Development of Silver Doped Hydroxyapatite Thin Films for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Silver Doped Hydroxyapatite Thin Films Using Spin-Coating Technique
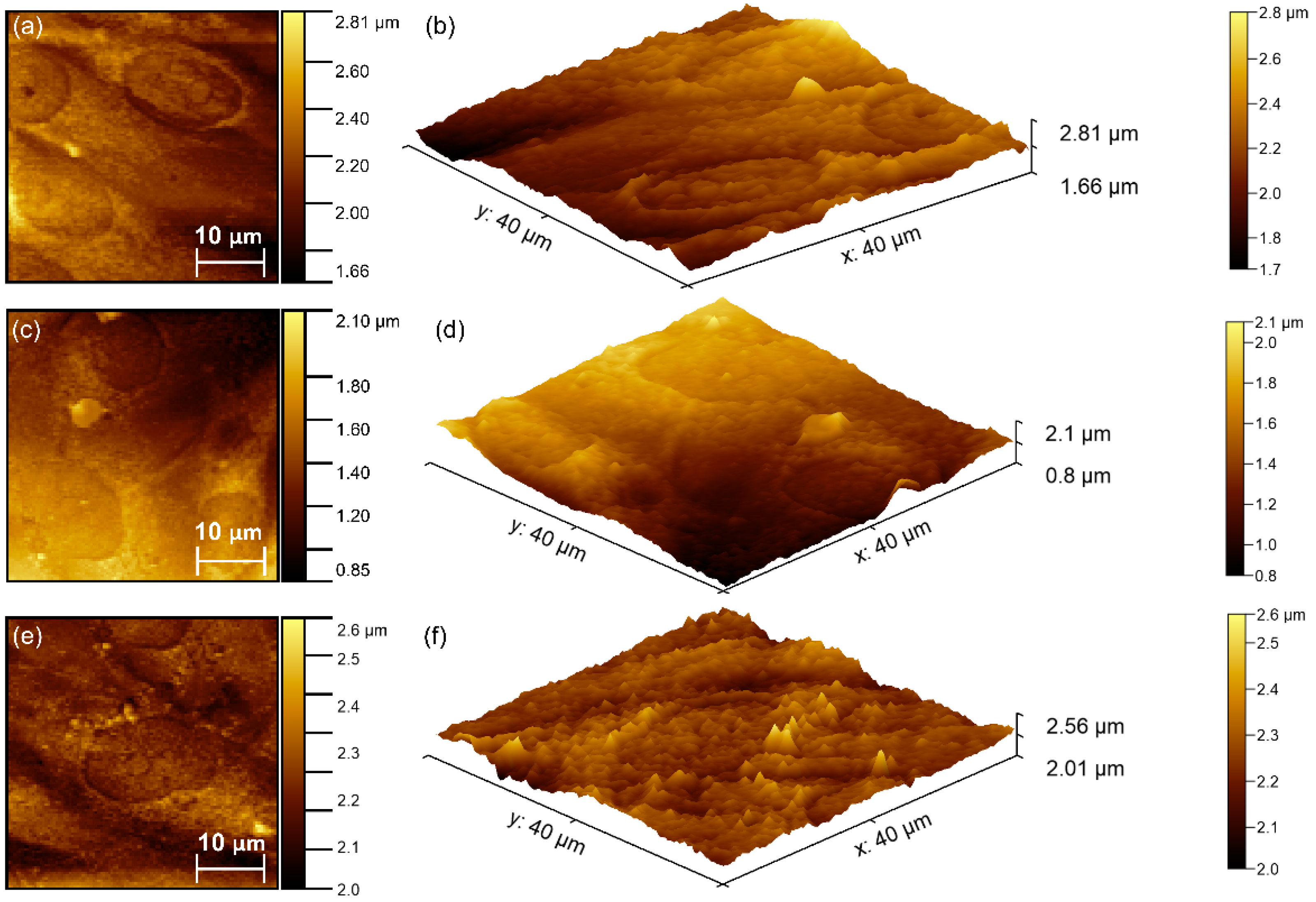
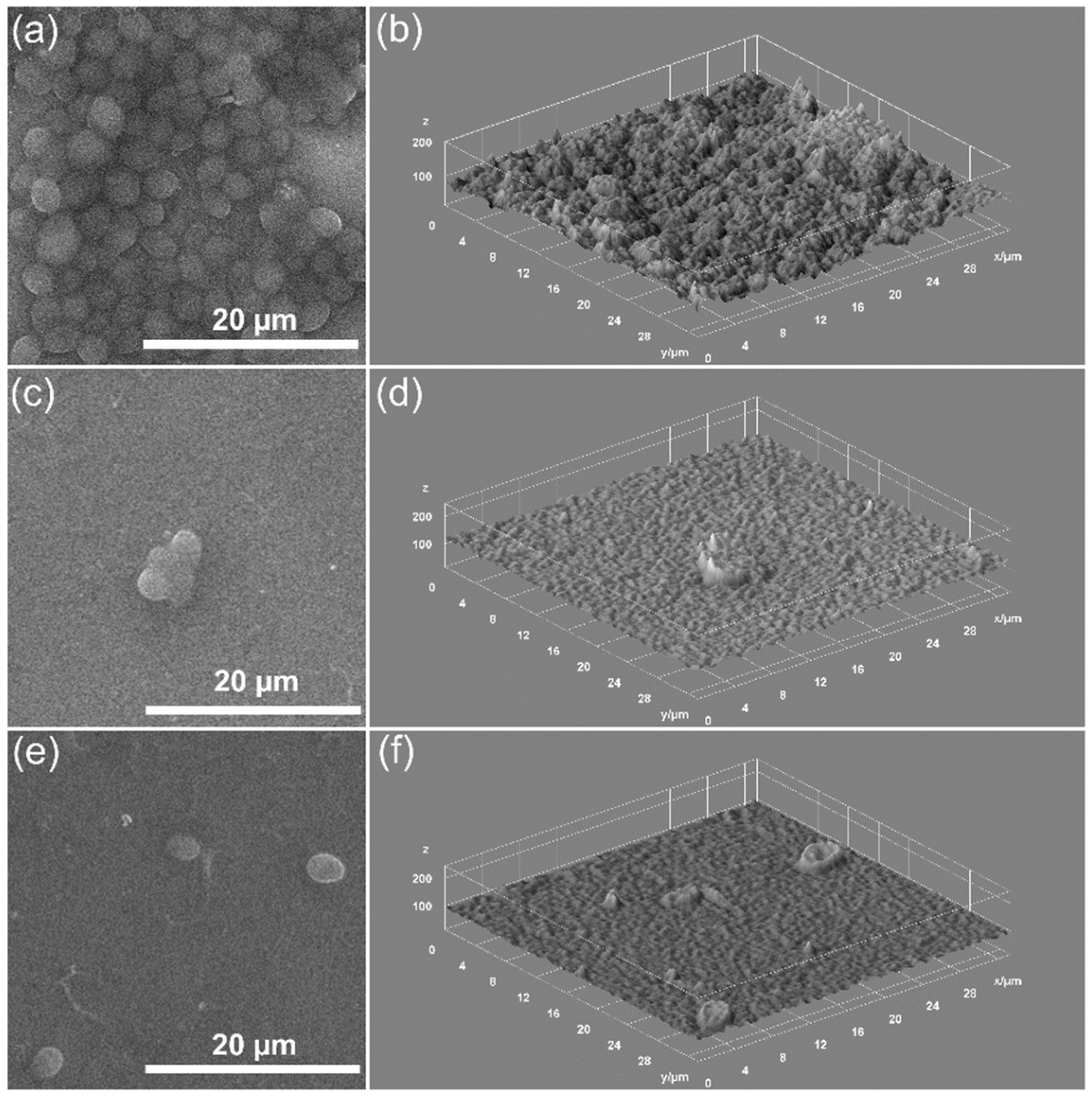
2.3. Characterization Methods
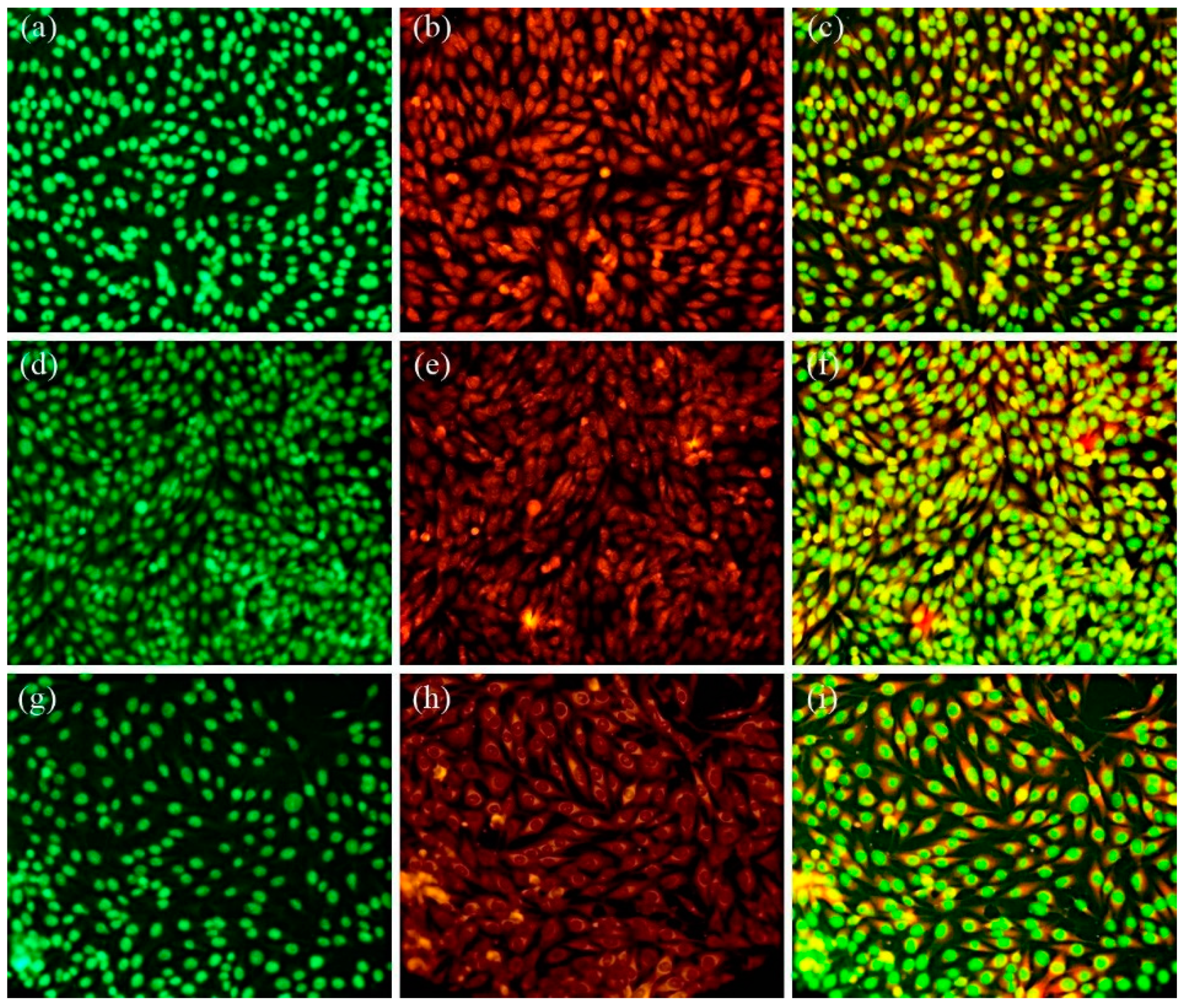
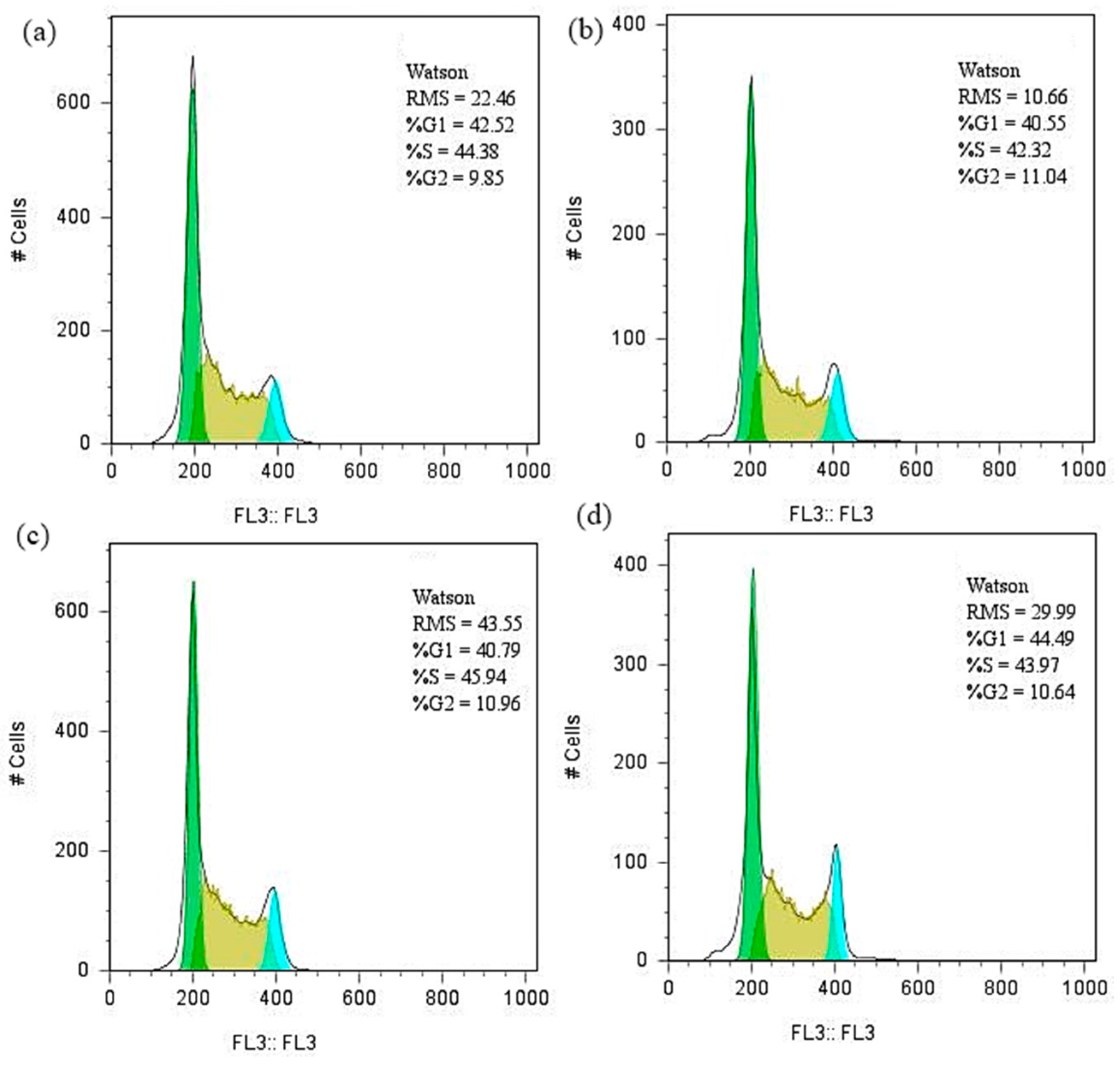
2.4. Biological Evaluation
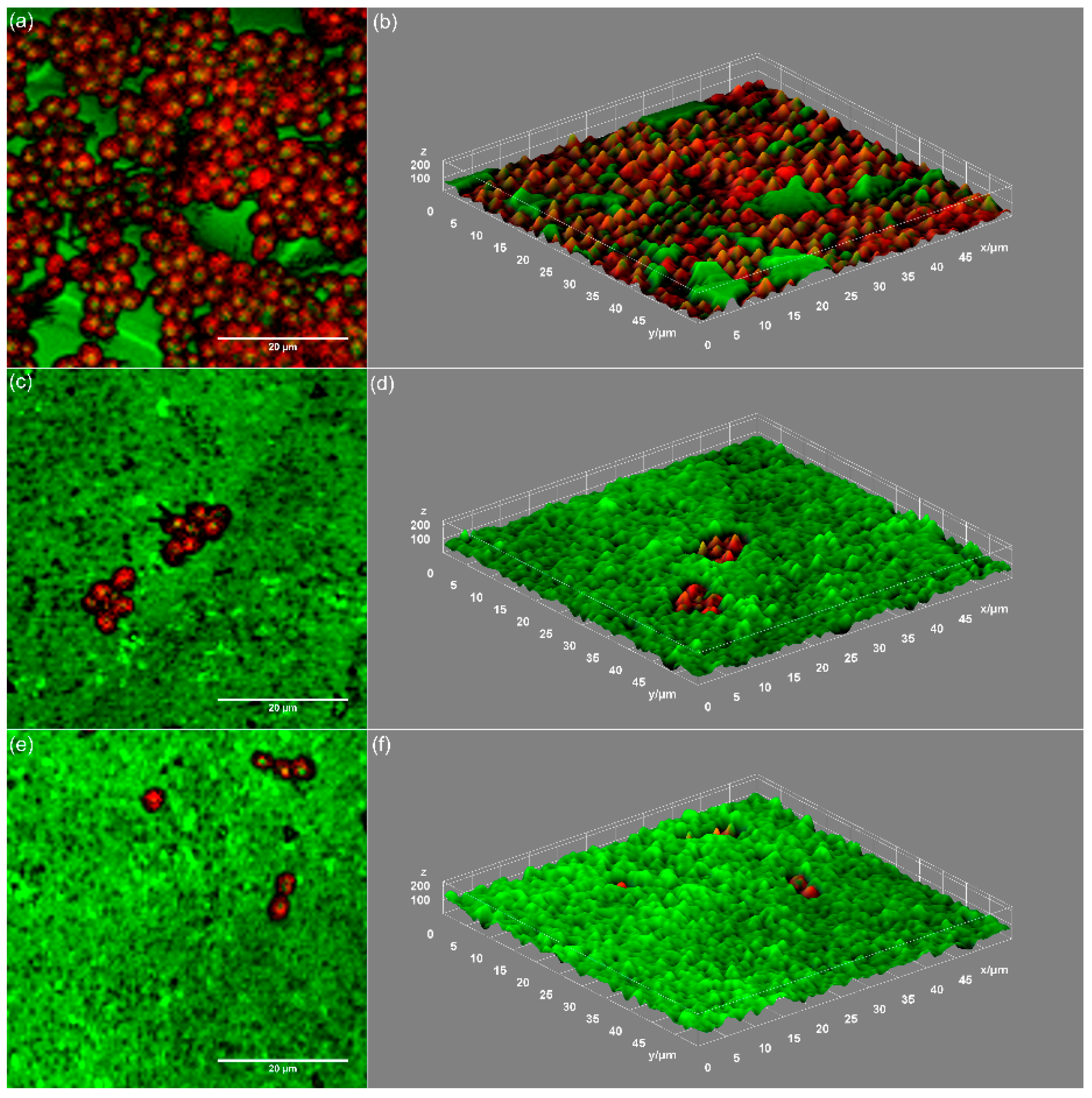
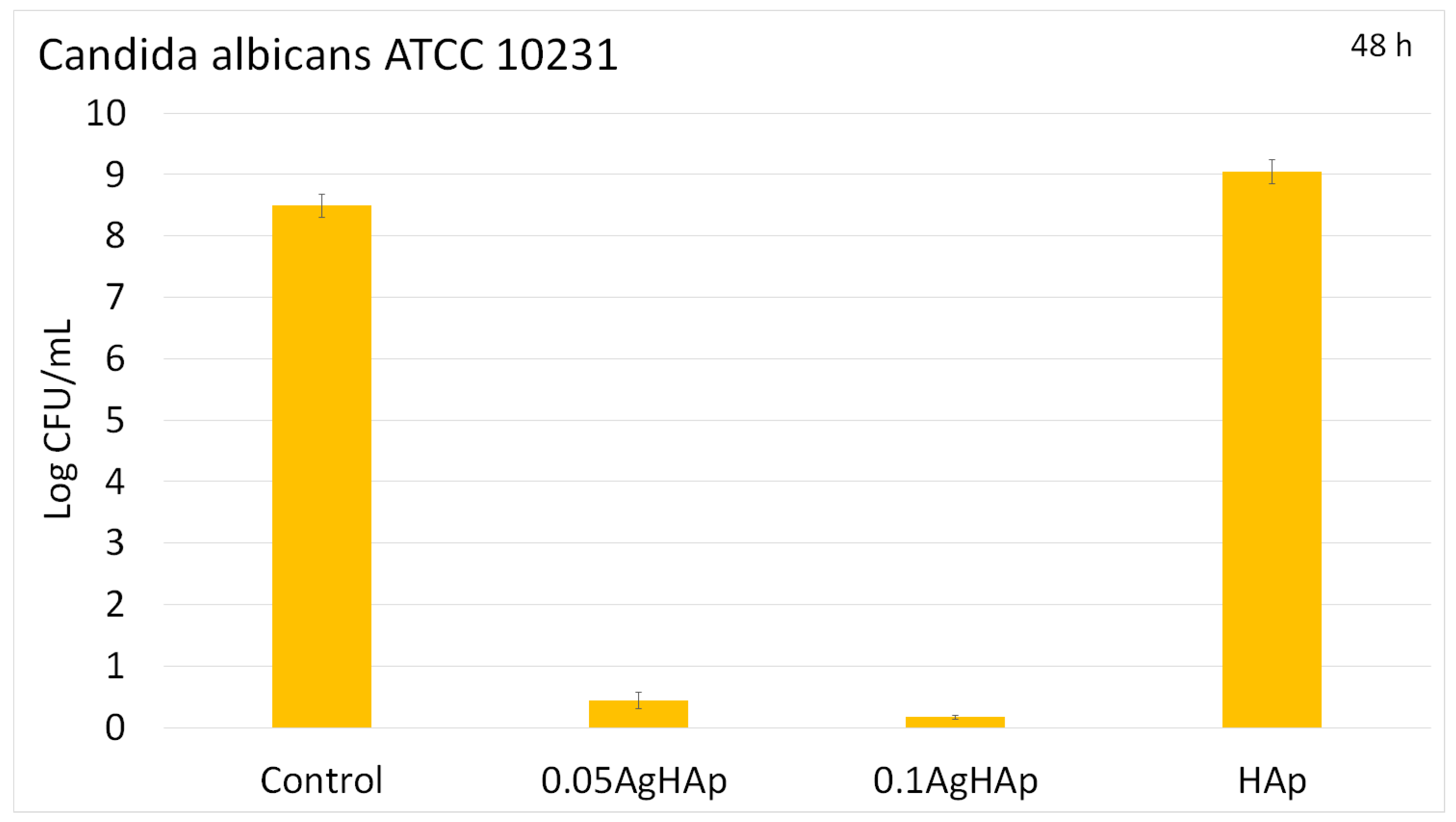
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, C.; Gao, J.; Wang, M.; Fu, J.; Wang, D.; Zhu, Y. Ultra-trace silver-doped hydroxyapatite with non-cytotoxicity and effective antibacterial activity. Mater. Sci. Eng. C 2015, 55, 497–505. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.Q.; Zhang, L.; Zheng, H.; Wang, Z. Hydroxyapatite nanocrystals for biomedical applications. J. Phys. Chem. C 2010, 114, 18352–18357. [Google Scholar] [CrossRef]
- Huang, Y.; Qu, Y.; Yang, B.C.; Li, W.; Zhang, B.; Zhang, X.D. In vivo biological responses of plasma sprayed hydroxyapatite coatings with an electric polarized treatment in alkaline solution. Mater. Sci. Eng. C 2009, 29, 2411–2416. [Google Scholar] [CrossRef]
- Simchi, A.; Tamjid, E.; Pishbin, F.; Boccaccini, A.R. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomedicine 2011, 7, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Cui, F.Z.; Kim, T.N.; Kim, J.W. Ag-substituted hydroxyapatite coatings with both antimicrobial effects and biocompatibility. J. Mater. Sci. Lett. 1999, 18, 559–561. [Google Scholar] [CrossRef]
- Cheng, K.; Weng, W.; Wang, H.; Zhang, S. In vitro behavior of osteoblast-like cells on fluoridated hydroxyapatite coatings. Biomaterials 2005, 26, 6288–6295. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.B.; Khajuria, D.K.; Karasik, D.; Gedanken, A. Silver and gold doped hydroxyapatite nanocomposites for enhanced bone regeneration. Biomed. Mater. 2019, 14, 055002. [Google Scholar] [CrossRef]
