Effect of Edible Coating on the Quality and Antioxidant Enzymatic Activity of Postharvest Sweet Cherry (Prunusavium L.) during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Materials and Treatments
2.2. Extraction
2.3. Determination of Fruit Colour
2.4. Determination of Fruit Firmness
2.5. Determination of Respiration Rate
2.6. Determination of Antioxidant Contents
2.6.1. Total Phenol (TP) Content
2.6.2. Total Anthocyanin Content
2.7. Determination of Antioxidant Enzyme Activity
2.8. Statistical Analyses
3. Results and Discussion
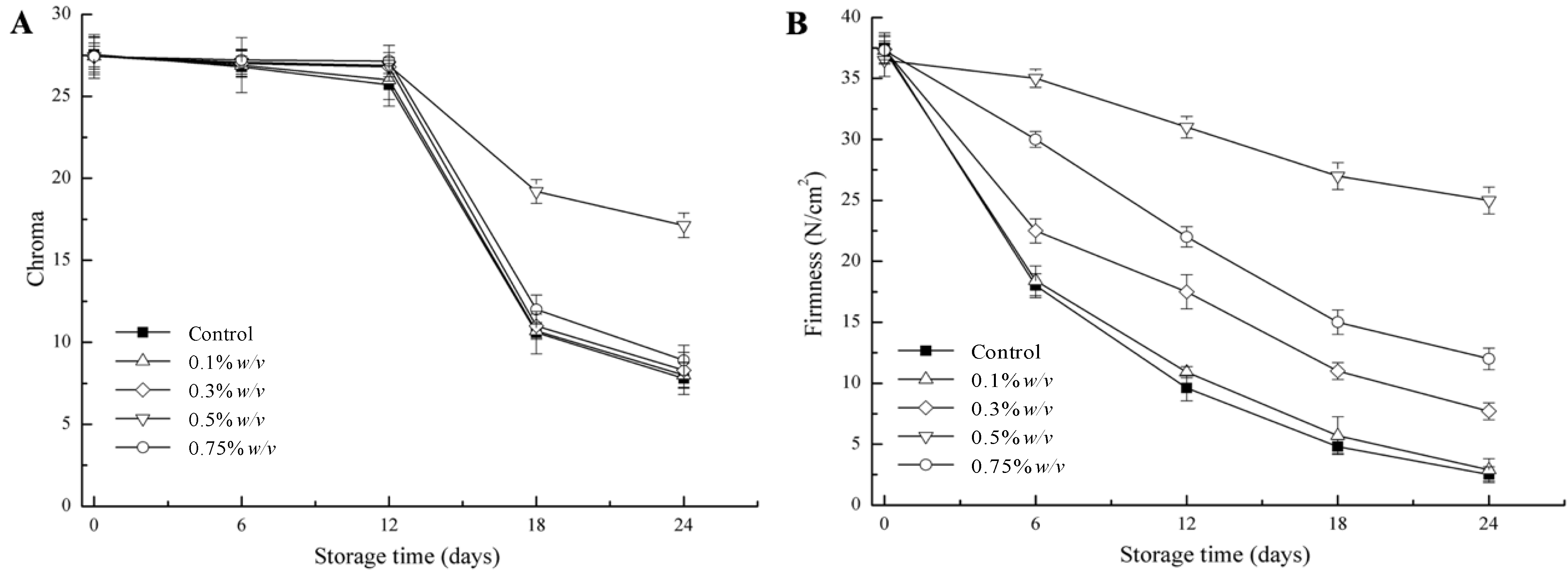
3.1. Colour and Firmness
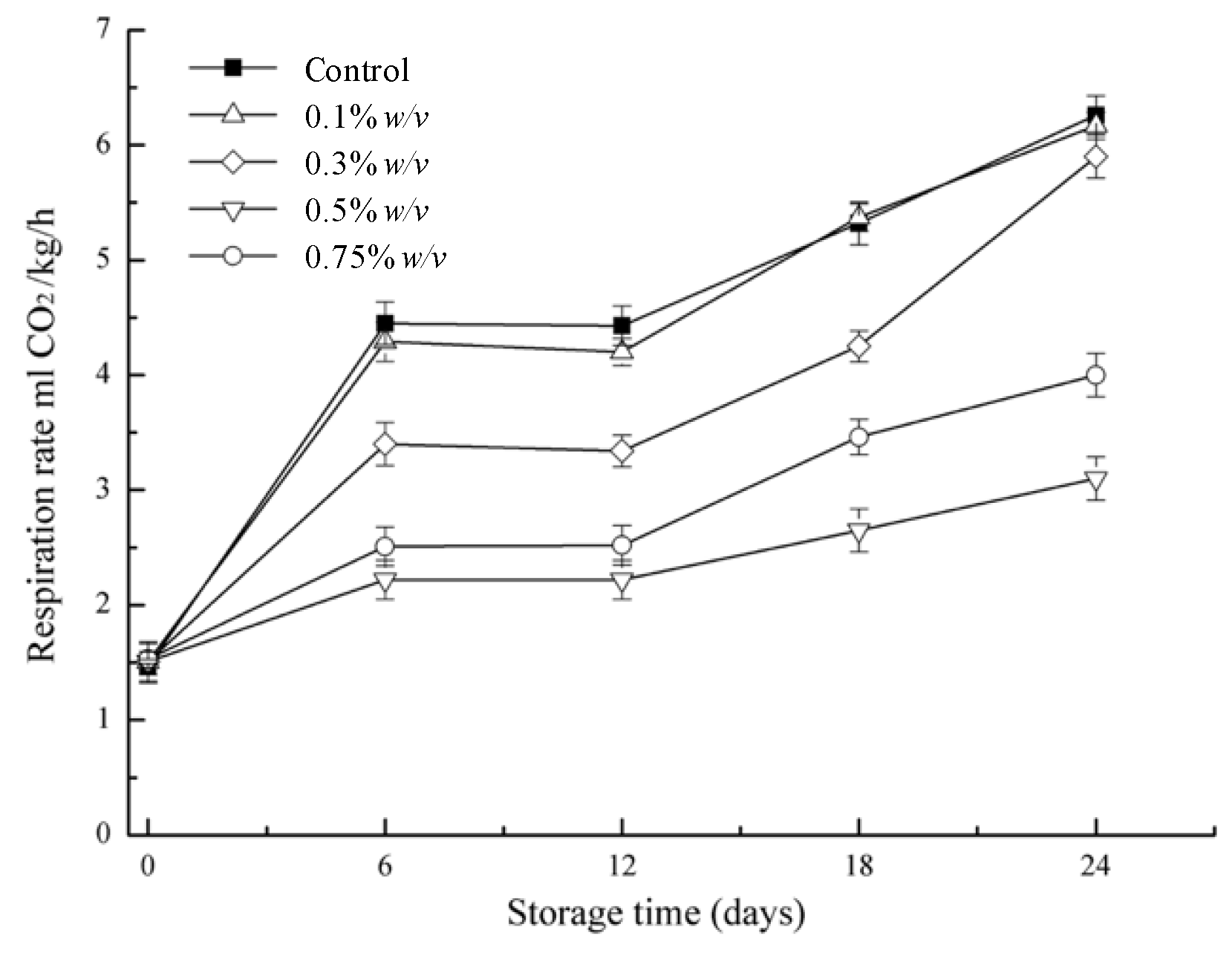
3.2. Respiration Rate
3.3. Effect on Phenolic and Anthocyanin Compounds
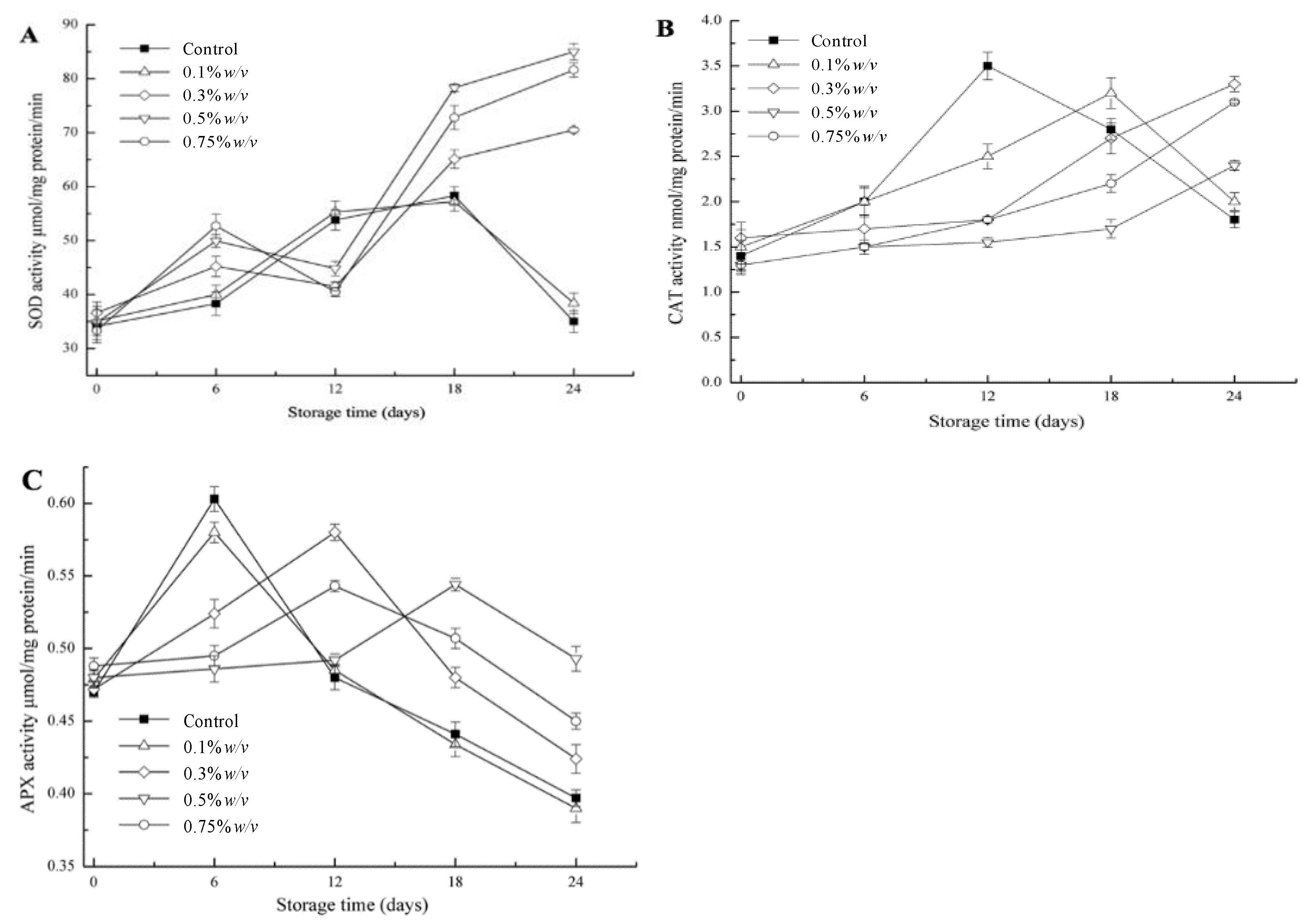
3.4. Antioxidant Enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, D.O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef]
- Michailidis, M.; Karagiannis, E.; Polychroniadou, C.; Tanou, G.; Karamanoli, K.; Molassiotis, A. Metabolic features underlying the response of sweet cherry fruit to postharvest UV-C irradiation. Plant Physiol. Biochem. 2019, 144, 49–57. [Google Scholar] [CrossRef]
- Bernalte, M.J.; Sabio, E.; Herna´ndez, M.T.; Gervasini, C. Influence of storage delay on quality of sweet cherry. Postharvest Biol. Technol. 2003, 28, 303–312. [Google Scholar] [CrossRef]
- Mirto, A.; Iannuzzi, F.; Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Fuggi, A. Metabolic characterization and antioxidant activity in sweet cherry (Prunus avium L.): Campania accessions Metabolic characterization of sweet cherry accessions. Food Chem. 2018, 240, 559–566. [Google Scholar] [CrossRef]
- Kokalj, D.; Zlatić, E.; Cigić, B.; Vidrih, R. Postharvest light-emitting diode irradiation of sweet cherries (Prunus avium L.) promotes accumulation of anthocyanins. Postharvest Biol. Technol. 2019, 148, 192–199. [Google Scholar] [CrossRef]
- Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D.; Serrano, M. Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. J. Sci. Food Agric. 2008, 88, 1287–1293. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, B.; Cui, K.; Cao, J.; Jiang, W. Improving postharvest quality and antioxidant capacity of sweet cherry fruit by storage at near-freezing temperature. Sci. Hortic. 2019, 246, 68–78. [Google Scholar] [CrossRef]
