Design and Molding of Thyroid Cartilage Prosthesis Based on 3D Printing Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Implant Design Requirements
2.2. Materials and Methods
2.3. Analysis Methods
3. Results and Discussion
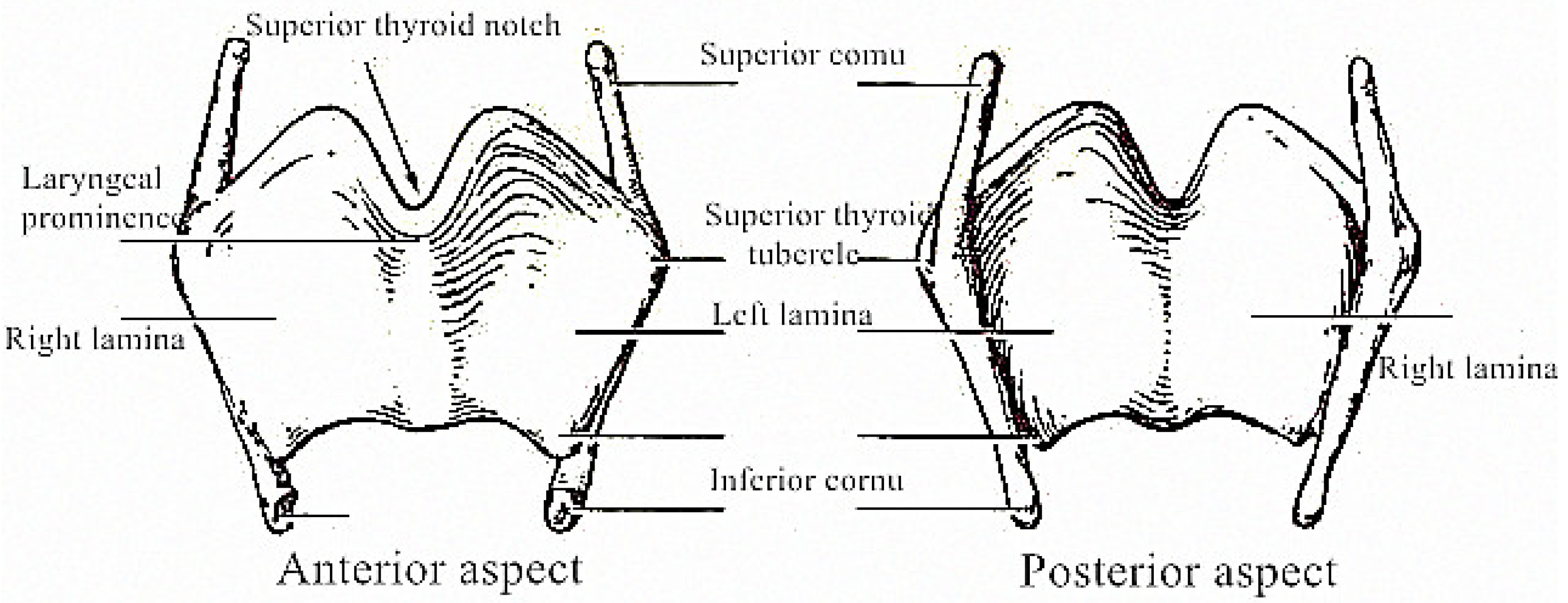
3.1. Design of Solid Thyroid Cartilage Prosthesis
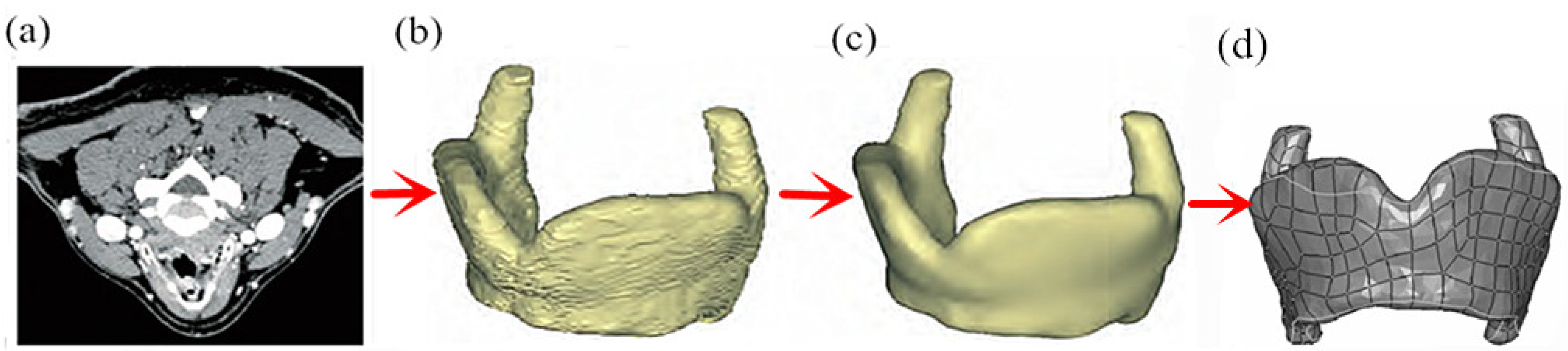
