Assessment of the Potential Diffusion Barriers between Tungsten and Silicon Carbide for Nuclear Fusion Application
Abstract
1. Introduction
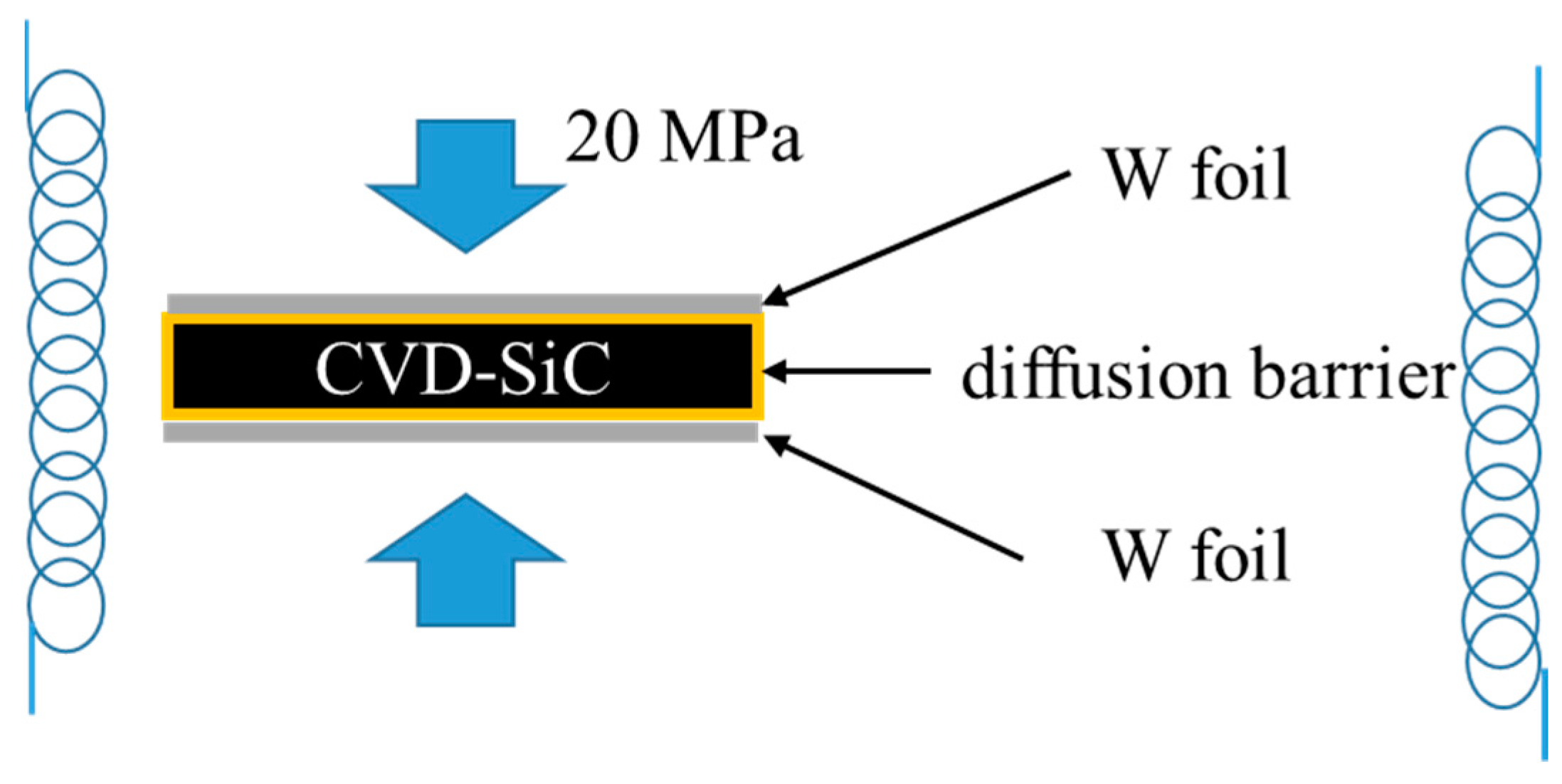
2. Materials and Methods
3. Results
3.1. Uncoated CVD-SiC Joined with W Foil
3.2. CVD-SiC and W Foil Joined with Diffusion Barrier
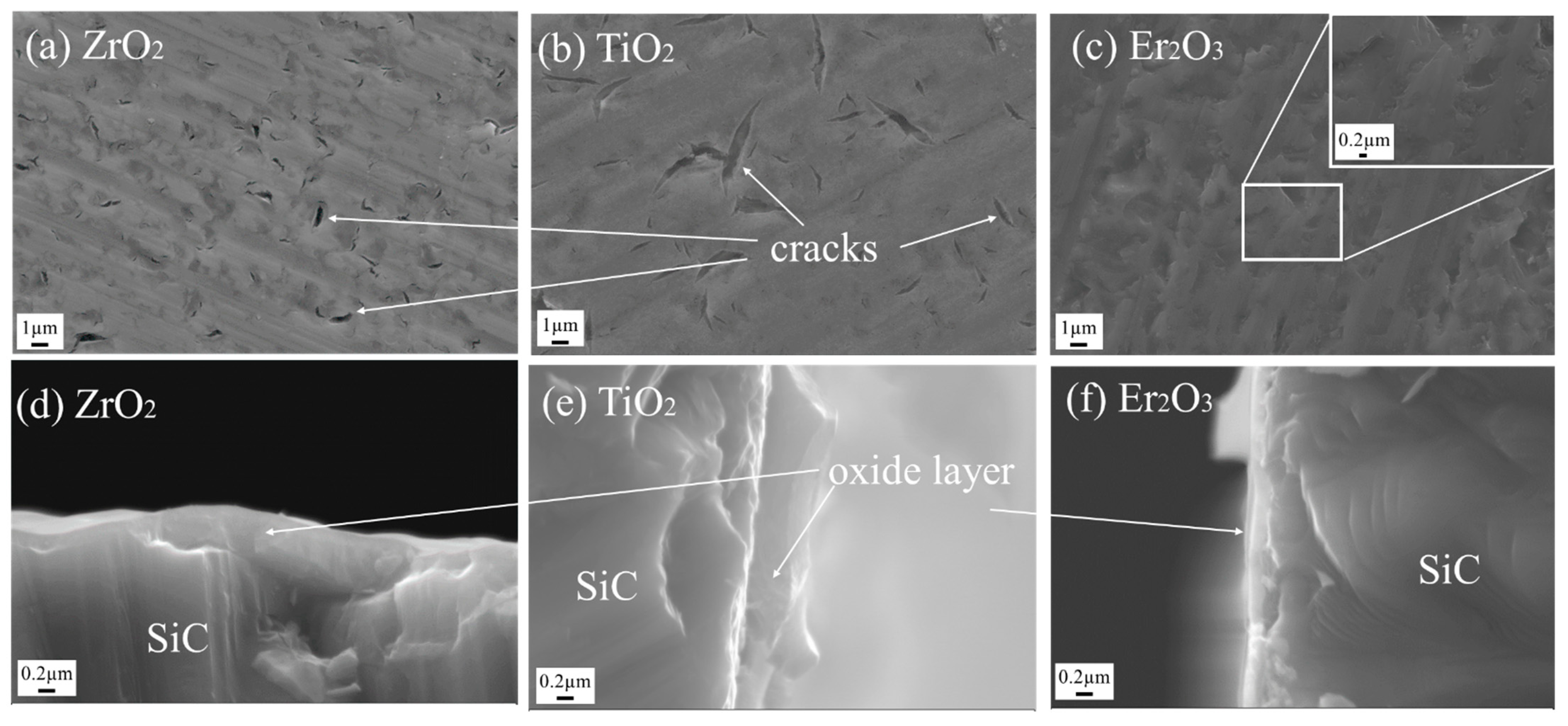
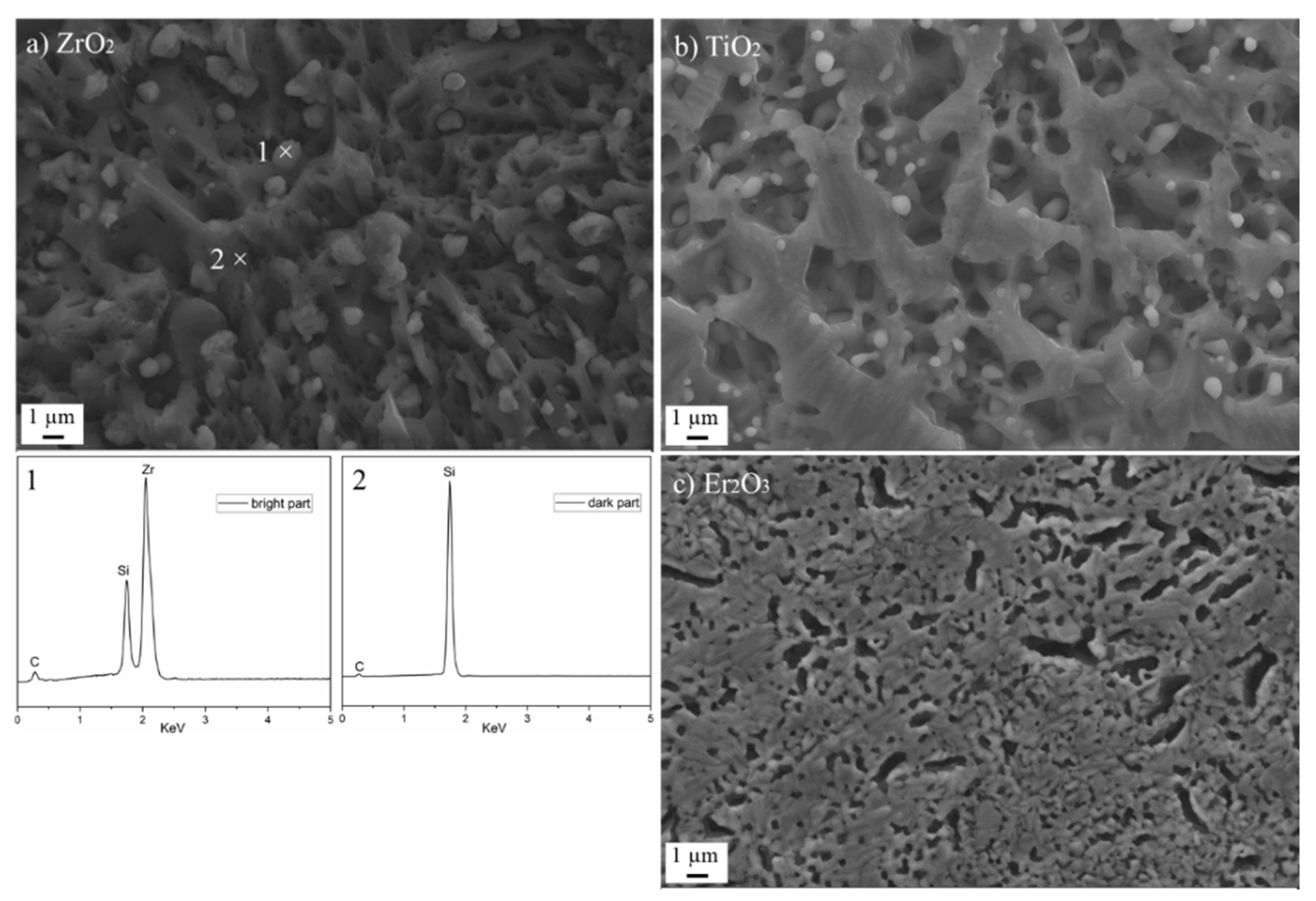
3.2.1. Oxide Coatings
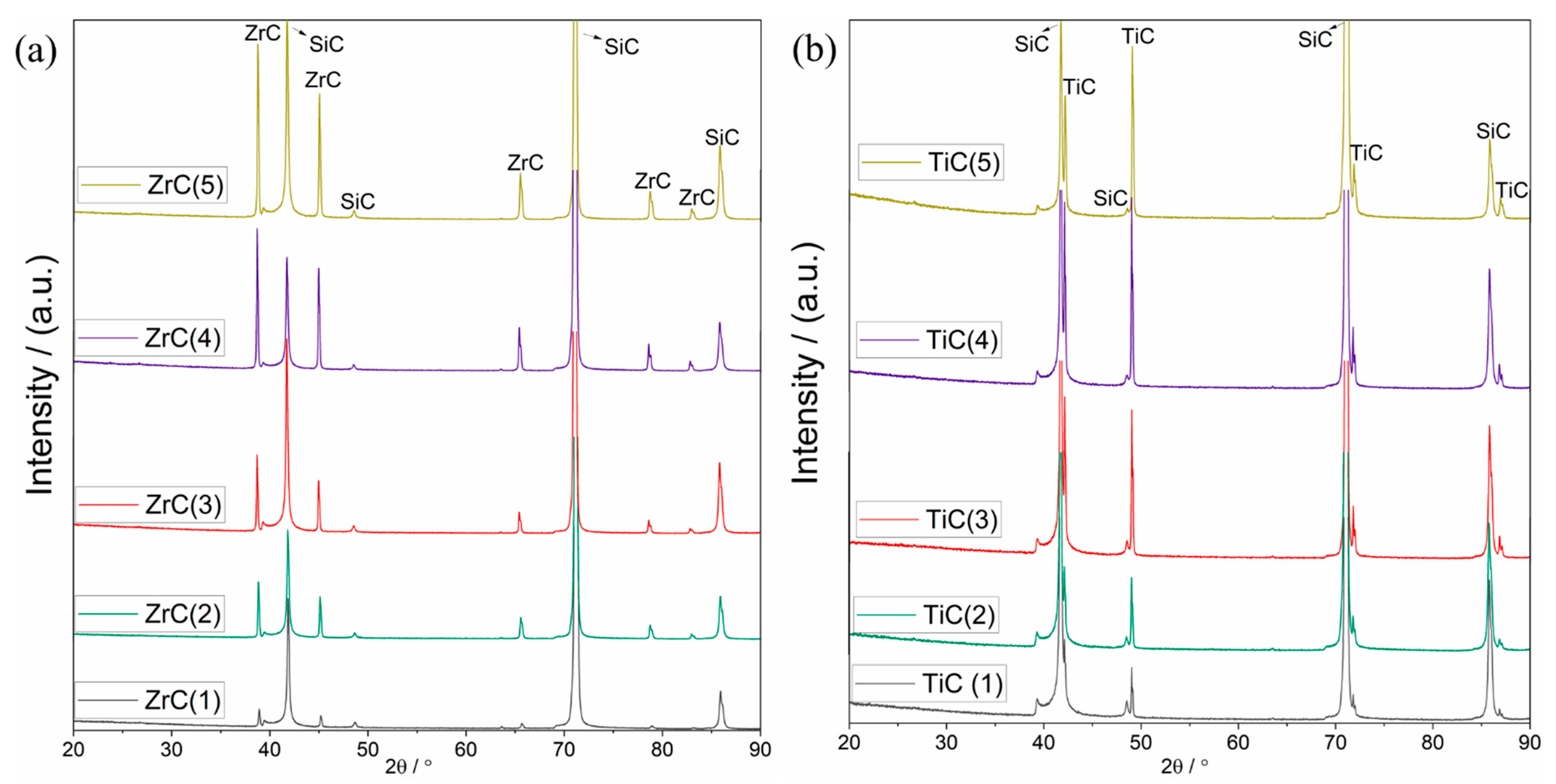
3.2.2. Carbide Coatings
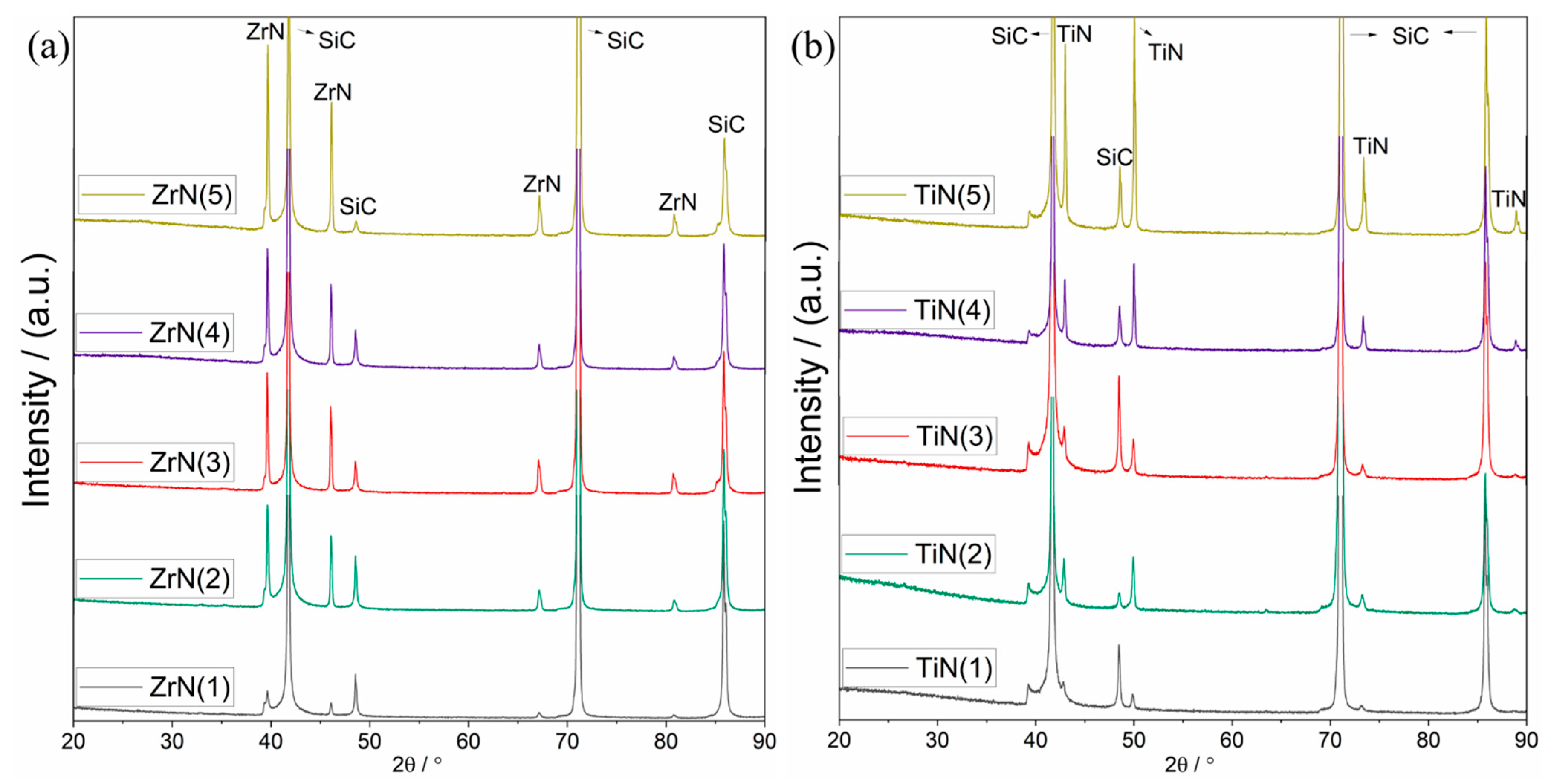
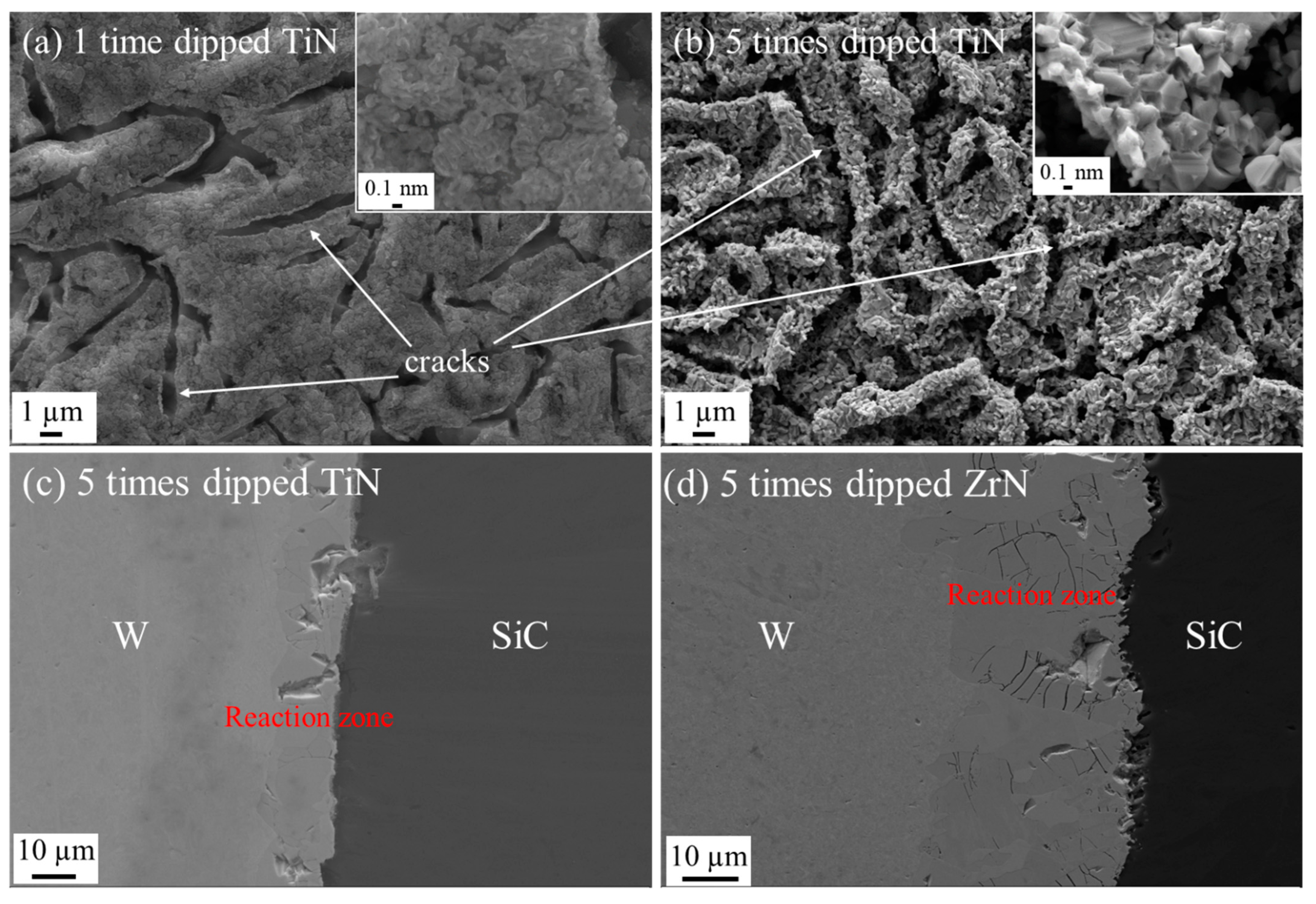
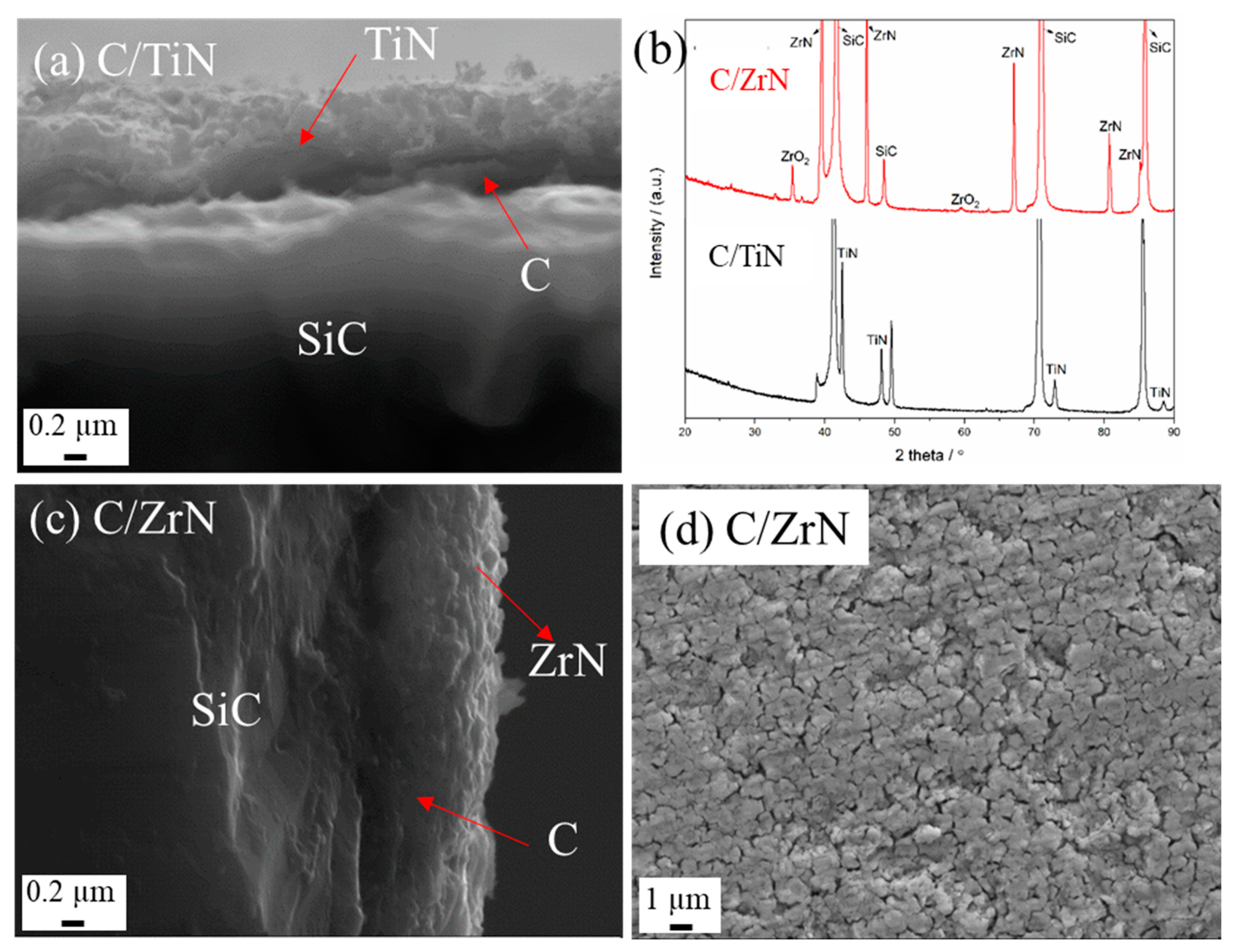
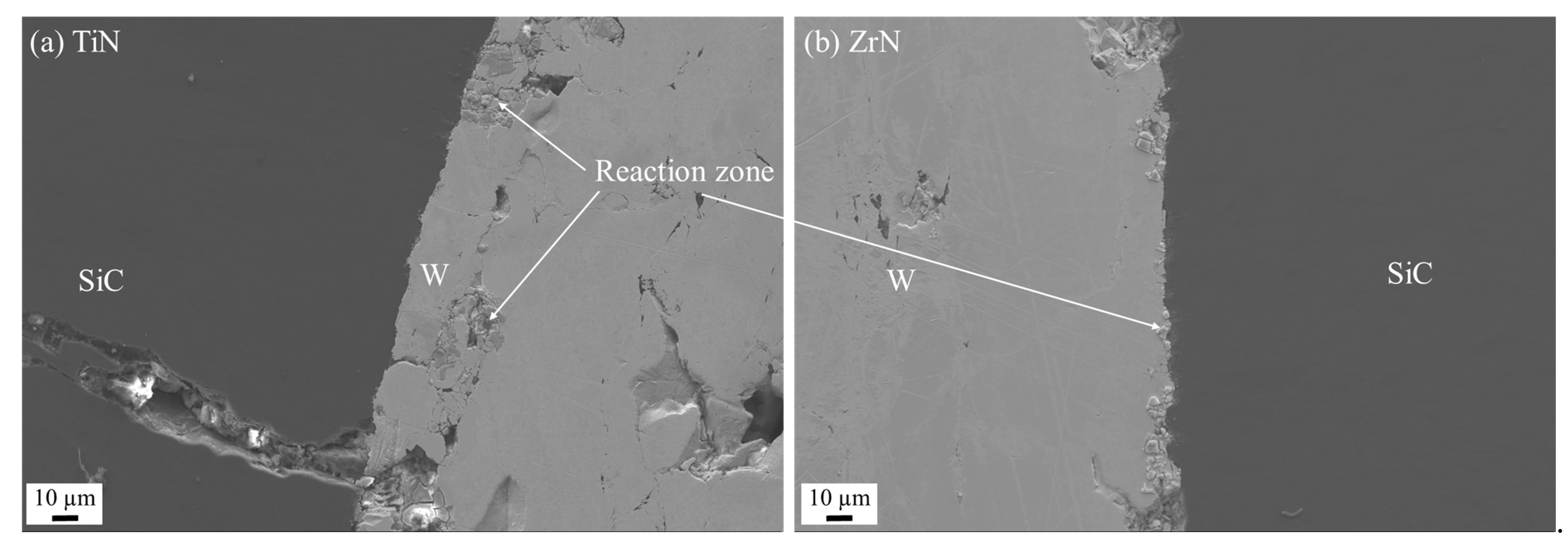
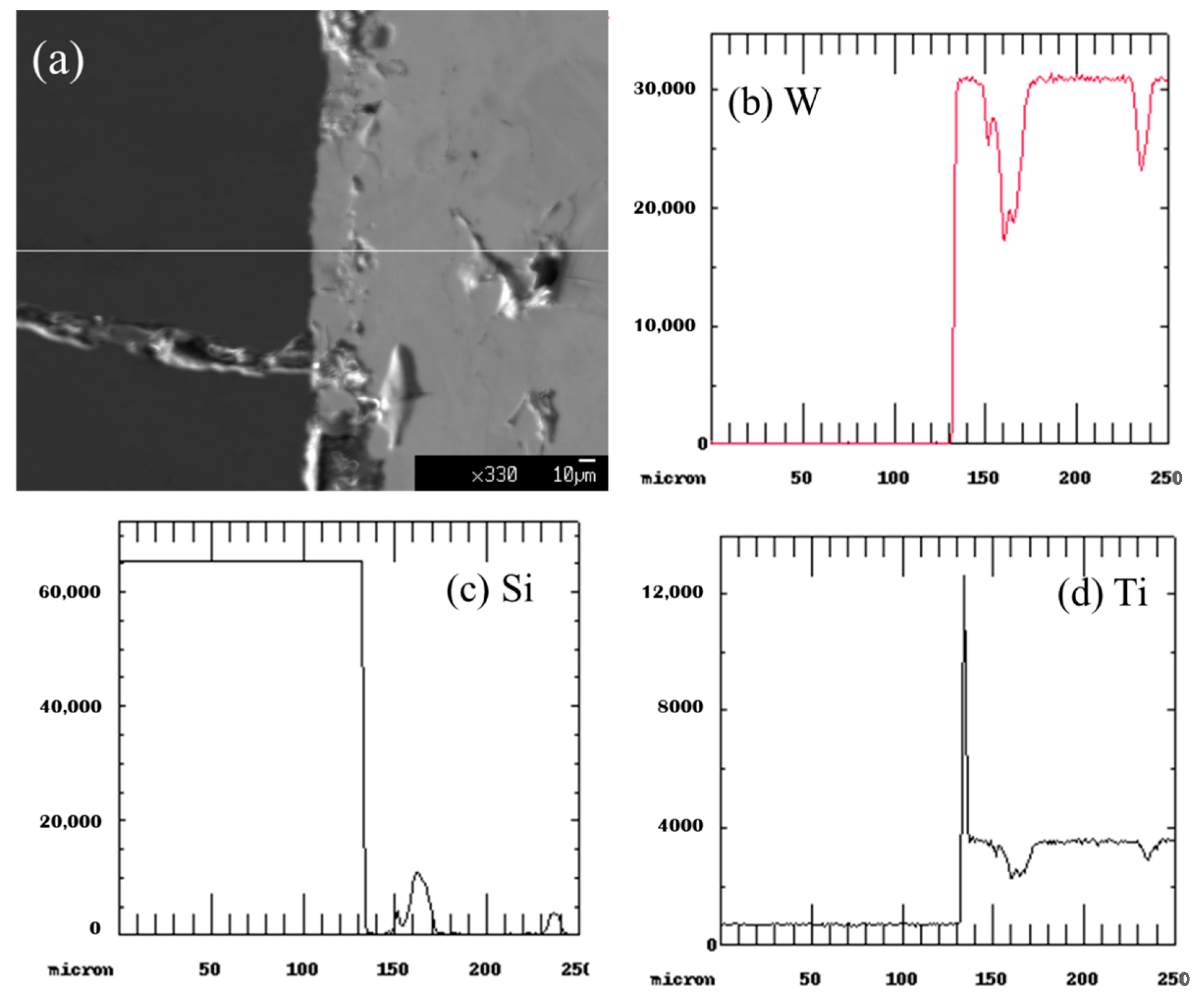
3.2.3. Nitride Coatings
Dipped Nitride Coatings
Sputtered Nitride Coatings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koyanagi, T.; Katoh, Y.; Nozawa, T.; Snead, L.L.; Kondo, S.; Henager, C.H.; Ferraris, M.; Hinoki, T.; Huang, Q. Recent progress in the development of SiC composites for nuclear fusion applications. J. Nucl. Mater. 2018, 511, 544–555. [Google Scholar] [CrossRef]