- Navarro-Tarazaga, M.L.; Sothornvit, R.; Pérez-Gago, M.B. Effect of plasticizer type and amount on hydroxypropyl methylcellulose-beeswax edible film properties and postharvest quality of coated plums (cv. Angeleno). J. Agric. Food Chem. 2008, 56, 9502–9509. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef]
- Tokatlı, K.; Demirdöven, A. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Sci. Hortic. 2020, 259, 108656. [Google Scholar] [CrossRef]
- Jeon, Y.I.; Kamil, J.Y.V.A.; Shahidi, F. Chitosan as an edible invisible film for quality preservation of herring and Atlantic cod. J. Agric. Food Chem. 2002, 20, 5167–5178. [Google Scholar] [CrossRef] [PubMed]
- Mohana, C.O.; Ravishankara, C.N.; Lalithab, K.V.; Srinivasa Gopalc, T.K. Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocoll. 2012, 26, 167–174. [Google Scholar] [CrossRef]
- Perdonesa, A.; Sánchez-Gonzáleza, L.; Chiralta, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.; Wang, J.; Zhao, Y.; Cao, J.; Jiang, W. Effects of chitosan coating on oxidative stress in bruised Yali pears (Pyrus bretschneideri Rehd.). Intern. J. Food Sci. Technol. 2010, 45, 2149–2154. [Google Scholar] [CrossRef]
- Petriccione, M.; De Sanctis, F.; Pasquariello, M.S.; Mastrobuoni, F.; Rega, P.; Scortichini, P.; Mencarelli, F. The effect of chitosan coating on the qualityand nutraceutical traits of sweet cherry during postharvest life. Food Bioprocess Technol. 2015, 8, 394–408. [Google Scholar] [CrossRef]
- Al-Said, F.A.; Opara, U.L.; Al-Yahyai, R.A. Physico-chemical and textural quality attributes of pomegranate cultivars (Punicagranatum L.) grown in the Sultanate of Oman. J. Food Eng. 2009, 90, 129–134. [Google Scholar] [CrossRef]
- Acero, N.; Gradillas, A.; Beltran, M.; García, A.; Mingarroc, D.M. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Schwartz, S.J., Eds.; John Wiley & Sons: New York, NY, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar]
