Abstract
Background: This study aimed to evaluate the effectiveness of an experimental bioactive enamel resin sealer in protecting the enamel adjacent to orthodontic brackets against erosion. Methods: Orthodontic brackets (n = 50) were bonded to freshly extracted, sound maxillary premolars using Transbond™ XT Primer (3M Unitek, Monrovia, CA, USA) and Transbond Plus Color Change adhesive (3M Unitek, USA). Five experimental groups (n = 10) had the following treatments applied: a resin bioactive sealer with 45S5 bioglass, 35% by weight; a resin sealer without bioactive glass; fluoride; the orthodontic sealer, Opal Seal (Opal-Orthodontics, South Jordan, UT, USA); and, in the control group, an untreated surface. All the specimens were stored for 18 min in 1% citric acid. All the specimens were examined by SEM and electron dispersive spectroscopy (EDS). The Wilcoxon signed-rank test was used to compare the enamel surfaces covered by the sealers before and after the acid challenge. Attenuated total reflectance Fourier transform infrared spectroscopy detected the degree of the experimental resins’ conversion to verify their suitability for clinical use. Results: The percentage of the bioactive resin sealer and Opal Seal groups’ protection against enamel erosion was 100%, which was significantly more than the other groups, p < 0.05. The degree of conversion for the bioactive and unfilled resins was 42.4% ± 3.6% and 48.57% ± 5%, respectively. Conclusion: The bioactive resin sealer and the Opal Seal both protected the enamel from erosion.
1. Introduction
Dental enamel is a highly mineralized tissue, in which the inorganic components comprise more than 98%; however, having faulty dietary habits increases the risk of exposing the enamel to dietary acidic erosion [1]. Orthodontic patients who suffer from these erosive challenges may suffer from multiple dental complications that could affect the progress of their orthodontic treatment plans, because these patients are at a high risk of developing caries [2,3] around their orthodontic brackets [4,5,6,7]. The consumption of soft drinks that have a low pH [8] may convert the incipient enamel lesions into erosive lesions that necessitate restorative treatment, rather than remineralization [7,8].
The literature shows that the protection of enamel next to orthodontic brackets is of prime importance [7,9,10,11,12]; however, there is still some controversy regarding the technique that should be followed to achieve optimum protection for enamel.
Cohen et al. [13] showed that using fluoride-releasing orthodontic bonding systems to bond orthodontic brackets to the enamel protected the enamel surrounding the orthodontic brackets against bacterial attack [13]; however, a clinical study showed that orthodontic brackets bonded to the tooth structure by fluoride- releasing agents did not show significant enamel protection [14].
Several strategies were employed to protect enamel surfaces, such as the use of nano particles to decrease the activity of the cariogenic biofilm that formed on the enamel surfaces in orthodontic patients [15,16,17]. Other strategies included the following: increasing the acid resistance and remineralizing the enamel by means of casein phosphopeptide- amorphous calcium phosphate [11], and by using a high concentration of chlorhexidine to diminish the high levels of streptococcus mutants in the orthodontic patients’ saliva [3]. Moreover, the application of probiotics, polyols, and resin infiltration showed promising results [15,16,17].
Professor Larry Hench introduced 45S5 bioglass in the late 1960s [18] to improve the biocompatibility of artificial protheses implanted in the patients’ bodies. This material had the ability to produce a rich layer of calcium and phosphate that was capable of bonding to various biologic tissues [18].
Recently, many studies have shown the possibility of using 45S5 bioglass to treat dental lesions [7,19,20,21]. Moreover, 45S5 bioglass showed its biocompatibility with dental pulp cells [22] and proved its resistance to brushing abrasion when applied onto tooth structures [23]. Additionally, previous research has shown the promising results of incorporating 45S5 particles in resin blends, as part of the restorative dental phase [24].
Previous research has shown the remineralization capacity of commercially available bonding systems doped with >10% bioactive glass [25]. Other reports demonstrated the capability of >5% fluoride-containing bioactive glass resins to be used as orthodontic primers [26]. Moreover, bioactive acidic (resin- free) pastes were utilized as orthodontic sealers [12]. However, in the current study, a (fluoride-free) bioactive glass–resin blend, with a 30% weight filler load, was tested. Moreover, a customized resin blend was utilized to determine the interaction of the 45S5 bioglass with each component. Doping commercially available adhesive systems may pose some difficulties in determining the exact interaction of the added bioactive glasses with their components, because many of the commercially available adhesive systems’ exact weight percentages are not fully described by the manufacturers.