- Min, J.; Choi, K.Y.; Dreaden, E.C.; Padera, R.F.; Braatz, R.D.; Spector, M.; Hammond, P.T. Designer Dual Therapy Nanolayered Implant Coatings Eradicate Biofilms and Accelerate Bone Tissue Repair. ACS Nano 2016, 10, 4441–4450. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Ferraz, M.P.; Monteiro, F.J.; Fernandes, M.H.; Beppu, M.M.; Mantione, D.; Sardon, H. Antibacterial silk fibroin/nanohydroxyapatite hydrogels with silver and gold nanoparticles for bone regeneration. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 231–239. [Google Scholar] [CrossRef]
- Geng, H.; Poologasundarampillai, G.; Todd, N.; Devlin-Mullin, A.; Moore, K.L.; Golrokhi, Z.; Gilchrist, J.B.; Jones, E.; Potter, R.J.; Sutcliffe, C.; et al. Biotransformation of silver released from nanoparticle coated titanium implants revealed in regenerating bone. ACS Appl. Mater. Interfaces 2017, 9, 21169–21180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duran, N.; Duran, M.; De Jesus, M.B.; Seabra, A.B.; Favaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed.: Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Matsumoto, T. Synthesis and modification of apatite nanoparticles for use in dental and medical applications. Jpn. Dent. Sci. Rev. 2015, 51, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7, BTRI-S36138. [Google Scholar] [CrossRef] [Green Version]
- Gokcekaya, O.; Ueda, K.; Ogasawara, K.; Kanetaka, H.; Narushima, T. In vitro evaluation of Ag-containing calcium phosphates: Effectiveness of Ag-incorporated β-tricalcium phosphate. Mater. Sci. Eng. C 2017, 75, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Vu, A.A.; Bandyopadhyay, A.; Bose, S. Compositionally graded doped hydroxyapatite coating on titanium using laser and plasma spray deposition for bone implants. Acta Biomater. 