- Li, H.X.; Zhong, Z.H.; Zhang, H.B.; Zhu, Z.X.; Hua, P.; Chen, C.; Wu, Y.C. Microstructure characteristic and its influence on the strength of SiC ceramic joints diffusion bonded by spark plasma sintering. Ceram. Int. 2018, 44, 3937–3946. [Google Scholar] [CrossRef]
- Son, S.J.; Park, K.H.; Katoh, Y.; Kohyama, A. Interfacial reactions and mechanical properties of W-SiC in-situ joints for plasma facing components. J. Nucl. Mater. 2004, 329–333, 1549–1552. [Google Scholar] [CrossRef]
- Kishimoto, H.; Shibayama, T.; Shimoda, K.; Kobayashi, T.; Kohyama, A. Microstructural and mechanical characterization of W/SiC bonding for structural material in fusion. J. Nucl. Mater. 2011, 417, 387–390. [Google Scholar] [CrossRef]
- Umer, M.A.; Lee, D.; Waseem, O.A.; Ryu, H.J.; Hong, S.H. Fabrication of protective-coated SiC reinforced tungsten matrix composites with reduced reaction phases by spark plasma sintering. Met. Mater. Int. 2016, 22, 493–500. [Google Scholar] [CrossRef]
- Shi, X.; Wang, M.; Zhang, S.; Zhang, Q. Fabrication and properties of W-20Cu alloy reinforced by titanium nitride coated SiC fibers. Int. J. Refract. Met. Hard Mater. 2013, 41, 60–65. [Google Scholar] [CrossRef]