The current study tested the suitability of a resin blend, fortified by 45S5 bioactive glass particles, as an orthodontic sealer, and compared it to a commercially available orthodontic sealer and fluoride application. The null hypothesis of the current study was that the tested enamel sealers would not protect the enamel surface from an erosive challenge.
2. Materials and Methods
Figure 1 summarizes the procedures conducted in the current study.
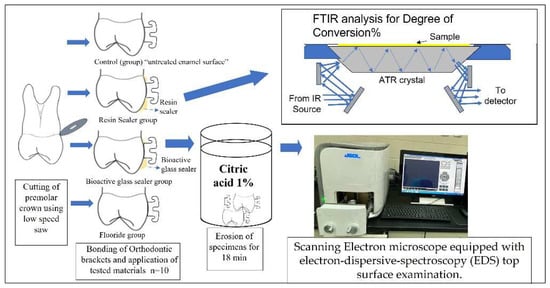
Figure 1.
Summary of the experimental procedures. Control group had untreated enamel surfaces.
2.1. Synthesis of Bioactive Orthodontic Sealer
The 45S5 bioactive glass powder was produced from 46.1 mol% SiO2, 26.9 mol% CaO, 24.4 mol% Na2O, and 2.5 mol% P2O5, according to the method described in a previous report [21]. A resin blend (see Table 1) was mixed together using a magnetic stirrer (Cimarec, A.J.cope, West Thurrock, UK) for 2 min and stored in a sealed dark container. The 45S5 bioglass was stirred with the resin blend until it was fully incorporated. The whole mixture was ultrasonicated in an ultrasonic bath for two minutes. The control group’s resin blend was prepared from the same chemicals, without adding the 45S5 bioglass.

Table 1.
Materials used in this study.
2.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (FT-IR/ATR) Analysis for the Utilized Resin Systems
FTIR/ATR (Figure 1) was used to detect the degree of conversion of the experimental resin sealers. An FT-IR spectrometer (Nicolet iS5 FT-IR, Thermo Electron Scientific Instruments LLC, Madison, WI, USA), equipped with an ATR attachment with multiple reflections, was employed to detect the spectra of the resins before and after light cure polymerization. The light curing unit was installed at a constant distance from the sample. A drop of each resin system (n = 5) was dispensed onto the diamond crystal, followed by light curing for 30 s by a calibrated light-emitting diode (LITEX 695, 1200 mW/cm2, Den-tamerica, San Jose Ave, CA, USA). All FTIR spectra were detected under standardized conditions [25]. The uncured resin in each group served as the control for the cured resin. The peak height was measured from the top of the peak to the baseline, utilizing a specific software installed by the FTIR spectrophotometer manufacturer. The degree of conversion (DC%) was recorded after applying the resin systems, both with and without the bioactive glass, polymerized and unpolymerized directly on the FTIR/ATR crystal. The ratio of the absorbance peak of the aliphatic carbon–carbon double bond was determined by measuring the 1637 cm−1 peak height and comparing it with the measurement of the 1720 cm−1 peak height (Figure 2).
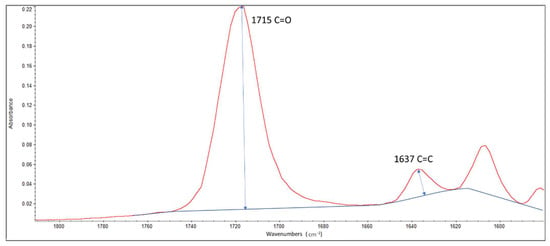
Figure 2.
The FTIR/ATR peaks employed in detecting the degree of conversion for the experimental sealers. The ratio of the absorbance peak at 1637 cm−1 peak height was compared to the peak measured at 1720 cm−1.
2.3. Specimen Preparation
The extracted sound human premolars were supplied upon receiving the necessary approval documents from the research ethical committee of the faculty. The total number of teeth obtained was forty. The teeth were extracted in the oral surgery department and immediately stored in 0.1% thymol for a maximum period of one week, until the start of the experiment, according to the guidelines approved by the university (approval code 055-02-20), and in accordance with the principles of the Declaration of Helsinki and its later amendments or comparable ethical standards. The sample size of the experimental groups was selected according to previous published articles and according to the threshold for significance, which was set at 0.05. Additionally, the sample size was verified from a previous pilot study and the power of test was set at 80%. Randomization of the specimens was performed and all teeth were examined by a light microscope to exclude any teeth with cracks, restorations, demineralization, or any other defects. Intra-examiner calibration was conducted before the results were recorded.