2019, 84, 414–423. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Fabrication of Silver- and Zinc-Doped Hydroxyapatite Coatings for Enhancing Antimicrobial Effect. Coatings 2020, 10, 905. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Chifiriuc, M.C.; Costescu, A.; Le Coustumer, P.; Predoi, D. Synthesis and antimicrobial activity of silver-doped hydroxyapatite nanoparticles. BioMed Res. Int. 2013, 2013, 916218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rau, J.V.; Cacciotti, I.; Laureti, S.; Fosca, M.; Varvaro, G.; Latini, A. Bioactive, nanostructured Si- substituted hydroxyapatite coatings on titanium prepared by pulsed laser deposition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Qin, J.; Ma, J. Electrophoretic deposition of biomimetic zinc substituted hydroxyapatite coatings with chitosan and carbon nanotubes on titanium. Ceram. Int. 2015, 41, 8878–8884. [Google Scholar] [CrossRef]
- Hoover, S.; Tarafder, S.; Bandyopadhyay, A.; Bose, S. Silver doped resorbable tricalcium phosphate scaffolds for bone graft applications. Mater. Sci. Eng. C 2017, 79, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, X.; Xie, Y.; Ji, H.; Ding, C.; Li, H.; Dai, K. Silver release from silver-containing hydroxyapatite coatings. Surf. Coat. Technol. 2010, 205, 1892–1896. [Google Scholar] [CrossRef]
- Roy, M.; Fielding, G.A.; Beyenal, H.; Bandyopadhyay, A.; Bose, S. Mechanical, in vitro antimicrobial, and biological properties of plasma-sprayed silver-doped hydroxyapatite coating. ACS Appl. Mater. Interfaces 2012, 4, 1341–1349. [Google Scholar] [CrossRef] [Green Version]