- Duan, X.; Liu, T.; Zhang, D.; Su, X.; Lin, H.; Jiang, Y. Effect of pure oxygen atmosphere on antioxidant enzyme and antioxidant activity of harvested litchi fruit during storage. Food Res. Intern. 2011, 44, 1905–1911. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Karamanoli, K.; Lazaridou, A.; Minas, I.S.; Molassiotis, A. Postharvest responses of sweet cherry fruit and stem tissues revealed by metabolomic profiling. Plant Physiol. Biochem. 2018, 127, 478–484. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of ‘Brooks’ and ‘Bing’ cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Chen, C.; Hu, W.; He, Y.; Jiang, A.; Zhang, R. Effect of citric acid combined with UV-C on the quality of fresh-cut apples. Postharvest Biol. Technol. 2016, 111, 126–131. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Zapata, P.J.; Castillo, S.; Serrano, M. Postharvest methyl salicylate treatments delay ripening and maintain quality attributes and antioxidant compounds of ‘Early Lory’ sweet cherry. Postharvest Biol. Technol. 2016, 117, 102–109. [Google Scholar] [CrossRef]
- Ji, L.; Pang, J.; Li, S.; Xiong, B.; Cai, L.G. Application of new physical storage technology in fruit and vegetable industry. Afr. J. Biotechnol. 2012, 11, 6718–6722. [Google Scholar]
- Dong, F.; Wang, X. Guar gum and ginseng extract coatings maintain the quality of sweet cherry. LWT—Food Sci. Technol. 2018, 89, 117–122. [Google Scholar] [CrossRef]
- Mozetic, B.; Simcic, M.; Trebse, P. Anthocyanins and hydroxycinnamic acids of Lambert Compact cherries (Prunus avium L.) after cold storage and 1-methylcyclopropene treatment. Food Chem. 2006, 97, 302–309. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Kiprovski, B.; Borković, B.; Malenčić, Đ.; Veberič, R.; Štampar, F.; Mikulič-Petkovšek, M. Postharvest changes in primary and secondary metabolites of sweet cherry cultivars induced by Monilinia laxa. Postharvest Biol. Technol. 2018, 144, 46–54. [Google Scholar] [CrossRef]
- Liu, X.; Ji, D.; Cui, X.; Zhang, Z.; Li, B.; Xu, Y.; Chen, T.; Tian, S. p-Coumaric acid induces antioxidant capacity and defense responses of sweet cherry fruit to fungal pathogens. Postharvest Biol. Technol. 2020, 169, 111297. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, B.; Zhang, W.; Cao, J.; Jiang, W. Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage. Postharvest Biol. Technol. 2019, 147, 113–122. [Google Scholar] [CrossRef]
