Study on AgCl/Al2O3 Catalyst Coating on Metal Workpiece Surface by Electrophoretic Deposition and Its Overall Catalytic Performance
Abstract
:1. Introduction
2. Materials and Methods
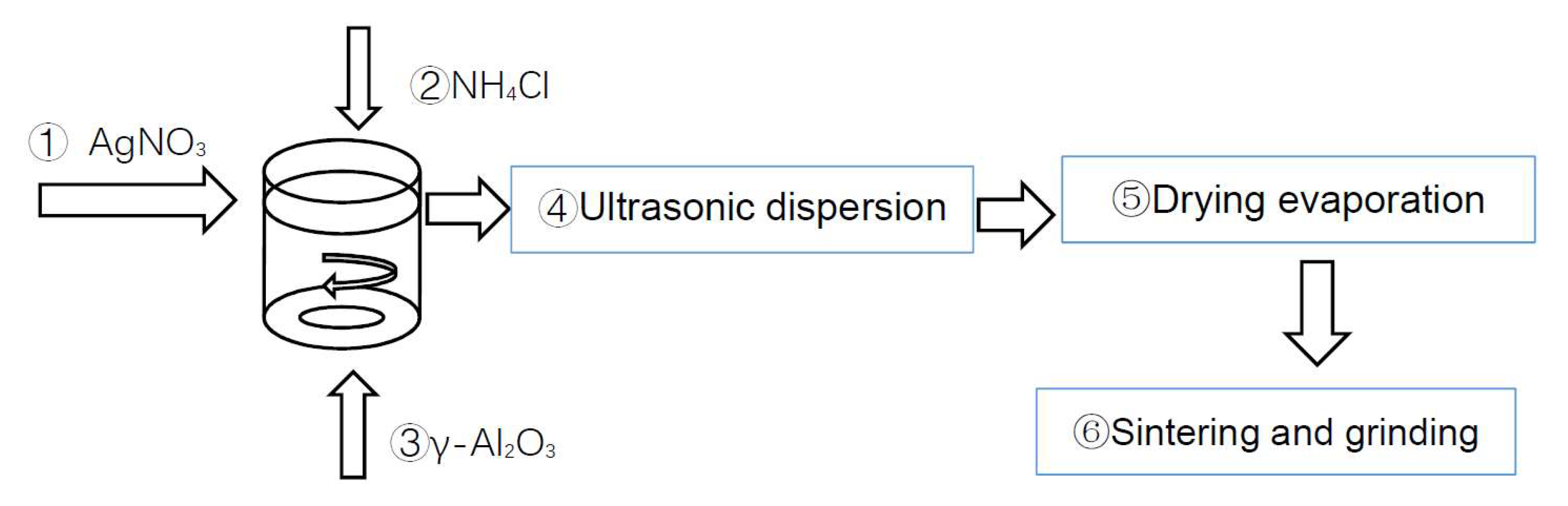
2.1. Preparation of Catalyst Materials
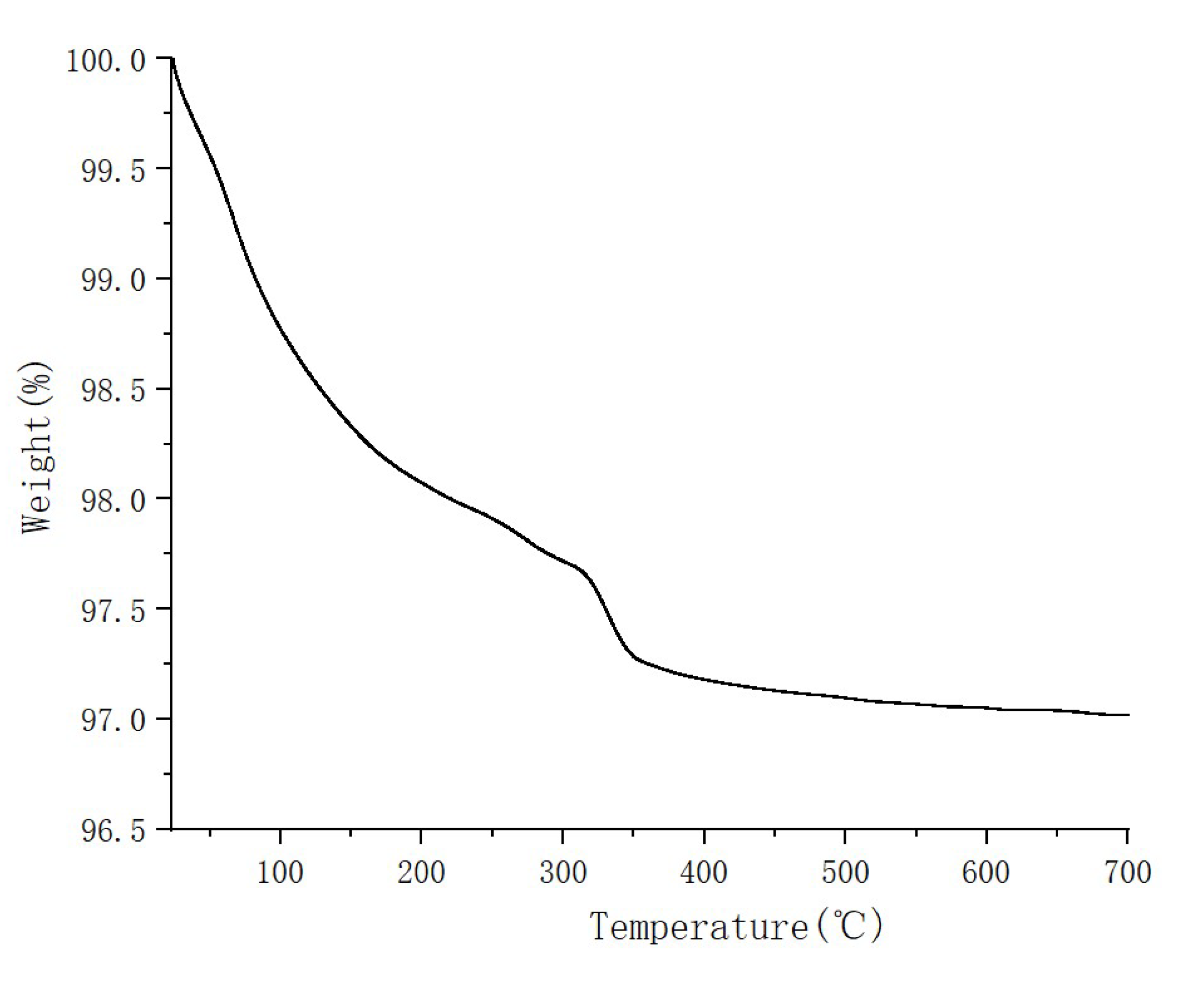
2.2. AgCl/Al2O3 Catalyst Material Performance Test
3. Electrophoretic Deposition Coating Experiment
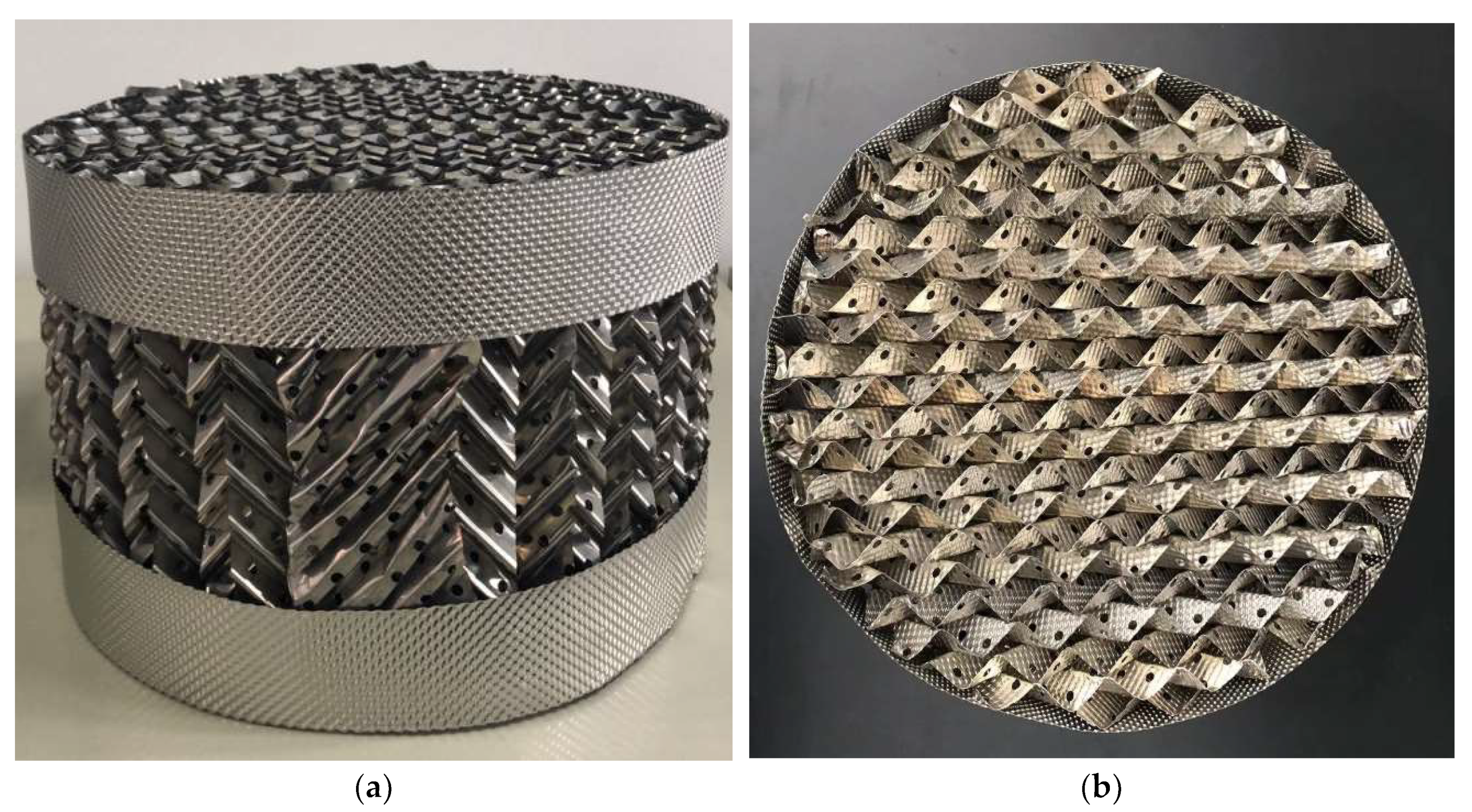
3.1. Design of Carrier Workpiece
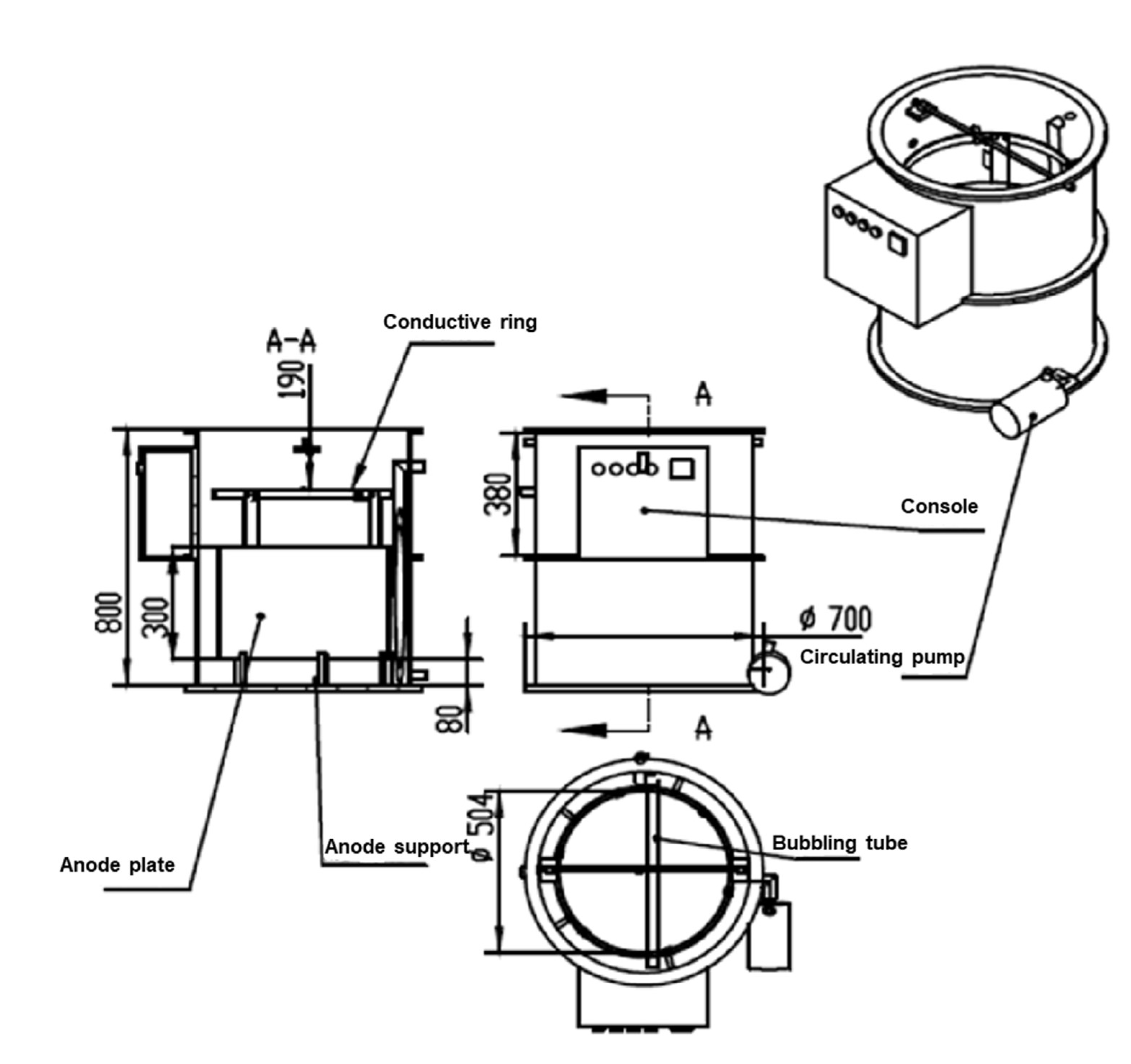
3.2. Design of Electrophoretic Deposition Device
3.3. Coating Experiment
- Water washing: place the workpiece to be deposited in the water washing bucket, wash it continuously with deionized water, and take it out after placing it in the water washing bucket for 30 min.