- Jamuna-Thevi, K.; Bakar, S.A.; Ibrahim, S.; Shahab, N.; Toff, M.R.M. Quantification of silver ion release, in vitro cytotoxicity and antibacterial properties of nanostuctured Ag doped TiO2 coatings on stainless steel deposited by RF magnetron sputtering. Vacuum 2011, 86, 235–241. [Google Scholar] [CrossRef]
- Hardes, J.; Ahrens, H.; Gebert, C.; Streitbuerger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Wedemeyer, C.; Saxler, G.; Winkelmann, W.; et al. Lack of toxicological side effects in silver-coated mega prostheses in humans. Biomaterials 2007, 28, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Qing, T.; Mahmood, M.; Zheng, Y.; Biris, A.S.; Shi, L.; Casciano, D.A. A genomic characterization of the influence of silver nanoparticles on bone differentiation in MC3T3-E1 cells. J. Appl. Toxicol. 2018, 38, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhu, C.; An, Z.; Jiang, Y.; Zhao, Y.; Wang, J.; Liu, X.; Hui, B.; Zhang, X.; Wang, Y. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int. J. Nanomed. 2014, 9, 2469–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, M.; Li, Z.; Casciano, D.; Khodakovskaya, M.V.; Chen, T.; Karmakar, A.; Dervishi, E.; Xu, Y.; Mustafa, T.; Watanabe, F.; et al. Nanostructural materials increase mineralization in bone cells and affect gene expression through miRNA regulation. J. Cell. Mol. Med. 2011, 15, 2297–2306. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, C.S.; Iconaru, S.L.; Massuyeau, F.; Constantin, L.V.; Costescu, A.; Predoi, D. Synthesis, structure and luminescent properties of europium-doped hydroxyapatite nanocrystalline powders. J. Nanomater. 2012, 2012, 942801. [Google Scholar] [CrossRef] [Green Version]
- Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Predoi, D.; Buton, N.; Megier, C.; Stan, G.E. Dextran-Thyme Magnesium-Doped Hydroxyapatite Composite Antimicrobial Coatings. Coatings 2020, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- ImageJ. Available online: http://imagej.nih.gov/ij (accessed on 10 January 2021).