- Du, Y.; Hinoki, T. Effect of tungsten matrix on the mechanical property of SiC fiber reinforced tungsten composites with foils fabricated at 1700 °C. Nucl. Mater. Energy 2022, 31, 101142. [Google Scholar] [CrossRef]
- Seng, W.F.; Barnes, P.A. Calculations of cobalt suicide and carbide formation on SiC using the Gibbs free energy. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2000, 76, 225–231. [Google Scholar] [CrossRef]
- Kang, H.K. Microstructures of high volume SiC reinforced tungsten composites produced by plasma spray. Scr. Mater. 2004, 51, 1051–1055. [Google Scholar] [CrossRef]
- Hinoki, T.; Snead, L.L.; Blue, C.A. Development of refractory armored silicon carbide by infrared transient liquid phase processing. J. Nucl. Mater. 2005, 347, 207–216. [Google Scholar] [CrossRef]
- Wang, Z.; DelaCruz, S.; Tsai, D.S.; Maboudian, R. W/TaC/SiC sandwich stack for high temperature applications. Ceram. Int. 2019, 45, 22292–22297. [Google Scholar] [CrossRef]
- DelaCruz, S.; Wang, Z.; Cheng, P.; Carraro, C.; Maboudian, R. TiN diffusion barrier for stable W/SiC(0001) interfaces in inert ambient at high temperature. Thin Solid Films 2019, 670, 54–59. [Google Scholar] [CrossRef]
- Roger, J.; Audubert, F.; le Petitcorps, Y. Thermal stability of W-xRe/TiC/SiC systems (x = 0, 5 and 25 at % Re) at high temperature. Adv. Eng. Mater. 2009, 11, 399–407. [Google Scholar] [CrossRef]
- Roger, J.; Audubert, F.; Le Petitcorps, Y. Reactivity of M/TiN/SiC systems (M = W and Mo) at high temperature. J. Mater. Sci. 2010, 45, 3073–3079. [Google Scholar] [CrossRef]
- Golestani Fard, M.A.; Baharvandi, H. Development of W–ZrC composite coating on graphite by a TIG-aided surface cladding process. Ceram. Int. 2021, 47, 27958–27971. [Google Scholar] [CrossRef]
- Liu, R.; Xie, Z.M.; Yang, J.F.; Zhang, T.; Hao, T.; Wang, X.P.; Fang, Q.F.; Liu, C.S. Recent progress on the R&D of W-ZrC alloys for plasma facing components in fusion devices. Nucl. Mater. Energy 2018, 16, 191–206. [Google Scholar] [CrossRef]
