxide during deformation at room temperature. Nano Lett. 2018, 18, 2492–2497. [Google Scholar] [CrossRef]
- Xu, Z.F.; Rong, J.; Yu, X.H.; Meng, K.; Zhan, Z.L.; Wang, X.; Zhang, Y.N. The interface structure of high-temperature oxidation–resistant Aluminum-based coatings on Titanium billet surface. JOM 2017, 69, 1848–1852. [Google Scholar] [CrossRef]
- Christoph, L.; Manfred, P. Titanium and Titanium Alloys: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Xiang, Z.D.; Rose, S.; Datta, P.K. Pack deposition of coherent aluminide coatings on γ-TiAl for enhancing its high temperature oxidation resistance. Surf. Coat. Technol. 2002, 161, 286–292. [Google Scholar] [CrossRef]
- Tsaur, C.C.; Rock, J.C.; Chang, Y.Y. The effect of NaCl deposit and thermal cycle on an aluminide layer coated on 310 stainless steel. Mater. Chem. Phys. 2005, 91, 330–337. [Google Scholar] [CrossRef]
- Wang, Y.S.; Xiong, J.; Yan, J.; Fan, H.Y.; Wang, J. Oxidation resistance and corrosion behavior of hot-dip aluminized coatings on commercial-purity titanium. Surf. Coat. Technol. 2011, 206, 1277–1282. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Peng, Y.P.; Mao, Y.L.; Pang, C.J.; Lu, L.Y. Effect of hot-dip aluminizing on the oxidation resistance of Ti–6Al–4V alloy at high temperatures. Corros. Sci. 2012, 55, 187–193. [Google Scholar] [CrossRef]
- Jeng, S.C. Oxidation behavior and microstructural evolution of hot-dipped aluminum coating on Ti-6Al-4V alloy at 800 °C. Surf. Coat. Technol. 2013, 235, 867–874. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, A.H.; Zhang, Z.; Zheng, R.R.; Xia, H.B.; Wang, Y.N. Laser alloying of Ti–Si compound coating on Ti–6Al–4V alloy for the improvement of bioactivity. Appl. Surf. Sci. 2014, 305, 16–23. [Google Scholar] [CrossRef]
- Sadeq, F.O.; Sharifitabar, M.; Afarani, M.S. Synthesis of Ti–Si–Al coatings on the surface of Ti–6Al–4V alloy via hot dip siliconizing route. Surf. Coat. Technol. 2018, 337, 349–356. [Google Scholar] [CrossRef]
- Dai, J.J.; Zhang, F.Y.; Wang, A.M.; Yu, H.J.; Chen, C.Z. Microstructure and properties of Ti-Al coating and Ti-Al-Si system coatings on Ti-6Al-4V fabricated by laser surface alloying. Surf. Coat. Technol. 2017, 309, 805–813. [Google Scholar] [CrossRef]
- Chen, C.; Feng, X.M.; Shen, Y.F. Oxidation behavior of a high Si content Al-Si composite coating fabricated on Ti-6Al-4V substrate by mechanical alloying method. J. Alloy. Compd. 2017, 701, 27–36. [Google Scholar] [CrossRef]
- Hu, X.Y.; Li, F.G.; Shi, D.M.; Xie, Y.; Li, Z.; Yin, F.C. A design of self-generated Ti–Al–Si gradient coatings on Ti–6Al–4V alloy based on silicon concentration gradient. J. Alloy. Compd. 2020, 830, 154670. [Google Scholar] [CrossRef]
- Rahmel, A.; Schütze, M. Mechanical aspects of the rare-earth effect. Oxid. Met. 1992, 38, 255–266. [Google Scholar] [CrossRef]
- Thanneeru, R.; Patil, S.; Deshpande, S.; Seal, S. Effect of trivalent rare earth dopants in nanocrystalline ceria coatings for high-temperature oxidation resistance. Acta Mater. 2007, 55, 3457–3466. [Google Scholar] [CrossRef]