- Gwyddion. Available online: http://gwyddion.net/ (accessed on 20 January 2021).
- Ciobanu, C.S.; Iconaru, S.L.; Predoi, D.; Trușcă, R.-D.; Prodan, A.M.; Groza, A.; Chifiriuc, M.C.; Beuran, M. Fabrication of Novel Chitosan–Hydroxyapatite Nanostructured Thin Films for Biomedical Applications. Coatings 2021, 11, 1561. [Google Scholar] [CrossRef]
- Debye, P.; Scherrer, P. Interference of irregularly oriented particles in X-rays. Phys. Zeit. 1916, 17, 277–283. [Google Scholar]
- Debye, P.; Scherrer, P. Interference on inordinate orientated particles in X-ray light. III. Phys. Zeit. 1917, 18, 291–301. [Google Scholar]
- Danilchenko, S.N.; Kukharenko, O.G.; Moseke, C.; Protsenko, I.Y.; Sukhodub, L.F.; Sulkio-Cleff, B. Determinat ion of the bone mineral crystallite size and lattice strain from diffraction line broadening. Cryst. Res. Technol. 2002, 37, 1234–1240. [Google Scholar] [CrossRef]
- Richardson, J.W.; Faber, J., Jr. Advances in X-ray Analysis; Barrett, C.S., Cohen, J.B., Faber, J., Jr., Jenkins, R., Leyden, D.E., Russ, J.C., Predecki, P.K., Eds.; Plenum Publishing: New York, NY, USA, 1986; Volume 29. [Google Scholar]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 °C. Nanoscale Res. Lett. 2011, 6, 613. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, S.; Viswanathan, B.; Varadarajan, T.K. A novel single step chemical route for noble metal nanoparticles embedded organic–inorganic composite films. Mater. Chem. Phys. 2006, 95, 51–55. [Google Scholar] [CrossRef]
- Markovic, M.; Fowler, B.O.; Tung, M.S. Preparation and Comprehensive Characterization of a Calcium Hydroxyapatite Reference Material. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553–568. [Google Scholar] [CrossRef]