- Serrano, M.; Díaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Valverde, J.M.; Valero, D. Maturity stage at harvest determines the fruit quality and antioxidant potential after storage of sweet cherry cultivars. J. Agric. Food Chem. 2009, 57, 3240–3246. [Google Scholar] [CrossRef] [PubMed]
- Varasteh, F.; Arzani, K.; Barzegar, M.; Zamani, Z. Changes in anthocyanins in arils of chitosan-coated pomegranate (Punica granatum L. cv. Rabbab-e-Neyriz) fruit during cold storage. Food Chem. 2012, 130, 267–272. [Google Scholar] [CrossRef]
- El Ghaouth, A.; Ponnampalam, R.; Boulet, M. Chitosan coating effect on storability and quality of fresh strawberries. J. Food Sci. 1991, 56, 1618–1621. [Google Scholar] [CrossRef]
- Goncalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984. [Google Scholar] [CrossRef]
- Artes, F.; Tudela, J.A.; Villaescusa, R. Thermal postharvest treatments for improving pomegranate quality and shelf life. Postharvest Biol. Technol. 2000, 18, 245–251. [Google Scholar] [CrossRef]
- Holcroft, D.M.; Gil, M.I.; Kader, A.A. Effect of carbon dioxide on anthocyanins, phenylalanine ammonia lyase and glucosyltransferase in the arils of stored pomegranates. J. Am. Soc. Hortic. Sci. 1998, 123, 136–140. [Google Scholar] [CrossRef]
- Miguel, G.; Fontes, C.; Antunes, D.; Neves, A.; Martins, D. Anthocyanin concentration of ‘Assaria’ pomegranate fruits during different cold storage conditions. J. Biomed. Biotechnol. 2004, 5, 338–342. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Zhao, Y.; Leonard, S.W.; Traber, M.G. Edible coating to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria ananassa) and raspberries (Rubus ideaus). Postharvest Biol. Technol. 2004, 33, 67–78. [Google Scholar] [CrossRef]
- Azarabadi, S.; Abdollahi, H.; Torabi, M.; Salehi, Z.; Jaber, N. ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in response to Erwinia amylovora in pear (Pyrus communis L). Eur. J. Plant Pathol. 2017, 147, 279–294. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. Oxidative stress antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cisneros-Zevallos, L.; Jacobo-Velaźquez, D.A. Controlled abiotic stresses revisited: From homeostasis through hormesis to extreme stresses and the impact on nutraceuticals and quality during pre- and postharvest applications in horticultural crops. J. Agric. Food Chem. 2020, 68, 11877–11879. [Google Scholar] [CrossRef]