- Pickling: immerse the stainless-steel workpiece in the pickling solution. Set the temperature of the pickling solution at 20–30 °C, and use an immersion time of 50 min to 60 min.
- Water washing: take out the pickled workpiece, put it into the water washing bucket again for washing, wash off the residual acid washing solution on the surface, and take it out after putting it in the water washing bucket for 30 min.
- Use Al (NO3) 3 · 9H2O as binder to heat treat Al (NO3) 3 · 9H2O. After heating at 135 °C for 1 h, obtain 60 wt.% aluminum salt. Dissolve the aluminum salt after heat treatment in the mixed solution of deionized water and absolute ethanol (deionized water: ethanol = 1:1) and conduct ultrasonic dispersion at room temperature for 30 min until the material is completely dissolved.
- Preparation of deposition solution: mix ethanol and deionized water 1:1 into the deposition tank, and mix the deposition solution evenly using a gas stirring device and circulating device.
- Add the catalyst material into the deposition liquid, and evenly mix the deposition liquid using the gas stirring device and the circulating device.
- Connect the pretreated workpiece using a hanger and put into the deposition tank. Connect the cathode of the rectifier to the plating racks, and connect the anode of the rectifier to the anode plate. Open the bubbling device and circulating water device, measure the temperature and pH of the deposition solution before deposition, adjust the voltage to 120 V, perform electrophoretic deposition for a certain time, and end the experiment. Disconnect the power supply, and take the workpiece out of the electrophoretic deposition tank.
- Put the deposited workpiece into the drying oven, and take it out after drying at 130 °C for 1 h.
- Sintering: set the sintering temperature at 600 °C in the experiment, put the workpiece in the muffle furnace, set the program, raise the temperature to 600 °C, and set the heating time as 1 h. After sintering at 600 °C for 4 h, stop the system and take out the workpiece after the temperature drops to room temperature. This coating process is based on the specific conditions of this study [11,12].