- Bai, X.; More, K.; Rouleau, C.M.; Rabiei, A. Functionally graded hydroxyapatite coatings doped with antibacterial components. Acta Biomater. 2010, 6, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Pasuk, I.; Vasile, B.S.; Lupu, A.R.; Hermenean, A.; Dinischiotu, A.; Predoi, D. Structural properties of silver doped hydroxyapatite and their biocompatibility. Mater. Sci. Eng. C 2013, 33, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Elliot, J. Structural and Chemistry of Apatites and Other Calcium Ortophosphates; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Predoi, D. Vibrational Investigations of Silver-Doped Hydroxyapatite with Antibacterial Properties. J. Spectrosc. 2013, 2013, 471061. [Google Scholar] [CrossRef]
- Salaie, R.N.; Besinis, A.; Le, H.; Tredwin, C.; Handy, R.D. The biocompatibility of silver and nanohydroxyapatite coatings on titanium dental implants with human primary osteoblast cells. Mater. Sci. Eng. C 2020, 107, 110210. [Google Scholar] [CrossRef] [PubMed]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Johnson, A.J.W. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Kudo, T.A.; Kanetaka, H.; Miyazaki, T.; Hashimoto, M.; Kawashita, M. Fibronectin adsorption on osteoconductive hydroxyapatite and non-osteoconductive α-alumina. Biomed. Mater. 2016, 11, 045006. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Kovtun, A.; Peetsch, A.; Goroja, S.N.; Sharonova, A.A.; Pichugin, V.F.; Grubova, I.Y.; Ivanova, A.A.; Teresov, A.D.; Koval, N.N.; et al. Preparation of a silicate-containing hydroxyapatite based coating by magnetron sputtering: Structure and osteoblast-like MG63 cells in vitro study. RSC Adv. 2013, 3, 11240–11246. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Y.; Hosoi, A.; Morita, Y.; Ju, Y. Microwave Atomic Force Microscope: MG63 Osteoblast-like Cells Analysis on Nanometer Scale. Microsyst. Technol. 2016, 22, 603–608. [Google Scholar] [CrossRef]