3.2. Parametric Modeling of Total Resection of Thyroid Cartilage Prosthesis
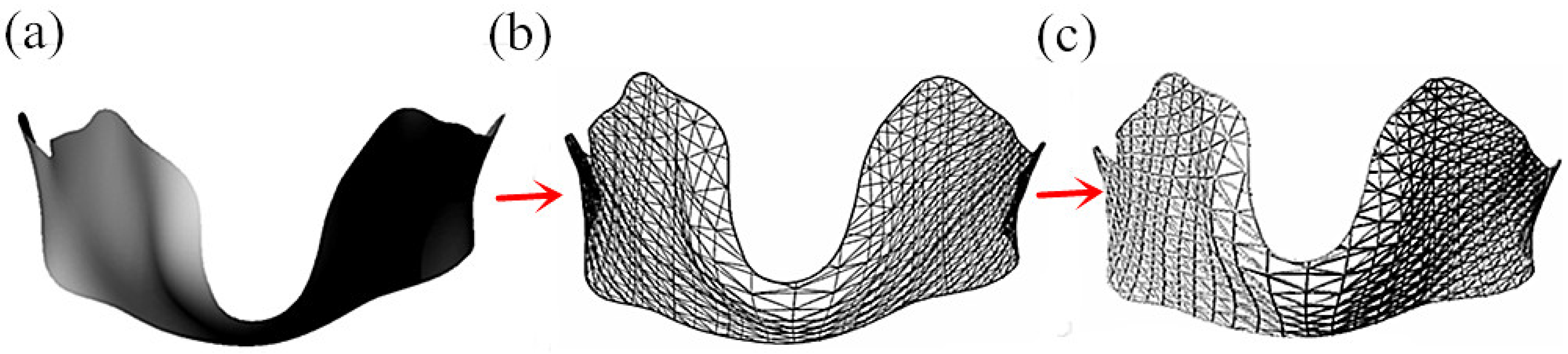
3.2.1. Single-Layer Grid Structure of Thyroid Cartilage
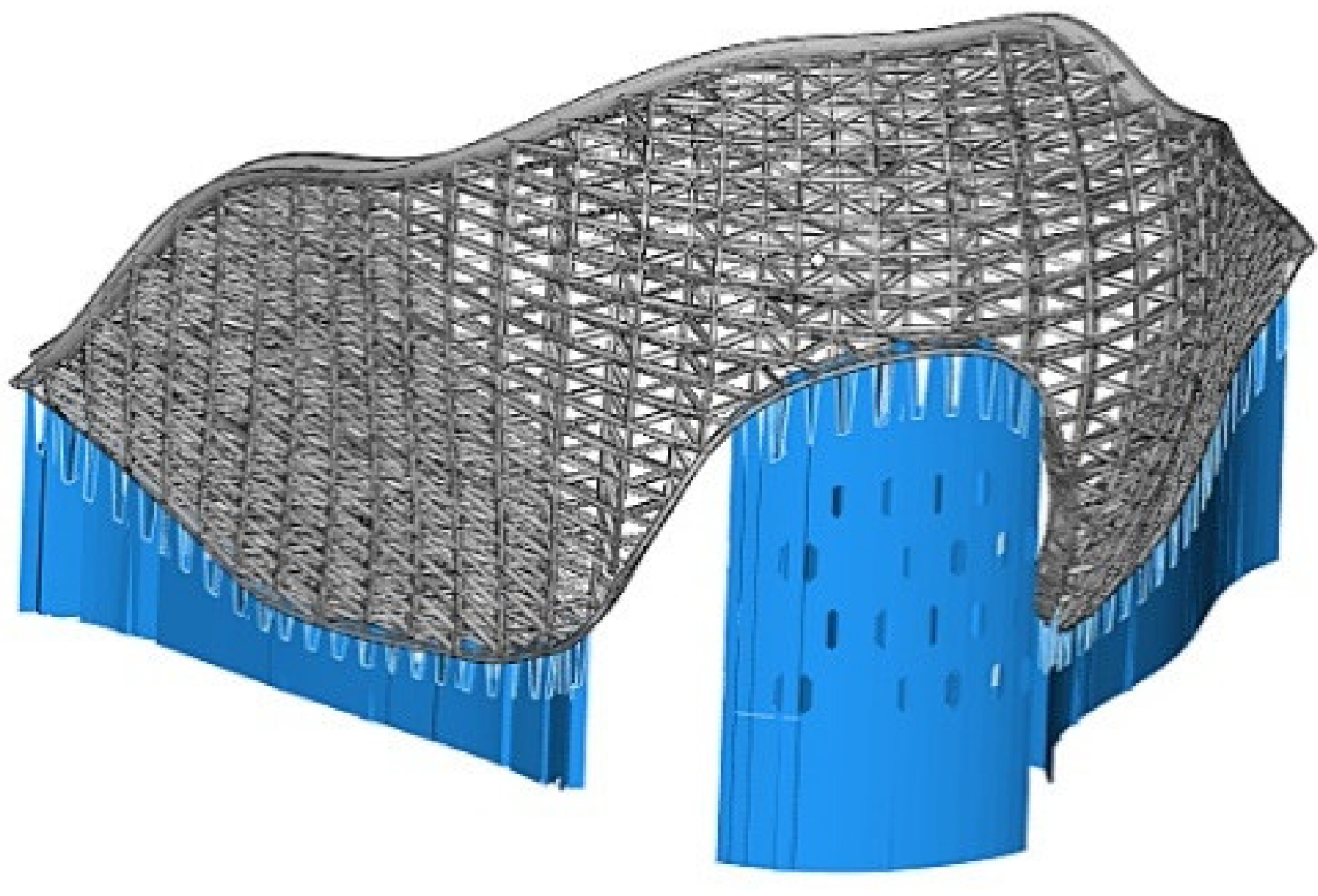
3.2.2. Multi-Layer Thyroid Cartilage Structure
3.3. Parametric Modeling of Prosthesis of Thyroid Cartilage with Partial Resection
3.4. Research on the Technology of SLM Molding Thyroid Cartilage Prosthesis