3.4. Exhaust Purification Test Experiment
3.4.1. Design of Exhaust Purification Test System
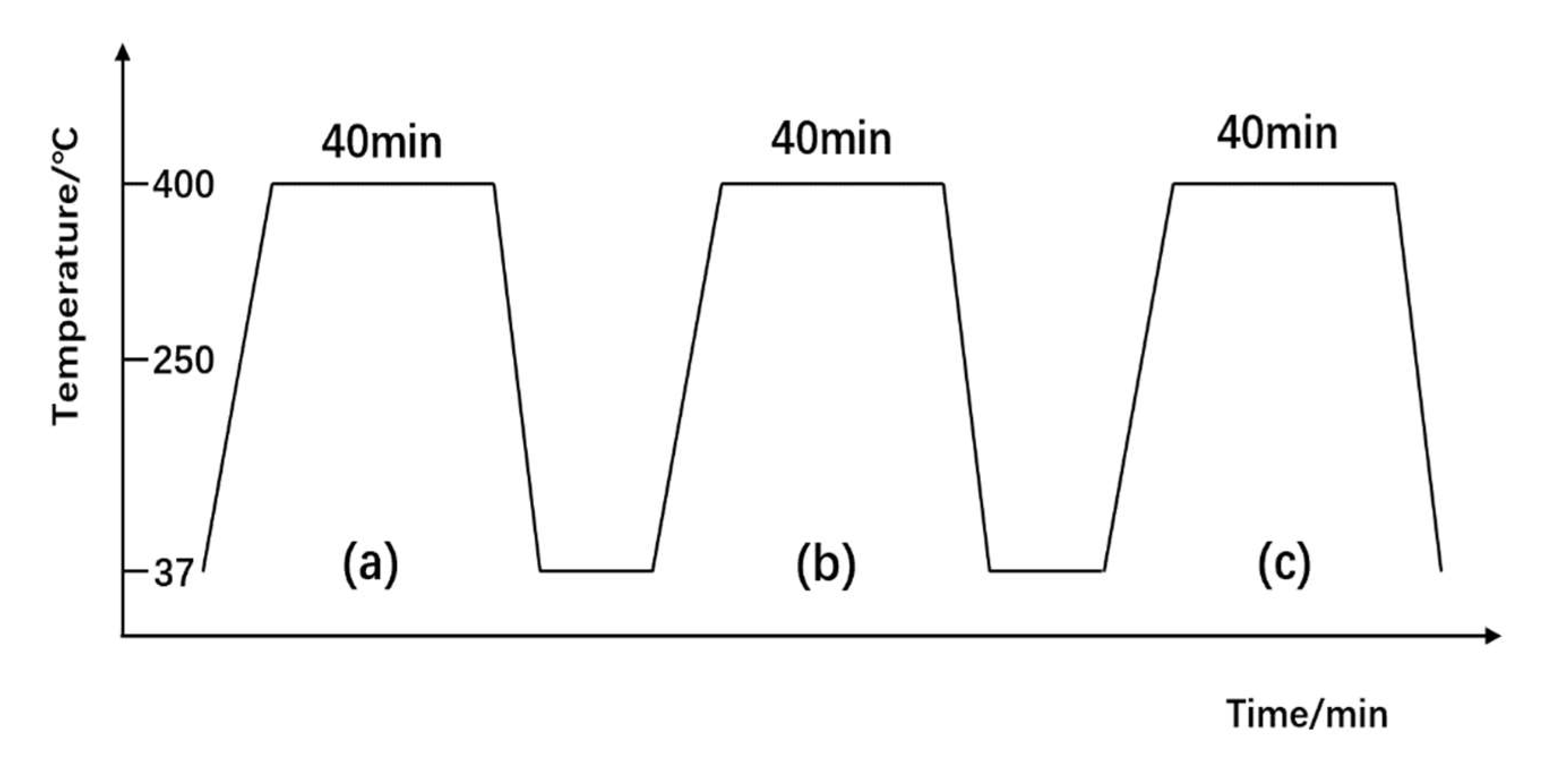
3.4.2. Experimental Process
4. Results and Discussion
4.1. Electrophoretic Deposition Experiment
4.2. Analysis of Test Results of Exhaust Purification
5. Conclusions
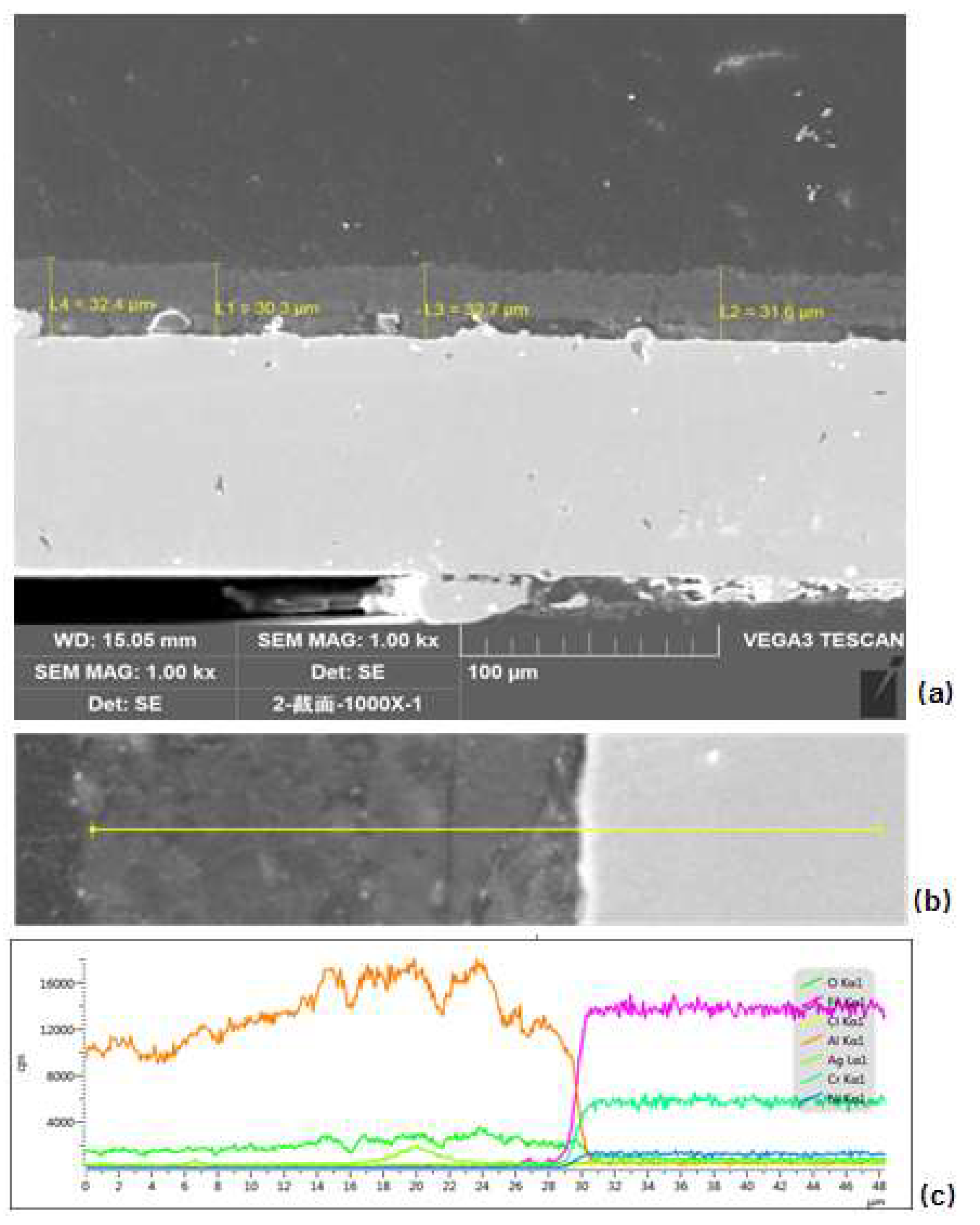
- After electrophoretic deposition, a AgCl/Al2O3 catalyst was successfully coated on a stainless-steel workpiece to make an integrated post-treatment device. After SEM observation, a uniform and dense catalyst coating was formed on the surface of the workpiece. By observing the cross section, the thickness of the catalyst coating was about 30 μm.