- Alves, S.F.; Wassall, T. In vitro evaluation of osteoblastic cell adhesion on machined osseointegrated implants. Braz. Oral Res. 2009, 23, 131–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of sur-face characteristics on bone integration of tita-nium implants: A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Borsari, V.; Giavaresi, G.; Fini, M.; Torricelli, P.; Salito, A.; Chiesa, R.; Chiusoli, L.; Volpert, A.; Rimondini, L.; Giardino, R. Physical characterization of different-roughness titanium sur-faces, with and without hydroxyapatite coating, and their effect on human osteoblast-like cells. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 75, 359–368. [Google Scholar] [CrossRef]
- Tsukamoto, M.; Miyamoto, H.; Ando, Y.; Noda, I.; Eto, S.; Akiyama, T.; Yonekura, Y.; Sonohata, M.; Mawatari, M. Acute and Subacute Toxicity In Vivo of Thermal-Sprayed Silver Containing Hydroxyapatite Coating in Rat Tibia. Biomed. Res. Int. 2014, 2014, 902343. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Buton, N.; Motelica-Heino, M. Zinc Doped Hydroxyapatite Thin Films Prepared by Sol–Gel Spin Coating Procedure. Coatings 2019, 9, 156. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Groza, A.; Gaiaschi, S.; Rokosz, K.; Raaen, S.; Negrila, C.C.; Prodan, A.-M.; Costescu, A.; et al. Development of Cerium-Doped Hydroxyapatite Coatings with Antimicrobial Properties for Biomedical Applications. Coatings 2020, 10, 516. [Google Scholar] [CrossRef]
- Ciobanu, S.C.; Iconaru, S.L.; Predoi, D.; Prodan, A.M.; Predoi, M.V. Physico-Chemical Properties and In Vitro Antifungal Evaluation of Samarium Doped Hydroxyapatite Coatings. Coatings 2020, 10, 827. [Google Scholar] [CrossRef]