2.4. Bracket Bonding
The teeth had bonding sites on their buccal surface, etched with 37% phosphoric acid, followed by the application of Transbond™ XT Primer (3M Unitek, USA), according to the manufacturer’s instructions. A Transbond PLUS color-change adhesive (3M Unitek) was applied on the base of the orthodontic brackets (Unitek™ Gemini Metal Brackets, 3M Unitek) and the brackets were then bonded to the enamel of the teeth. All of the excess materials were carefully removed under magnification and then light cured using an LED curing unit, according to the manufacturer’s recommendations.
2.5. Tested Material Application
All the specimens’ enamel surfaces had a treatment window of 2 mm surrounding the orthodontic brackets. The treatment window was created by masking the whole specimen’s surfaces with nail varnish, leaving the treatment window exposed to the various experimental procedures. All specimens were divided into four equal groups: the first group (BGRS) had a bioactive glass sealer applied to their surfaces; the second group (RS) had resin sealer (the same resin composition of the bioactive sealer, but devoid of bioactive glass) applied; the third group (fluoride) had fluoride (Nupro® Acidulated Phosphate Fluoride, Dentsply, Germany) applied; the fourth group had Opal Seal orthodontic enamel sealer applied (Opal Orthodontics, South Jordan, UT, USA); and the control group had nothing applied to the enamel and served as a negative control. A summary of the materials utilized in this study is available in Table 1.
2.6. Erosion Challenge
Five specimens from each group were exposed to an erosive acidic challenge by storing the specimens in a dark sealed container containing 50 mL of 1% citric acid, with pH 2.1. The specimens and the acidic solution were continuously stirred using a magnetic stirrer for 18 min at room temperature [27].
2.7. Scanning Electron Microscope, Equipped with Electron Dispersive Spectroscopy (EDS), Top Surface Examination
The specimens were examined before and after exposure to the citric acid solution using the SEM (JCM-6000 NeoScope, Jeol, Akishima, Japan). The treatment window was divided into 8 sections (A–H), as shown in Figure 3, and the areas of the sections were calculated. With the aid of landmarks placed on the brackets, the boxes were checked under the SEM. The ratio between the areas showing intact enamel surfaces or surfaces covered with resin were compared to the total surface area of the experimental window. After the erosion challenge, the percentage of intact enamel area was used to evaluate the protective potential of the applied agents against the erosion challenge. Where areas showed enamel erosion, it meant that the applied protective agent had been dissolved and had exposed the underlying enamel to erosion.
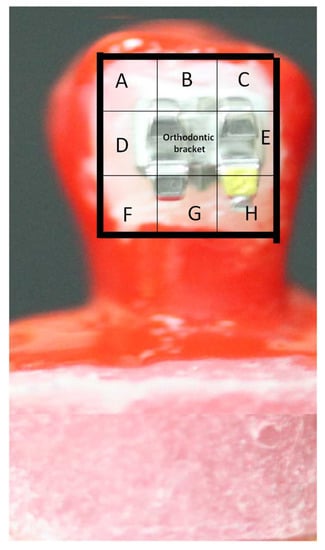
Figure 3.
The treatment window was divided into 8 sections (A–H) and the areas of the sections were calculated. With the aid of landmarks placed on the bracket, the boxes were checked under the SEM. After the acidic challenge, the areas that were covered with resin were considered protected by the resin and resistant to the erosion challenge.
All specimens were stored in ethanol solutions with different concentrations, varying from 50 to 100%, to render the specimens dehydrated. The specimens’ surfaces were gold coated to render them conductive and suitable for SEM observation. Chemical characterization of the specimens’ surfaces was conducted using EDS.
2.8. Statistical Analysis
The enamel surfaces’ protection percentage for all groups was compared using the Wilcoxon signed-rank test [28] before and after exposure to the erosive challenge. Finding no signs of erosion of the enamel surrounding the orthodontic brackets (after the erosive challenge) was considered to show 100% protection of the enamel. The Kruskal–Wallis test was utilized to compare the degree of protection among the groups. Statistically significant differences between the groups were detected at the level of 0.05. The degree of conversion readings for the experimental resins were obtained. SPSS (v24, IBM, Armonk, NY, USA) was employed to conduct the statistical analysis process.
3. Results
3.1. Degree of Conversion
The bioactive resin sealer’s degree of conversion was 42.4% ± 3.6%, and for the resin sealer, this was 48.57% ± 5%.