- Through the repeatability experiment of the integrated post-treatment workpiece, it is proved that the catalyst coating coated on the workpiece surface had good stability, the catalyst maintained high catalytic activity after multiple reactions, and the conversion efficiency could reach more than 70%, thus effectively converting NOx in the tail gas into nitrogen. This study provides a new idea for the development of a marine exhaust purification catalyst.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, N.K.; Majumder, C.B. Novel biofiltration methods for the treatment of heavy metals from industrial waste water. J. Hazard Mater. 2008, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; He, H.; Ma, Z.-C. Hydrogen promotes the selective catalytic reduction of NOx by ethanol over Ag/Al2O3. Catal. Commun. 2007, 2, 187–192. [Google Scholar] [CrossRef]
- He, H.; Yu, Y.-B. Selective catalytic reduction of NOx over Ag/Al2O3 catalyst: From reaction mechanism to diesel engine test. Catal. Today 2005, 100, 37–47. [Google Scholar] [CrossRef]
- Yu, Y.-B.; He, H.; Feng, Q. Novel enolic surface species formed during partial oxidation of CH3CHO, C2H5OH, and C3H6 on Ag/Al2O3: An in situ DRIFTS study. J. Phys. Chem. B. 2003, 47, 13090–13092. [Google Scholar] [CrossRef]
- Yoshida, Q.K. Characterization of an AgCl/Al2O3 catalyst for lean NO conversion. Phys. Chem. Chem. Phys. 1999, 14, 3365–3367. [Google Scholar]
- Yu, Y.-M.; Sato, S.; Zhou, W.-M.; Tian, H.; Ju, D.-Y. Study on the Effect of Different Preparation Methods on the Performance of AgCl/Al2O3 Catalyst Used for Diesel Exhaust Treatment. J. Thermodyn. Catal. 2020, 11, 208. [Google Scholar]
- Rehman, M.A.U.; Bastan, F.E.; Haider, B.; Boccaccini, A.R. Electrophoretic deposition of PEEK/bioactive glass composite coatings for orthopedicimplants: A design of experiments (DOE) study. J. Mater. Des. 2017, 130, 223–230. [Google Scholar] [CrossRef]
- Kahradeh, K.H.; Saievar-Iranizad, E.; Bayat, A. Electrophoretically deposited carbon micro and nanospheres thin films as superhydrophobic coatings. J. Surf. Coat. Technol. 2017, 319, 318–325. [Google Scholar] [CrossRef]
- Hafedh, D.; Nader, B.-J.; Florica, S.-L.; Joel, F.; Larbi, A.B.C.; Hicham, B. Effect of annealing temperature on the structural and mechanical properties of coatings prepared by electrophoretic deposition of TiO2 nano particles. J. Thin Solid Film. 2017, 638, 201–212. [Google Scholar]
- Zhao, W.-T.; Shi, J.-L.; Guo, Q.-G.; Song, J.-R.; Liu, L. SiC coating by electrophoretic deposition on graphite substrate. J. Space Mater. Technol. 2008, 4, 51–54. [Google Scholar]
- Sun, H.; Quan, X.; Chen, S.; Zhao, H.; Zhao, Y. Preparation of well-adhered γ-Al2O3 washcoat on metallic wire mesh monoliths by electrophoretic deposition. Appl. Surf. Sci. 2007, 253, 3303–3310. [Google Scholar] [CrossRef]
- Fan, X.-D.; Liang, H.; Xu, M.-X.; Xu, T.-X. Study on electrophoretic deposition processing of Al2O3 microporous membranes. J. Tianjin Univ. 2000, 33, 663–664. [Google Scholar]
- Wang, Z.-J.; Xu, G.-Y.; Liu, X.; Yu, Y.-B.; He, H. Investigation of Water and Sulfur Tolerance of Precipitable Silver Compound Ag/Al2O3 Catalysts in H2 -Assisted C3H6-SCR of NOx. J. ACS Omega 2020, 5, 29593–29600. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Ding, Y.; Ju, D. Study on AgCl/Al2O3 Catalyst Coating on Metal Workpiece Surface by Electrophoretic Deposition and Its Overall Catalytic Performance. Coatings 2022, 12, 553. https://doi.org/10.3390/coatings12050553
Yu Y, Ding Y, Ju D. Study on AgCl/Al2O3 Catalyst Coating on Metal Workpiece Surface by Electrophoretic Deposition and Its Overall Catalytic Performance. Coatings. 2022; 12(5):553. https://doi.org/10.3390/coatings12050553
Chicago/Turabian StyleYu, Yimin, Yunlong Ding, and Dongying Ju. 2022. "Study on AgCl/Al2O3 Catalyst Coating on Metal Workpiece Surface by Electrophoretic Deposition and Its Overall Catalytic Performance" Coatings 12, no. 5: 553. https://doi.org/10.3390/coatings12050553
APA StyleYu, Y., Ding, Y., & Ju, D. (2022). Study on AgCl/Al2O3 Catalyst Coating on Metal Workpiece Surface by Electrophoretic Deposition and Its Overall Catalytic Performance. Coatings, 12(5), 553. https://doi.org/10.3390/coatings12050553