- Sharma, M.; Jacob, J.K.; Subramanian, J.; Paliyath, G. Hexanal and 1-MCP treatments for enhancing the shelf life and quality of sweet cherry (Prunus avium L.). Sci. Hortic. 2010, 125, 239–247. [Google Scholar] [CrossRef]
- Jin, P.; Shang, H.; Chen, J.; Hong, Z.; Zhao, Y.; Zheng, Y. Effect of 1-methylcyclopropene on chilling injury and quality of peach fruit during coldstorage. J. Food Sci. 2011, 76, 485–491. [Google Scholar] [CrossRef]
- Srednicka-Tober, D.; Ponder, A.; Hallmann, E.; Głowacka, A.; Rozpara, E.l.Z. The profile and content of polyphenols and carotenoids in local and commercial sweet cherry fruits (Prunus avium L.) and their antioxidant activity in vitro. Antioxidants 2019, 8, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racchi, M.L. Antioxidant defences in plants with attention to Prunus and Citrus spp. Antioxidants 2013, 2, 340–369. [Google Scholar] [CrossRef]
- Cao, J.; Tang, D.; Wang, Y.; Li, X.; Li, H.; Sun, C. Characteristics and immune-enhancing activity of pectic polysaccharides from sweet cherry (Prunus avium L). Food Chem. 2018, 25, 447–454. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Sarengaowa; Feng, K. Effect of Edible Coating on the Quality and Antioxidant Enzymatic Activity of Postharvest Sweet Cherry (Prunusavium L.) during Storage. Coatings 2022, 12, 581. https://doi.org/10.3390/coatings12050581
Hu W, Sarengaowa, Feng K. Effect of Edible Coating on the Quality and Antioxidant Enzymatic Activity of Postharvest Sweet Cherry (Prunusavium L.) during Storage. Coatings. 2022; 12(5):581. https://doi.org/10.3390/coatings12050581
Chicago/Turabian StyleHu, Wenzhong, Sarengaowa, and Ke Feng. 2022. "Effect of Edible Coating on the Quality and Antioxidant Enzymatic Activity of Postharvest Sweet Cherry (Prunusavium L.) during Storage" Coatings 12, no. 5: 581. https://doi.org/10.3390/coatings12050581
APA StyleHu, W., Sarengaowa, & Feng, K. (2022). Effect of Edible Coating on the Quality and Antioxidant Enzymatic Activity of Postharvest Sweet Cherry (Prunusavium L.) during Storage. Coatings, 12(5), 581. https://doi.org/10.3390/coatings12050581