- Xie, X.F.; Jing, K.; Xie, Z.M.; Liu, R.; Yang, J.F.; Fang, Q.F.; Liu, C.S.; Wu, X. Mechanical properties and microstructures of W–TiC and W–Y2O3 alloys fabricated by hot-pressing sintering. Mater. Sci. Eng. A 2021, 819, 141496. [Google Scholar] [CrossRef]
- Kurishita, H.; Matsuo, S.; Arakawa, H.; Sakamoto, T.; Kobayashi, S.; Nakai, K.; Takida, T.; Kato, M.; Kawai, M.; Yoshida, N. Development of re-crystallized W-1.1%TiC with enhanced room-temperature ductility and radiation performance. J. Nucl. Mater. 2010, 398, 87–92. [Google Scholar] [CrossRef]
- Lee, D.; Umer, M.A.; Shin, Y.; Jeon, S.; Hong, S. The effect of sintering conditions and ZrN volume fraction on the mechanical properties of spark plasma sintered W/ZrN composites. Mater. Sci. Eng. A 2012, 552, 481–485. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.; Kang, J.; Song, S.H.; Lee, D. Effects of ZrN and W Particle Sizes on the Mechanical and Ablation Properties of ZrN/W Composites. Met. Mater. Int. 2019, 25, 733–739. [Google Scholar] [CrossRef]
- Adachi, J.; Kurosaki, K.; Uno, M.; Yamanaka, S. Thermal and electrical properties of zirconium nitride. J. Alloys Compd. 2005, 399, 242–244. [Google Scholar] [CrossRef]