3.2. Scanning Electron Microscope, Equipped with Electron Dispersive Spectroscopy (EDS), Top Surface Examination Results
The enamel surface that was not treated by any bioactive glass (control group) before exposure to the erosion challenge revealed a smooth surface (Figure 4A,B). The control group (Figure 4C,D), where the enamel was exposed to erosion, revealed a surface in which the enamel prisms’ borders were clearly observed. The RS group, with resin-treated specimens, showed that the whole surface was covered by a homogenous layer of resin (Figure 5A,B) before exposure to the acid challenge; however, the resin layer was mostly disintegrated by the action of the acid challenge (Figure 5C,D). The BGRS group, treated with the bioactive glass resin, showed a homogenous layer of bioglass particles embedded in the resin matrix (Figure 6A,B) before the erosion challenge and after exposure to the acid challenge (Figure 6C,D). The enamel surfaces in the fluoride group, before exposure to the erosion challenge (Figure 7A,B), showed a smooth enamel surface; however, after exposure to erosion, the borders of the enamel prisms were clearly observed (Figure 7C,D).
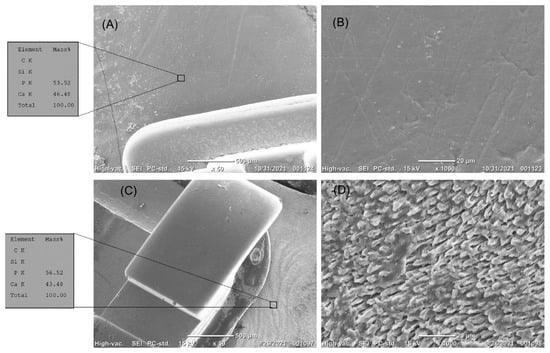
Figure 4.
(A–D) The top surface of intact enamel before erosion. (A) The enamel surface is smooth. EDS analysis showed a high content of calcium and phosphate. (B) High magnification of the enamel surface showed the smooth surface of the enamel. (C,D) The top surface of treated enamel next to the orthodontic brackets after erosion. (C) The enamel surface did not resist erosion. EDS analysis showed a high content of calcium and phosphate. (D) High magnification showed the eroded enamel prisms.
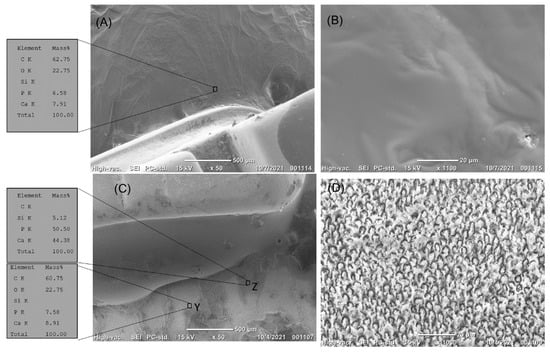
Figure 5.
(A–D) The top surface of enamel treated by resin sealer next to the orthodontic brackets before erosion. (A) The enamel surface was covered with the resin sealer. EDS analysis showed a high content of carbon, with calcium and phosphate content hardly detected. (B) High magnification of the treated enamel surface showed complete coverage of the enamel surface by the bioactive resin, with evidence of the dispersion of the bioactive particles in the resin matrix. (C,D) The top surface of enamel treated by resin sealer next to the orthodontic brackets after erosion. (C) The resin sealer did not resist erosion, as 50% of the surface was still covered by the sealer. EDS analysis showed a high content of carbon in areas of the resin sealer (area Z), while areas devoid of resin sealer (area Y) had a high content of calcium and phosphate. (D) High magnification of area Y showed erosion of enamel.
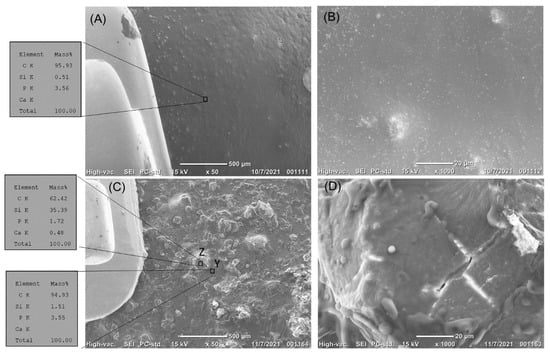
Figure 6.
(A–D) The top surface of the enamel treated by bioactive glass resin sealer next to the orthodontic brackets before erosion. (A) The enamel surface is covered with the bioactive glass resin sealer. EDS analysis showed a high content of carbon, with the calcium and phosphate content hardly detected. (B) High magnification of the treated enamel surface showed complete coverage of the enamel surface by the bioactive resin, with evidence of the dispersion of the bioactive particles in the resin matrix. (C,D) The top surface of the enamel treated by bioactive glass resin sealer next to the orthodontic brackets after erosion. (C) The bioactive glass resin sealer resisted erosion. EDS analysis showed a high content of silica in areas of the bioactive glass dispersion (area Z), while areas of the resin matrix had carbon peaks clearly detected (area Y), with the calcium and phosphate content hardly detected. (D) High magnification of area Z showed silica-filler top surface.