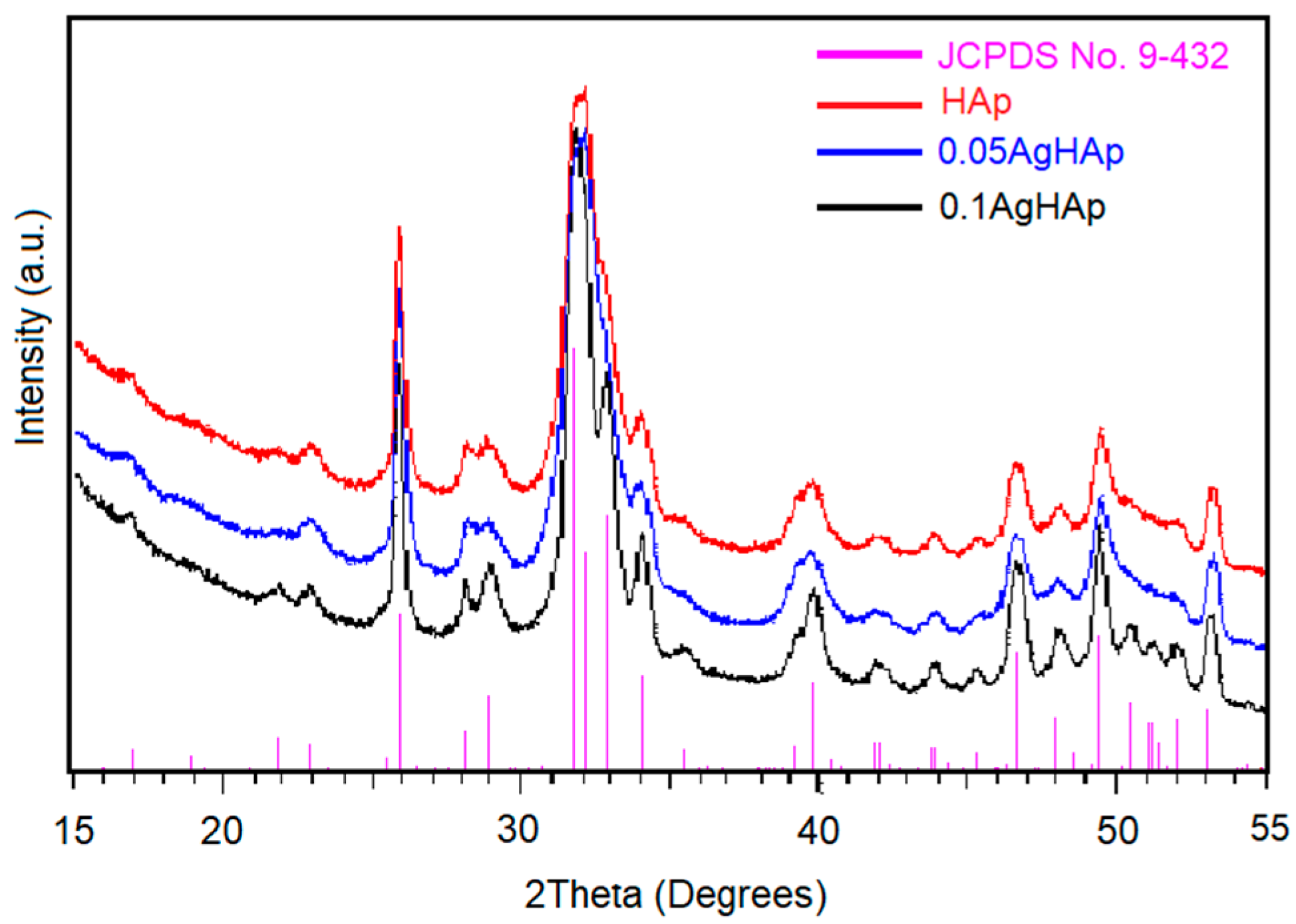
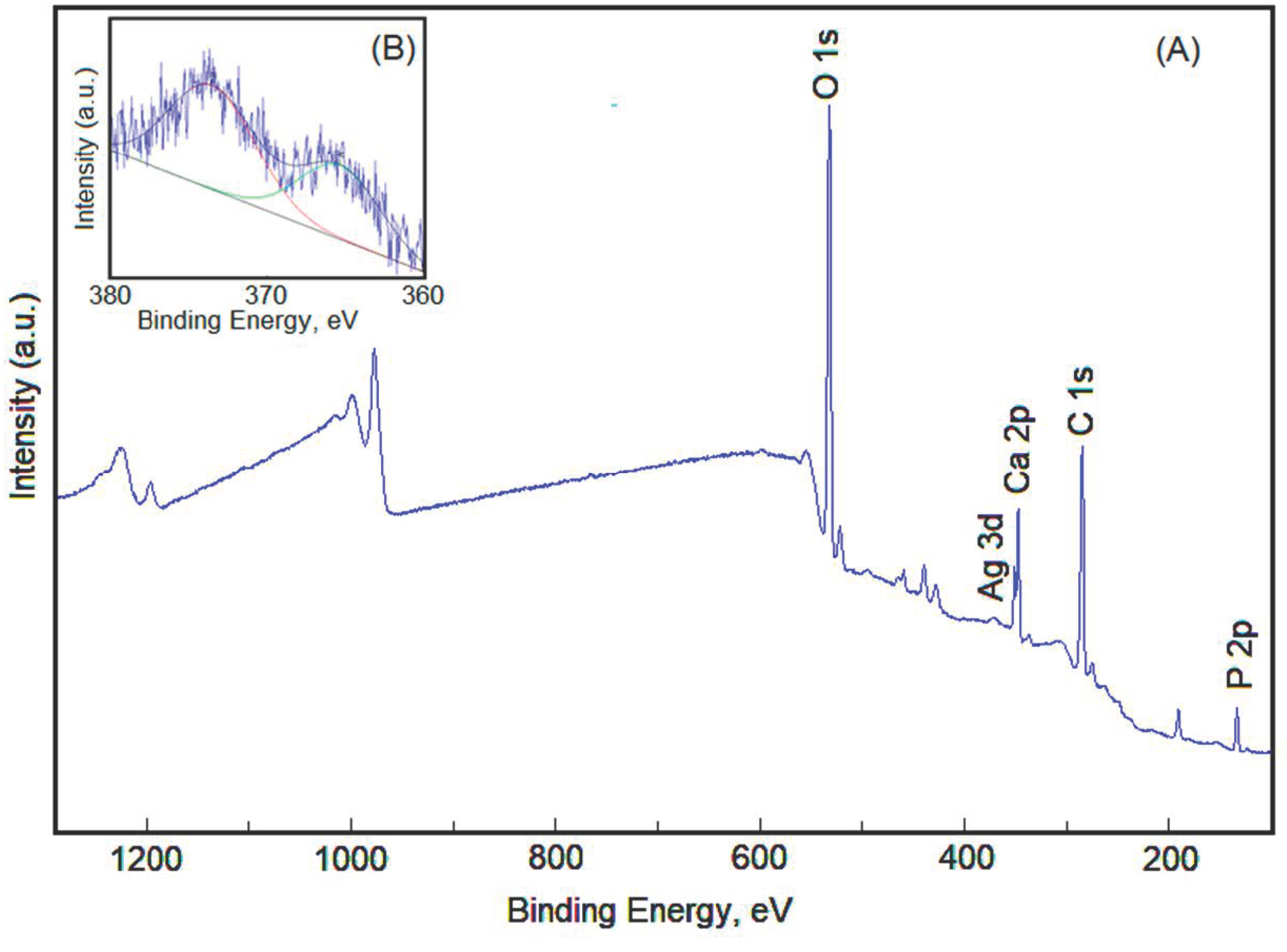
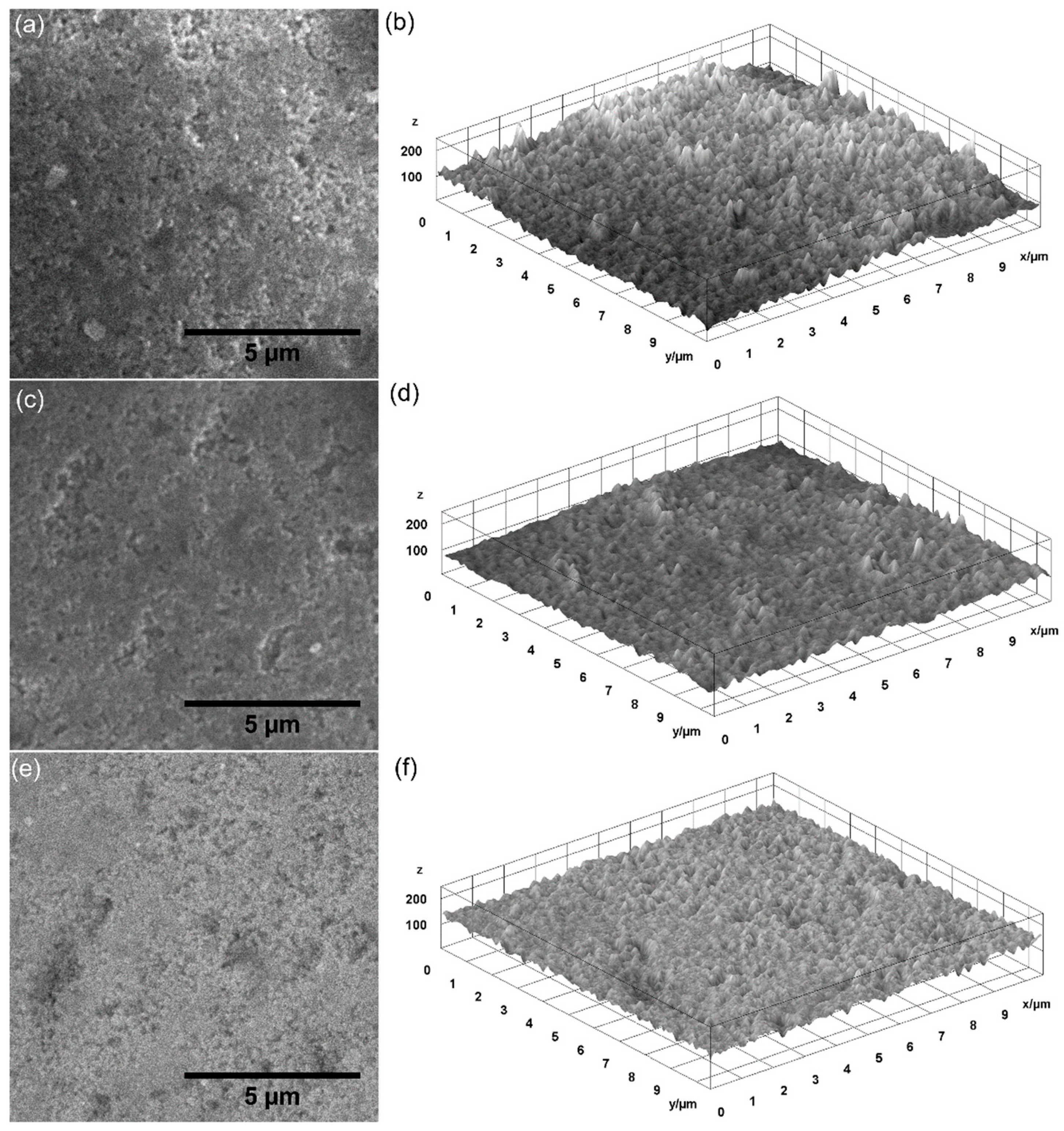
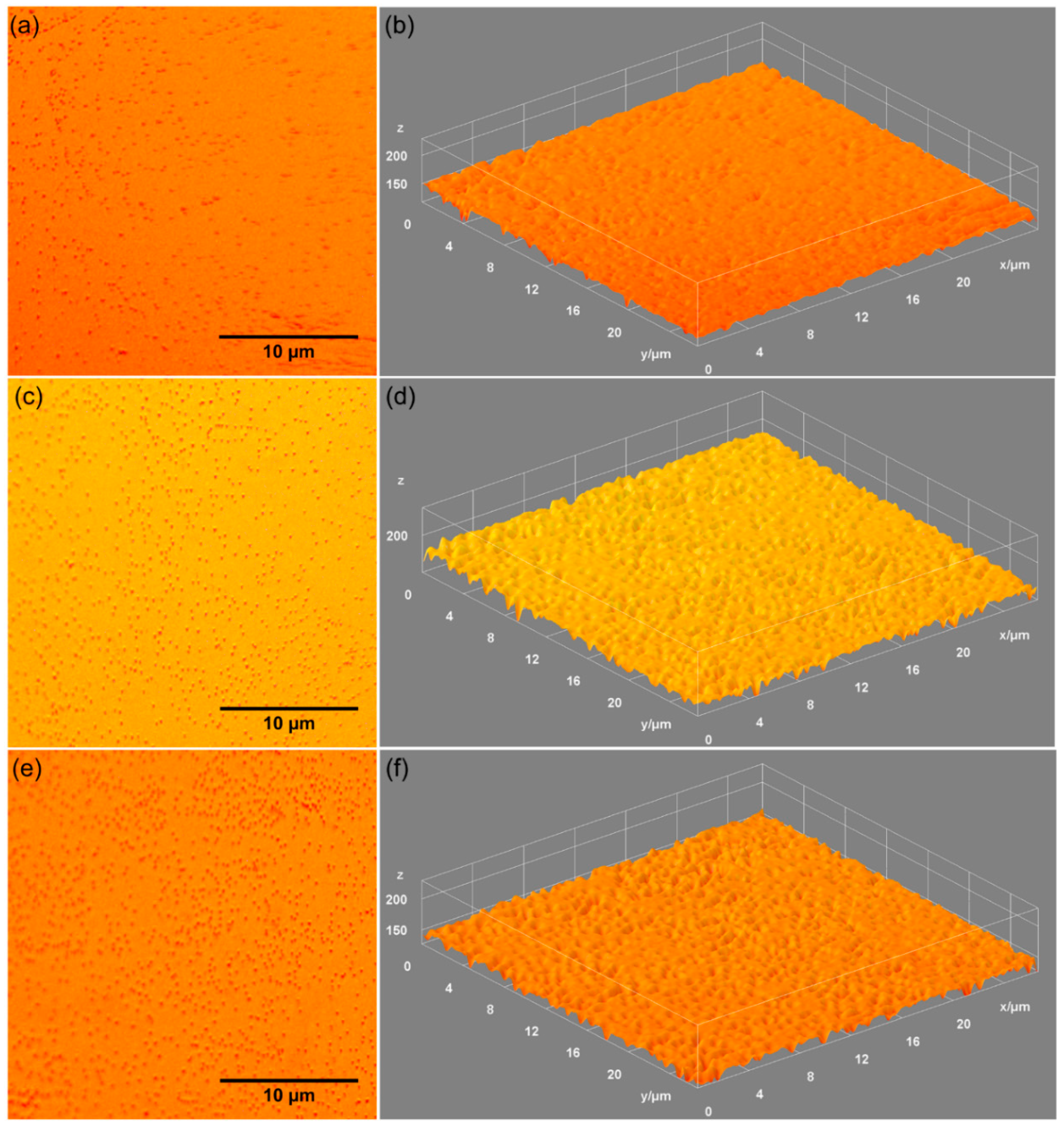
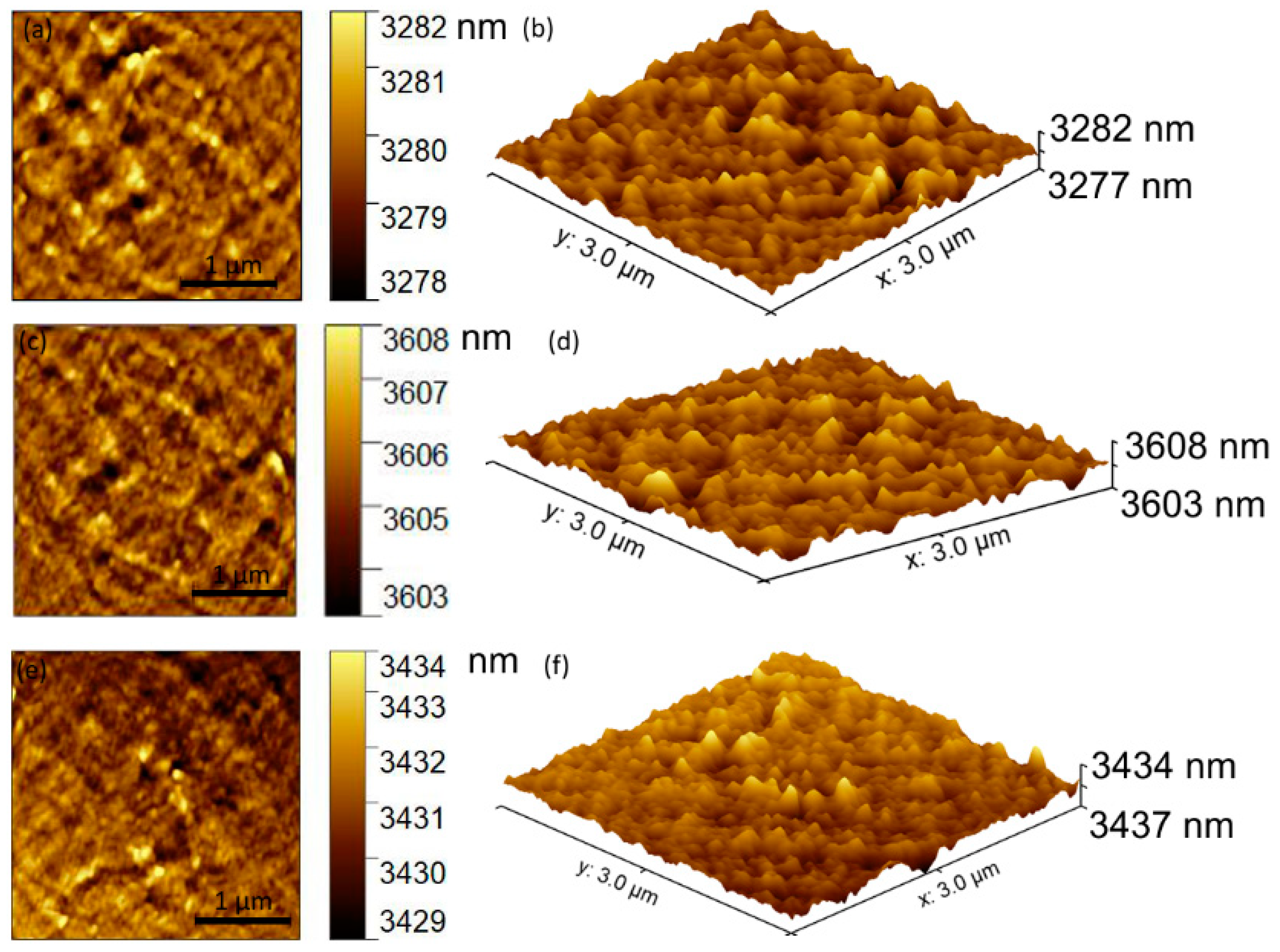
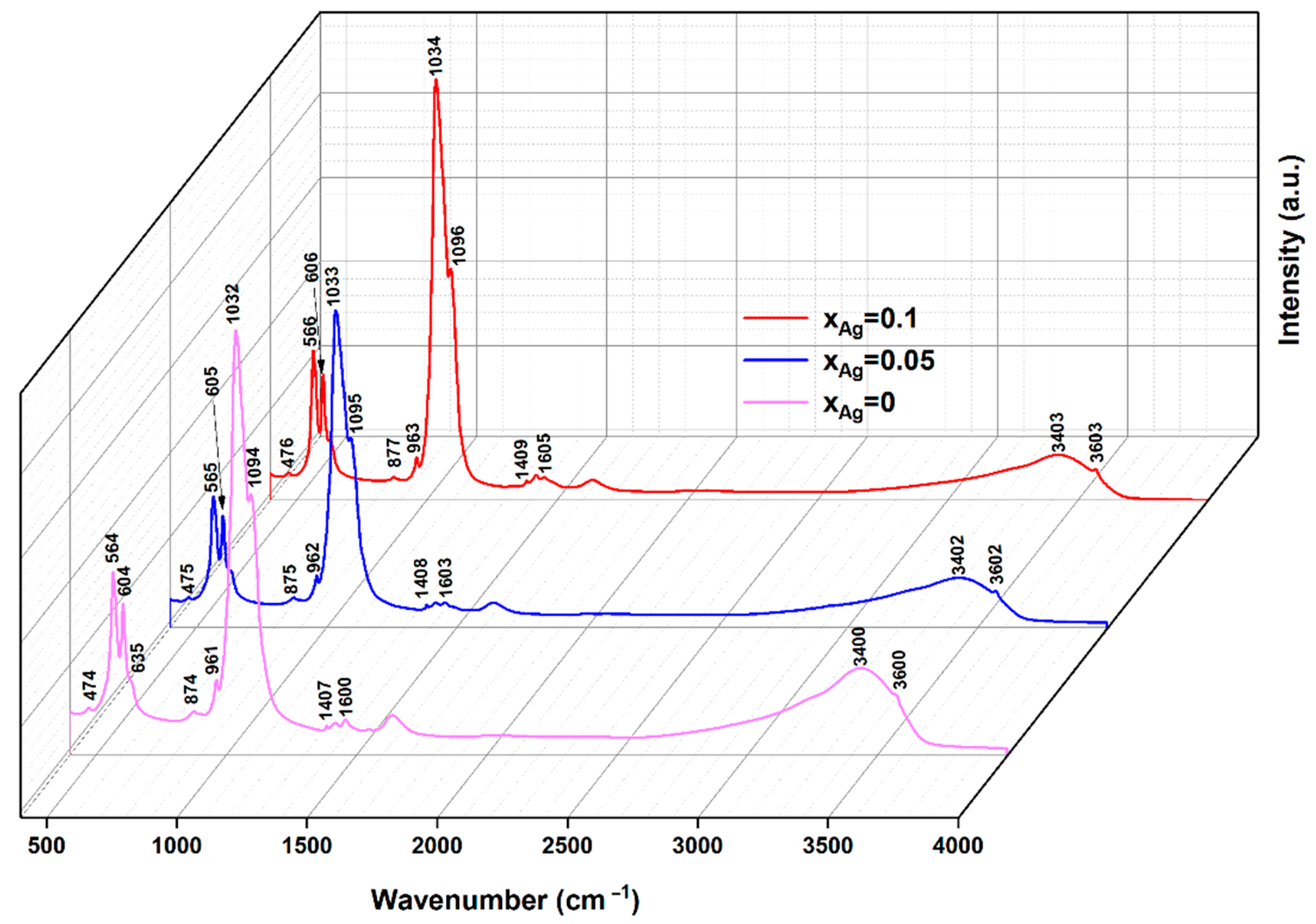
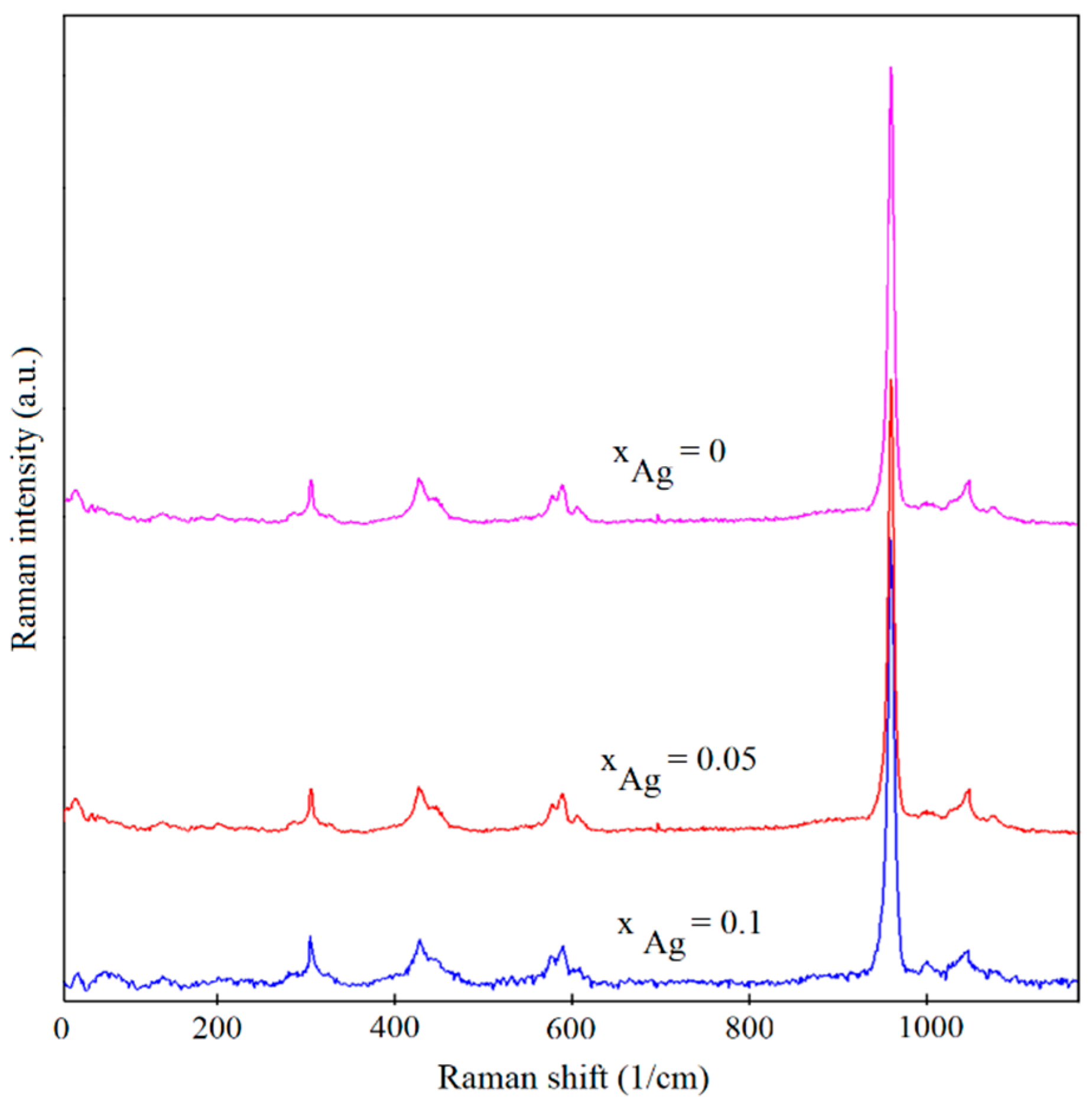

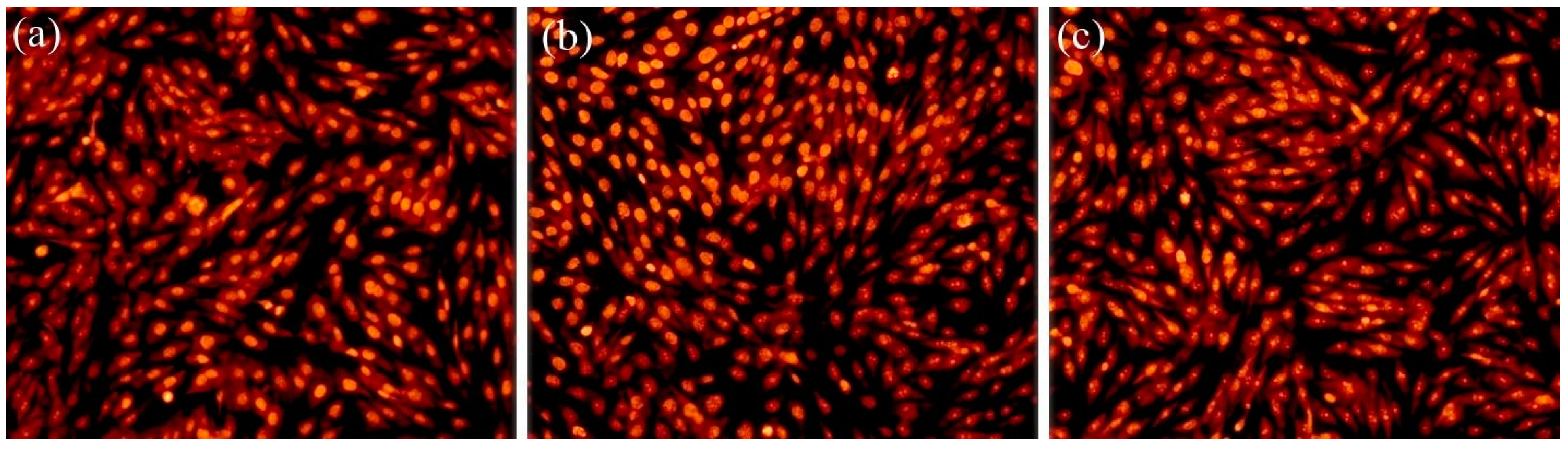
| Sample | Lattice Constant | Crystalline Size | ||
|---|---|---|---|---|
| a (Å) | c (Å) | d300 (Å) | d002 (Å) | |
| HAp (JCPDS card No. 09-432) | 9.416 | 6.874 | - | - |
| HAp | 9.418 | 6. 878 | 17.98 | 24.87 |
| 0.05 AgHAp | 9.421 | 6.883 | 16.34 | 22.91 |
| 0.1 AgHAp | 9.421 | 6.883 | 15.15 | 21.31 |
| Sample | RRMS |
|---|---|
| HAp | 7.92 nm |
| 0.05 AgHAp | 6.26 nm |
| 0.1 AgHAp | 6.20 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iconaru, S.L.; Predoi, D.; Ciobanu, C.S.; Motelica-Heino, M.; Guegan, R.; Bleotu, C. Development of Silver Doped Hydroxyapatite Thin Films for Biomedical Applications. Coatings 2022, 12, 341. https://doi.org/10.3390/coatings12030341
Iconaru SL, Predoi D, Ciobanu CS, Motelica-Heino M, Guegan R, Bleotu C. Development of Silver Doped Hydroxyapatite Thin Films for Biomedical Applications. Coatings. 2022; 12(3):341. https://doi.org/10.3390/coatings12030341
Chicago/Turabian StyleIconaru, Simona Liliana, Daniela Predoi, Carmen Steluta Ciobanu, Mikael Motelica-Heino, Régis Guegan, and Coralia Bleotu. 2022. "Development of Silver Doped Hydroxyapatite Thin Films for Biomedical Applications" Coatings 12, no. 3: 341. https://doi.org/10.3390/coatings12030341
APA StyleIconaru, S. L., Predoi, D., Ciobanu, C. S., Motelica-Heino, M., Guegan, R., & Bleotu, C. (2022). Development of Silver Doped Hydroxyapatite Thin Films for Biomedical Applications. Coatings, 12(3), 341. https://doi.org/10.3390/coatings12030341