- Lengauer, W. Transition Metal Carbides, Nitrides, and Carbonitrides. Handb. Ceram. Hard Mater. 2008, 265, 202–252. [Google Scholar] [CrossRef]
- Harrison, R.W.; Lee, W.E. Processing and properties of ZrC, ZrN and ZrCN ceramics: A review. Adv. Appl. Ceram. 2016, 115, 294–307. [Google Scholar] [CrossRef]
- Chikada, T.; Suzuki, A.; Yao, Z.; Sawada, A.; Terai, T.; Muroga, T. Basic study on self-healing of Er2O3 coating for vanadium-lithium blanket system. Fusion Eng. Des. 2007, 82, 2572–2577. [Google Scholar] [CrossRef][Green Version]
- Xiao, F.; Miao, Q.; Wei, S.; Li, Z.; Sun, T.; Xu, L. Microstructure and mechanical properties of W-ZrO2 alloys by different preparation techniques. J. Alloys Compd. 2019, 774, 210–221. [Google Scholar] [CrossRef]
- Jasper, B.; Schoenen, S.; Du, J.; Hoeschen, T.; Koch, F.; Linsmeier, C.; Neu, R.; Riesch, J.; Terra, A.; Coenen, J.W. Behavior of tungsten fiber-reinforced tungsten based on single fiber push-out study. Nucl. Mater. Energy. 2016, 9, 416–421. [Google Scholar] [CrossRef]