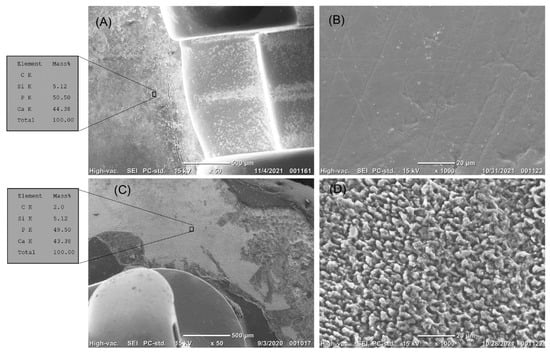
Figure 7.
(A,B) The top surface of enamel treated by fluoride next to the orthodontic brackets before erosion. (A) The enamel surface was smooth. EDS analysis showed a high content of calcium and phosphate. (B) High magnification of the treated enamel surface showed the smooth surface of the enamel. (C,D) The top surface of the enamel treated by fluoride next to the orthodontic brackets after erosion. (C) The enamel surface treated with fluoride did not resist erosion. EDS analysis showed a high content of calcium and phosphate. (D) High magnification showed the eroded enamel prisms.
The enamel surfaces in the Opal Seal group showed a homogenous layer of resin (Figure 8A,B) before the erosion challenge and after exposure to the acid challenge (Figure 8C,D).
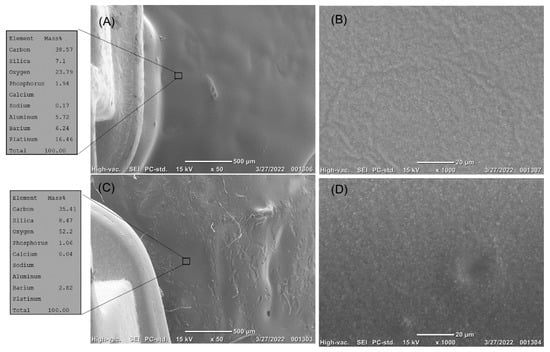
Figure 8.
(A,B) The top surface of the enamel treated by Opal Seal sealer next to the orthodontic brackets before erosion. (A) The enamel surface is covered with the Opal Seal. EDS analysis showed a high content of carbon, with the calcium and phosphorus content hardly detected. (B) High magnification of the treated enamel surface showed complete coverage of the enamel surface by the Opal Seal resin, with evidence of the dispersion of the filler particles in the resin matrix. (C,D) The top surface of enamel treated by Opal Seal next to the orthodontic brackets after erosion. (C) The sealer resisted erosion. EDS analysis showed a high content of carbon, with nearly the same content of silica. (D) High magnification showed that the filler content was more clearly observed.
The erosion challenge did not have a significant effect on the bioglass resin sealer and Opal Seal groups, p > 0.05; Table 2 however, the erosion challenge significantly affected the enamel surface in the rest of the groups, p < 0.05.

Table 2.
Protective potential of the tested agents.
4. Discussion
The adopted null hypothesis was rejected. The application of the bioactive resin sealer and Opal Seal protected the enamel from the erosive challenge.
In the current experiment, the specimens were eroded by citric acid because of its abundance in various fruit juices [29] and soft drinks [29].
The unfilled resin coating material utilized in the current experiment disintegrated, due to its storage in the citric acid [30], causing the underlying enamel to be exposed to demineralization.
The same enamel features were observed in the samples treated with fluoride, because fluoride has a high affinity to bind to the superficial layers of enamel, forming acid-resistant fluorapatite compounds [31,32]. It seems that the acidic erosive challenge applied in the current experiment was too strong, meaning that it caused complete dissolution of the outer fluorapatite-formed layer.
The untreated enamel surfaces also showed complete disintegration of the outer enamel layer and exposure of the enamel rods’ peripheries, which emphasizes the importance of protecting the enamel surfaces next to orthodontic brackets, as they are vulnerable to dissolution, due to their exposure to acidic attacks [3].