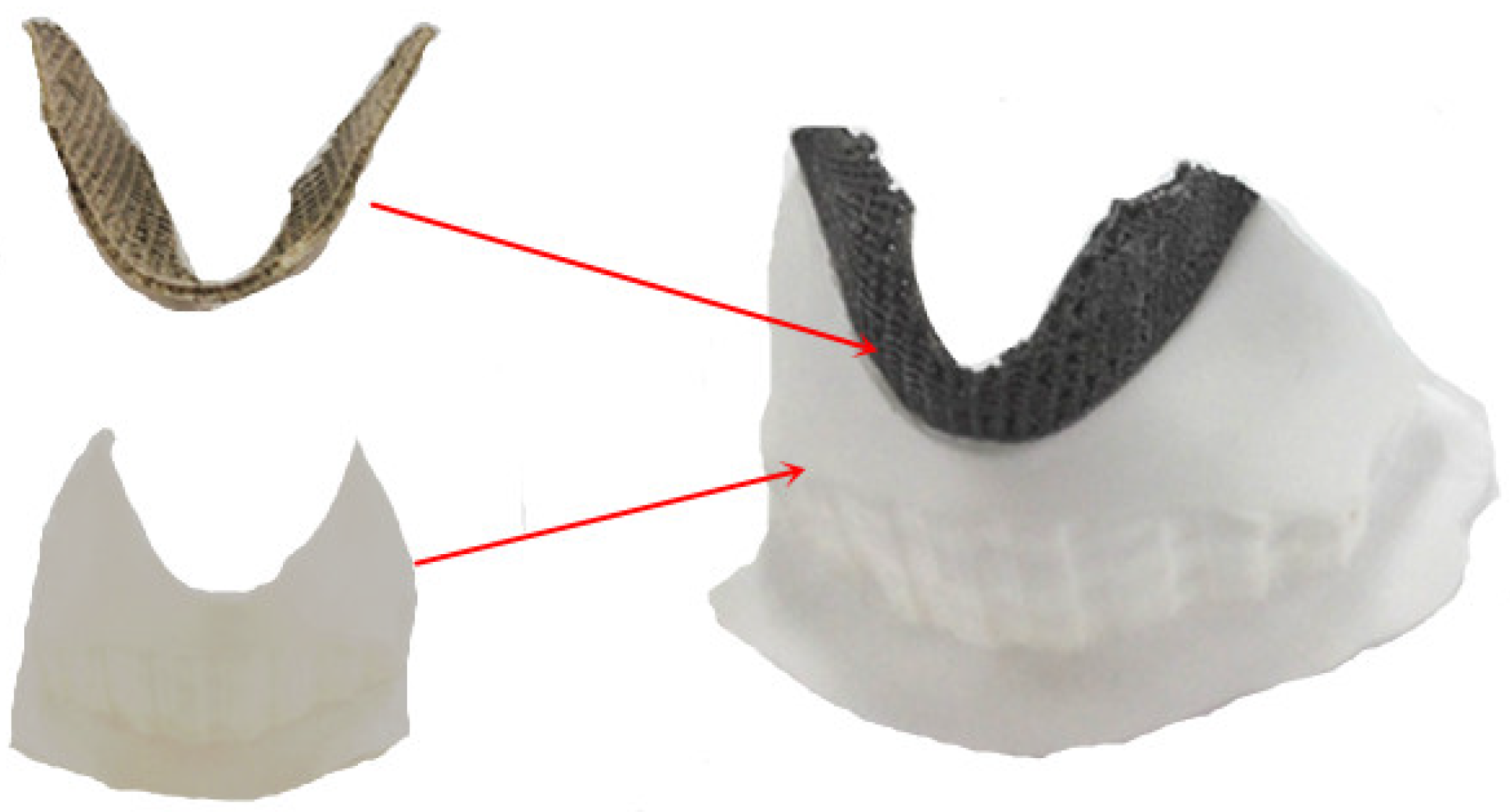
3.5. Matching Test of SLM Molding Thyroid Cartilage Prosthesis
4. Conclusions
- A thyroid cartilage prosthesis was designed using a parameterized method with total or partial resection. The modeling effect was good, with good matching to unaffected sites, good overlap between pillars, appropriate pore size that meets the requirements of biocompatibility, and good overlap suspension between pillars to meet the requirements of SLM manufacturing.
- The thyroid cartilage prosthesis prepared by SLM molding has better overlap between porous pillars, better connectivity between pores, less powder adhesion on the surface, and no obvious nodulation between porous pillars. The resulting prosthesis can be directly used after simple sandblasting and polishing.