- Huang, N.; Hu, S.; Xie, G.; Zeng, P.; Ru, Q. Effect of rare earth element cerium on mechanical properties and morphology of TiN coating prepared by arc ion plating. J. Rare Earth. 2003, 21, 380–383. [Google Scholar]
- Li, Z.; Liao, C.L.; Liu, Y.X.; Wang, X.M.; Wu, Y.; Zhao, M.X.; Long, Z.H.; Yin, F.C. 700 °C Isothermal section of the Al-Ti-Si ternary phase diagram. J. Phase Equilib. 2014, 35, 564–574. [Google Scholar] [CrossRef]
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Ma, S.M.; Li, N.; Zhang, C.; Wang, X.M. Evolution of intermetallic phases in an Al-Si-Ti alloy during solution treatment. J. Alloy. Compd. 2020, 831, 154872. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.G.; Li, W.; Qin, Q.D.; Tian, B.; Hu, S.W. Al–Si coating fused by Al + Si powders formed on Ti–6Al–4V alloy and its oxidation resistance. Mater. Sci. Eng. A 2006, 430, 142–150. [Google Scholar] [CrossRef]
- Knaislová, A.; Novák, P.; Kopeček, J.; Průša, F. Properties comparison of Ti-Al-Si alloys produced by various metallurgy methods. Materials 2019, 12, 3084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knaislová, A.; Novák, P.; Linhart, J.; Szurman, I.; Skotnicová, K.; Juřica, J.; Čegan, T. Structure and properties of cast Ti-Al-Si alloys. Materials 2021, 14, 813. [Google Scholar] [CrossRef] [PubMed]
- Knaislová, A.; Novák, P.; Cabibbo, M.; Jaworska, L.; Vojtěch, D. Development of TiAl–Si Alloys—A Review. Materials 2021, 14, 1030. [Google Scholar] [CrossRef]


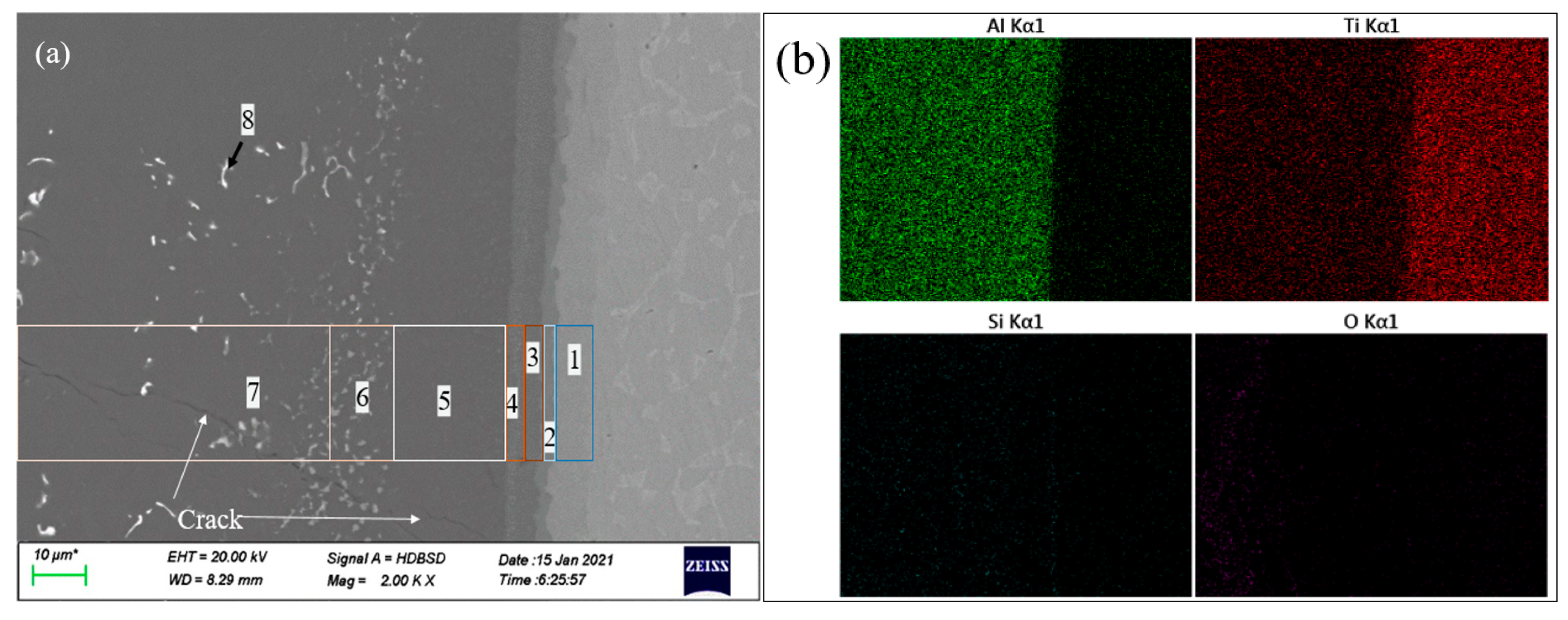
| Phase Layer in Figure 6 | Ti | Al | Si | O | Ce | Compounds |
|---|---|---|---|---|---|---|
| 1# | 76.46 | 23.54 | 0 | 0 | 0 | Ti3Al |
| 2# | 64.76 | 35.24 | 0 | 0 | 0 | Ti2Al |
| 3# | 47.40 | 52.60 | 0 | 0 | 0 | TiAl |
| 4# | 34.47 | 65.53 | 0 | 0 | 0 | TiAl2 |
| 5# | 25.43 | 67.07 | 0 | 7.50 | 0 | TiAl3 |
| 6# | 24.48 | 54.90 | 11.95 | 8.67 | 0 | Ti(Al,Si)3 + Al2O3 |
| 7# | 0.09 | 85.34 | 4.35 | 10.01 | 0.21 | L-(Al, Si, Ce) + Al2O3 |
| 8# | 8.77 | 57.17 | 14.08 | 3.77 | 16.21 | TiAl6Si2Ce2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, F.; Hu, X.; He, W.; Liu, Z.; Tan, Y. Preparation of Ti-Al-Si Gradient Coating Based on Silicon Concentration Gradient and Added-Ce. Coatings 2022, 12, 683. https://doi.org/10.3390/coatings12050683
Wang Z, Li F, Hu X, He W, Liu Z, Tan Y. Preparation of Ti-Al-Si Gradient Coating Based on Silicon Concentration Gradient and Added-Ce. Coatings. 2022; 12(5):683. https://doi.org/10.3390/coatings12050683
Chicago/Turabian StyleWang, Zihan, Faguo Li, Xiaoyuan Hu, Wei He, Zhan Liu, and Yao Tan. 2022. "Preparation of Ti-Al-Si Gradient Coating Based on Silicon Concentration Gradient and Added-Ce" Coatings 12, no. 5: 683. https://doi.org/10.3390/coatings12050683
APA StyleWang, Z., Li, F., Hu, X., He, W., Liu, Z., & Tan, Y. (2022). Preparation of Ti-Al-Si Gradient Coating Based on Silicon Concentration Gradient and Added-Ce. Coatings, 12(5), 683. https://doi.org/10.3390/coatings12050683