The specimens protected by the bioactive resin sealer showed complete coverage of the enamel surface before exposure to erosion, which indicates the suitability of the suggested resin for wetting the enamel surface without causing any problems related to viscosity. Moreover, the degree of conversion of the tested resin sealers in the current experiment was acceptable [33]. However, these values for the degree of conversion are relatively low, compared to the degree of conversion values obtained for the commercially available resin sealers, which may be attributed to the inhibitory effect exerted by the 45S5 bioactive glass on the BIS-GMA-based resins [24]. This may have been improved if different resin systems were utilized [24]. Moreover, the degree of conversion test was conducted in the current study at room temperature (20 °C), and was not exposed to any post-cure temperatures [24]. The SEM pictures could hardly identify the 45S5 bioglass particles embedded in the resin matrix before the acidic challenge; however, upon eroding the bioactive resin sealer, the filler particles became evident by using the SEM, which may be attributed to the dissolution of parts of the resin matrix. The bioactive resin tested in this study had 45S5 bioactive particles dispersed in the resin matrix without silanization, which might have rendered the 45S5 particles easier to interact with the surrounding storage media if the resin matrix had disintegrated. It may be hypothesized that the protective effect exerted by the bioactive resin sealer utilized in this study can be explained as follows: The bioactive resin sealer’s resin matrix was eroded by the citric acid [30], and, thus, some of the 45S5 fillers were exposed to the aqueous citric acid media, resulting in the formation of a layer on top of the glass particles, which was rich in silica [7,9,21], and in the formation of a layer on top that was rich in calcium and phosphate [7,9,21]. This layer of calcium and phosphate formed a barrier of acid-resistant crystals on top of the enamel surface (Figure 9). However, it could be suggested that parts of this calcium–phosphate-rich layer might have dissolved in the low pH acidity of the citric acid solution, releasing some of the calcium and phosphate ions from the bioglass particles into the surrounding acidic medium. The ions released from the bioactive resin, which were mainly calcium and phosphate ions, might have changed the chemical composition of the erosive solution [34], leading to a decrease in its erosive action [1,34]. The aforementioned hypothesis can be supported by the EDS analysis that was conducted in the current study, which showed that the average weight percentage of multiple areas of the bioactive resin (especially in areas showing the projection of fillers from the resin matrix) was 36% (with a detectable amount of phosphorus and calcium); while, for the Opal Seal group, no signs of filler projection were noted and the silica’s average weight percentage was approximately 9%. It is worth mentioning here that Opal Seal is an orthodontic enamel sealer, which is 38% filled with glass ionomer and nanofillers, while the bioactive resin sealer examined in the current study is 35% filled with 45S5 glass.
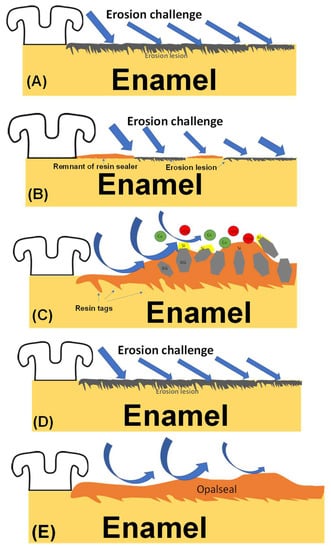
Figure 9.
(A–D) Suggested mechanisms for the tested groups. (A) Untreated enamel surface exposed to erosion challenge suffered from erosion of the enamel surface. (B) Resin sealer devoid of bioactive particles was dislodged from 50% of the total surface area, exposing areas of enamel that were eroded. (C) When the salivary pH drops, due to the consumption of erosive drinks, the bioactive sealer will have parts of its resin matrix eroded by the erosive solution, exposing parts of the bioactive fillers that had silica forming on the surface. The silica-rich layer will have calcium and phosphate compounds forming on top of it (as was described by the theory introduced by Professor Larry Hench). The calcium and phosphate ions will be released into the low pH saliva, causing a decrease in its dental erosion potential. (D) Fluoride-treated enamel surfaces showed erosion of the enamel surfaces after the erosive challenge. (E) The enamel surfaces treated with Opal Seal showed protection of the enamel surface after erosion.
The technique used in this study, of applying the 45S5 bioglass, has some advantages over previous techniques [7,9,20,21] that utilized an acidic paste, based on 45S5 bioglass particles, as the current technique is faster and easier to apply, and there is no need to wait for 24 h [7,9,20,21] to allow the formation of an “interaction layer” that can protect the enamel surface.
One limitation of this study was the in vitro setting of the experiment; thus, in vivo studies should be carried out to confirm the results obtained. Moreover, detailed chemical characterizations of the tested bioactive material should be conducted after long storage durations, to examine the chemical changes in the bioactive material and the effect of the silanization of its particles on its degradation. Additionally, the interface formed between the resin sealer and the enamel sealer should be investigated.