- Riesch, J.; Buffiere, J.Y.; Höschen, T.; Scheel, M.; Linsmeier, C.; You, J.H. Crack bridging in as-fabricated and embrittled tungsten single fibre-reinforced tungsten composites shown by a novel in-situ high energy synchrotron tomography bending test. Nucl. Mater. Energy 2018, 15, 1–12. [Google Scholar] [CrossRef]
- Morimoto, T.; Ogasawara, T. Potential strength of NicalonTM, Hi NicalonTM, and Hi Nicalon Type STM monofilaments of variable diameters. Compos. Part A Appl. Sci. Manuf. 2006, 37, 405–412. [Google Scholar] [CrossRef]
- Hummer, D.R.; Heaney, P.J.; Post, J.E. Thermal expansion of anatase and rutile between 300 and 575 K using synchrotron powder X-ray diffraction. Powder Diffr. 2007, 22, 352–357. [Google Scholar] [CrossRef]
- Dargis, R.; Williams, D.; Smith, R.; Arkun, E.; Roucka, R.; Clark, A.; Lebby, M. Structural and Thermal Properties of Single Crystalline Epitaxial Gd2O3 and Er2O3 Grown on Si(111). ECS J. Solid State Sci. Technol. 2012, 1, N24–N28. [Google Scholar] [CrossRef]






| Coating | Er2O3 | ZrO2 | TiO2 | ZrC | TiC | ZrN | TiN |
|---|---|---|---|---|---|---|---|
| dipping | √ | × | × | × | × | × | × |
| sputtering | ---- | ---- | ---- | ---- | ---- | √ | √ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Wang, B.; Zhong, Y.; Hinoki, T. Assessment of the Potential Diffusion Barriers between Tungsten and Silicon Carbide for Nuclear Fusion Application. Coatings 2022, 12, 639. https://doi.org/10.3390/coatings12050639
Du Y, Wang B, Zhong Y, Hinoki T. Assessment of the Potential Diffusion Barriers between Tungsten and Silicon Carbide for Nuclear Fusion Application. Coatings. 2022; 12(5):639. https://doi.org/10.3390/coatings12050639
Chicago/Turabian StyleDu, Yina, Baopu Wang, Yansong Zhong, and Tatsuya Hinoki. 2022. "Assessment of the Potential Diffusion Barriers between Tungsten and Silicon Carbide for Nuclear Fusion Application" Coatings 12, no. 5: 639. https://doi.org/10.3390/coatings12050639
APA StyleDu, Y., Wang, B., Zhong, Y., & Hinoki, T. (2022). Assessment of the Potential Diffusion Barriers between Tungsten and Silicon Carbide for Nuclear Fusion Application. Coatings, 12(5), 639. https://doi.org/10.3390/coatings12050639