5. Conclusions
Within the limitations of this in vitro study, it may be suggested that the bioactive resin sealer may serve as an orthodontic enamel sealer, which is capable of protecting the enamel surface against demineralization challenges.
Funding
This project was funded by the Science and Technology Unit-King Abdulaziz University, Kingdom of Saudi Arabia, award number UE-41-101.
Institutional Review Board Statement
The teeth were stored in 0.1% thymol until the start of the experiment, according to the guidelines approved by the university (approval code 055-02-20), and in accordance with the principles of the Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the manuscript.
Acknowledgments
This project was funded by the Science and Technology Unit-King Abdulaziz University, Kingdom of Saudi Arabia, award number UE-41-101.
Conflicts of Interest
The author declares no conflict of interest.
References
- Beyer, M.; Reichert, J.; Sigusch, B.W.; Watts, D.C.; Jandt, K.D. Morphology and structure of polymer layers protecting dental enamel against erosion. Dent. Mater. 2012, 28, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Al Mulla, A.H.; Kharsa, S.A.; Kjellberg, H.; Birkhed, D. Caries risk profiles in orthodontic patients at follow-up using Cariogram. Angle Orthod. 2009, 79, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Bazi, S.M.; Abbassy, M.A.; Bakry, A.S.; Merdad, L.A.; Hassan, A.H. Effects of chlorhexidine (gel) application on bacterial levels and orthodontic brackets during orthodontic treatment. J. Oral Sci. 2016, 58, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Mizrahi, E. Enamel demineralization following orthodontic treatment. Am. J. Orthod. 1982, 82, 62–67. [Google Scholar] [CrossRef]
- Mitchell, L. Decalcification during orthodontic treatment with fixed appliances—An overview. Br. J. Orthod. 1992, 19, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, R.G.; Tinanoff, N. Salivary Streptococcus mutans levels in patients before, during, and after orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 1991, 100, 35–37. [Google Scholar] [CrossRef]
- Abbassy, M.A.; Bakry, A.S.; Hill, R. The efficiency of fluoride bioactive glasses in protecting enamel surrounding orthodontic bracket. Biomed. Res. Int. 2021, 2021, 5544196. [Google Scholar] [CrossRef]
- Scully, M.; Morley, B.; Niven, P.; Crawford, D.; Pratt, I.S.; Wakefield, M. Factors associated with high consumption of soft drinks among Australian secondary-school students. Public Health Nutr. 2017, 20, 2340–2348. [Google Scholar] [CrossRef] [Green Version]
- Abbassy, M.A.; Bakry, A.S.; Alshehri, N.I.; Alghamdi, T.M.; Rafiq, S.A.; Aljeddawi, D.H.; Nujaim, D.S.; Hassan, A.H. 45S5 Bioglass paste is capable of protecting the enamel surrounding orthodontic brackets against erosive challenge. J. Orthod. Sci. 2019, 8, 5. [Google Scholar] [CrossRef]
- Bakhsh, T.A.; Bakry, A.S.; Mandurah, M.M.; Abbassy, M.A. Novel evaluation and treatment techniques for white spot lesions. An In Vitro study. Orthod. Craniofacial Res. 2017, 20, 170–176. [Google Scholar] [CrossRef]
- Bakry, A.S.; Abbassy, M.A. Increasing the efficiency of CPP-ACP to remineralize enamel white spot lesions. J. Dent. 2018, 76, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.S.; Abbassy, M.A.; Alharkan, H.F.; Basuhail, S.; Al-Ghamdi, K.; Hill, R. A novel fluoride containing bioactive glass paste is capable of re-mineralizing early caries lesions. Materials 2018, 11, 1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, W.J.; Wiltshire, W.A.; Dawes, C.; Lavelle, C.L. Long-term in vitro fluoride release and rerelease from orthodontic bonding materials containing fluoride. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Benson, P.E.; Alexander-Abt, J.; Cotter, S.; Dyer, F.M.V.; Fenesha, F.; Patel, A.; Campbell, C.; Crowley, N.; Millett, D.T. Resin-modified glass ionomer cement vs composite for orthodontic bonding: A multicenter, single-blind, randomized controlled trial. Am. J. Orthod. Dentofac. Orthop. 2019, 155, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Borzabadi-Farahani, A.; Borzabadi, E.; Lynch, E. Nanoparticles in orthodontics, a review of antimicrobial and anti-caries applications. Acta Odontol. Scand. 2014, 72, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, L.; Borzabadi-Farahani, A.; Karimi, S.; Saadat, S.; Badiee, M.R. Evaluation of the shear bond strength and antibacterial activity of orthodontic adhesive containing silver nanoparticle, an in-vitro study. Nanomaterials 2020, 10, 1466. [Google Scholar] [CrossRef]
- Khoroushi, M.; Kachuie, M. Prevention and treatment of white spot lesions in orthodontic patients. Contemp. Clin. Dent. 2017, 8, 11–19. [Google Scholar] [CrossRef]
- Hench, L.L. Special report: The interfacial behavior of biomaterials, 1979. J. Biomed. Mater. Res. 1980, 14, 803–811. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Berakdar, G.A.; Mkawi, E.M.; et al. Silica-based bioactive glasses and their applications in hard tissue regeneration: A review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef]
- Abbassy, M.A.; Bakry, A.S.; Hill, R.; Habib Hassan, A. Fluoride bioactive glass paste improves bond durability and remineralizes tooth structure prior to adhesive restoration. Dent. Mater. 2021, 37, 71–80. [Google Scholar] [CrossRef]
- Abbassy, M.A.; Bakry, A.S.; Almoabady, E.H.; Almusally, S.M.; Hassan, A.H. Characterization of a novel enamel sealer for bioactive remineralization of white spot lesions. J. Dent. 2021, 109, 103663. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.S.; Tamura, Y.; Otsuki, M.; Kasugai, S.; Ohya, K.; Tagami, J. Cytotoxicity of 45S5 bioglass paste used for dentine hypersensitivity treatment. J. Dent. 2011, 39, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.S.; Takahashi, H.; Otsuki, M.; Tagami, J. The durability of phosphoric acid promoted bioglass-dentin interaction layer. Dent. Mater. 2013, 29, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Spanovic, N.; Taubock, T.T.; Attin, T.; Tarle, Z. Degree of conversion of experimental resin composites containing bioactive glass 45S5: The effect of post-cure heating. Sci. Rep. 2019, 9, 17245. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Yoo, K.H.; Yoon, S.Y.; Kim, I.R.; Park, B.S.; Son, W.S.; Ko, C.C.; Son, S.A.; Kim, Y.I. Enamel Anti-demineralization effect of orthodontic adhesive containing bioactive glass and graphene oxide: An in-vitro study. Materials 2018, 11, 1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.; Kim, I.R.; Park, B.S.; Lee, D.J.; Ko, C.C.; Son, W.S.; Kim, Y.I. Remineralization property of an orthodontic primer containing a bioactive glass with silver and zinc. Materials 2017, 10, 1253. [Google Scholar] [CrossRef] [Green Version]
- Sales-Peres, S.H.; Brianezzi, L.F.; Marsicano, J.A.; Forim, M.R.; da Silva, M.F.; Sales-Peres, A. Evaluation of an experimental gel containing euclea natalensis: An in vitro study. Evid. Based Complement. Altern. Med. 2012, 2012, 184346. [Google Scholar] [CrossRef] [Green Version]
- Bakry, A.S.; Takahashi, H.; Otsuki, M.; Tagami, J. Evaluation of new treatment for incipient enamel demineralization using 45S5 bioglass. Dent. Mater. 2014, 30, 314–320. [Google Scholar] [CrossRef]
- Magalhaes, A.C.; Moraes, S.M.; Rios, D.; Wiegand, A.; Buzalaf, M.A. The erosive potential of 1% citric acid supplemented by different minerals: An in vitro study. Oral Health Prev. Dent. 2010, 8, 41–45. [Google Scholar]
- Yu, H.; Wegehaupt, F.J.; Wiegand, A.; Roos, M.; Attin, T.; Buchalla, W. Erosion and abrasion of tooth-colored restorative materials and human enamel. J. Dent. 2009, 37, 913–922. [Google Scholar] [CrossRef] [Green Version]
- Bakry, A.S.; Marghalani, H.Y.; Amin, O.A.; Tagami, J. The effect of a bioglass paste on enamel exposed to erosive challenge. J. Dent. 2014, 42, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.S.; Abbassy, M.A. The efficacy of a bioglass (45S5) paste temporary filling used to remineralize enamel surfaces prior to bonding procedures. J. Dent. 2019, 85, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Shinya, M.; Shinya, A.; Lassila, L.V.; Varrela, J.; Vallittu, P.K. Enhanced degree of monomer conversion of orthodontic adhesives using a glass-fiber layer under the bracket. Angle Orthod. 2009, 79, 546–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbour, M.E.; Parker, D.M.; Allen, G.C.; Jandt, K.D. Enamel dissolution in citric acid as a function of calcium and phosphate concentrations and degree of saturation with respect to hydroxyapatite. Eur. J. Oral Sci. 2003, 111, 428–433. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).