- The resulting thyroid cartilage prosthesis fits well with the arc-shaped groove of the unresected part of the thyroid cartilage and the convex part of the thyroid cartilage, providing a smooth transition between the prosthesis model and the unresected part so that the prosthesis can be directly used after heat treatment and anodizing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, Z.; Zhang, J.; MA, S.; MA, Z.; SI, Y.; Qiao, X. Allogenic chondrocytes-polyglycolic acid compound for repair of thyroid cartilage defects. Chin. J. Tissue Eng. Res. 2016, 20, 1711–1717. [Google Scholar]
- Mobbs, R.J.; Coughlan, M.; Thompson, R.; Sutterlin, C.E.; Phan, K. The utility of 3D printing for surgical planning and patient-specific implant design for complex spinal pathologies: Case report. J. Neurosurg. Spine 2017, 26, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Su, H.J.; Wei, K.C.; Guo, W.; Ma, L.W.; Yu, R.L.; Zhang, B.; Fu, H.Z. New development of laser rapid forming and its application in high performance materials processing. Chin. J. Nonferrous Met. 2013, 23, 1567–1574. [Google Scholar]
- Wei, C.; Li, L.; Zhang, X.; Chueh, Y.-H. 3D printing of multiple metallic materials via modified selective laser melting. CIRP Ann. 2018, 67, 245–248. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Krawczynska, A.; Rożniatowski, K.; Zdunek, J.; Kurzydlowski, K.; Swieszkowski, W. Microstructure and mechanical properties investigation of CP titanium processed by selective laser melting (SLM). J. Mater. Process. Technol. 2017, 241, 13–23. [Google Scholar] [CrossRef]
- Wang, L.; Lin, D.; Liang, C. The effect of several repair methods in partial laryngectomy. West China Med. 1997, 2, 82–83. [Google Scholar]
- Liu, Y.; Zhou, J. Bone morphogenetic protein complex combined with CAD/CAM technique in the in situ repair of laryngeal cartilage defects. Res. Tissue Eng. China 2015, 19, 8228–8233. [Google Scholar]
- Feng, H.; Chen, J.; Zhao, F. Application of two methods to reconstruction of laryngeal function after partial laryngectomy for laryngeal carcinoma. Chin. J. Mod. Med. 2016, 26, 132–136. [Google Scholar]
- Wang, Y.; Huang, Y.; Liu, A. Preparation of HA foam ceramics by direct foaming for repairing Thy-Roid cartilage damage. Mod. Hosp. 2014, 14, 20–22. [Google Scholar]
- Zhen, T.; Jianjun, Y.; Xiao, Z.; Xing, C.; Liang, Z.; Shuichao, G.; Xu, C.; Bo, S.; Xuekui, L. Application of digital technique combined with 3D printing technique in reconstruction of thyroid cartilage in partial laryngectomy. Chin. Otorhinolaryngol. Head Neck Surg. 2020, 27, 20–24. [Google Scholar]
- Zhang, L.; Hu, W.; Lin, W.; Cao, G.; Huang, H.; Xia, W.; Lu, M.; Wei, G. Experimental study on repair of thyroid cartilage defect with rhBMP-2/Cha. J. Huazhong Univ. Sci. Technol. 2014, 43, 316–320. [Google Scholar]
- Kranti, B.; Tyagi, I.; Ramani, M.K. Laryngotracheal reconstruction using iliac crest graft: An institutional experience. Indian J. Otolaryngol. Head Neck Surg. 2010, 62, 75–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hallak, B.; Von Wihl, S.; Boselie, F.; Bouayed, S. Repair of displaced thyroid cartilage fracture using miniplate osteosynthesis. BMJ Case Rep. 2018, 11, e226677. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Xu, J. Biocompatibility of hip prosthesis materials. J. Clin. Rehabil. Tissue Eng. Res. 2008, 12, 7682–7699. [Google Scholar]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; He, S.; He, D. Compression and energy absorption property of porous aluminum alloy gradient. Mater. Mech. Eng. 2010, 24, 77–78. [Google Scholar]
- Yan, N.; Hou, T. Porosity of Biomaterials and Bone Ingrowth. Chin. J. Biomed. Eng. 2008, 27, 612–613. [Google Scholar]
- Zhang, X.-W.; Li, C.; Zhang, Q.-M. Mechanical Properties of Porous Metal Materials and Their Dependence on Geometric Parameters. J. Mater. Eng. 2014, 4, 55–59. [Google Scholar]
- Spector, M.; Michno, M.J.; Smarook, W.H.; Kwiatkowski, G.T. A high-modulus polymer for porous orthopedic implants: Biomechanical compatibility of porous implants. J. Biomed. Mater. Res. 1978, 12, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D. Porous structure modeling and laser selective melting direct manufacturing for implants. Ph.D. Thesis, South China University of Technology, Guangzhou, China, March 2013. [Google Scholar]
- Wang, X.; Xiao, Z.; Zhang, G.; Guan, H.; Yang, Y. Effect of tilt angle on laser selective melting of Ti6Al4V alloy. Powder Metall. Mater. Sci. Eng. 2016, 21, 376–382. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Y.; Zhang, Z.; Song, C.; Wang, A.; Yu, J. Optimal Design of Support Structures in Selective Laser Melting of Parts. Chin. J. Lasers 2016, 43, 59–66. [Google Scholar]
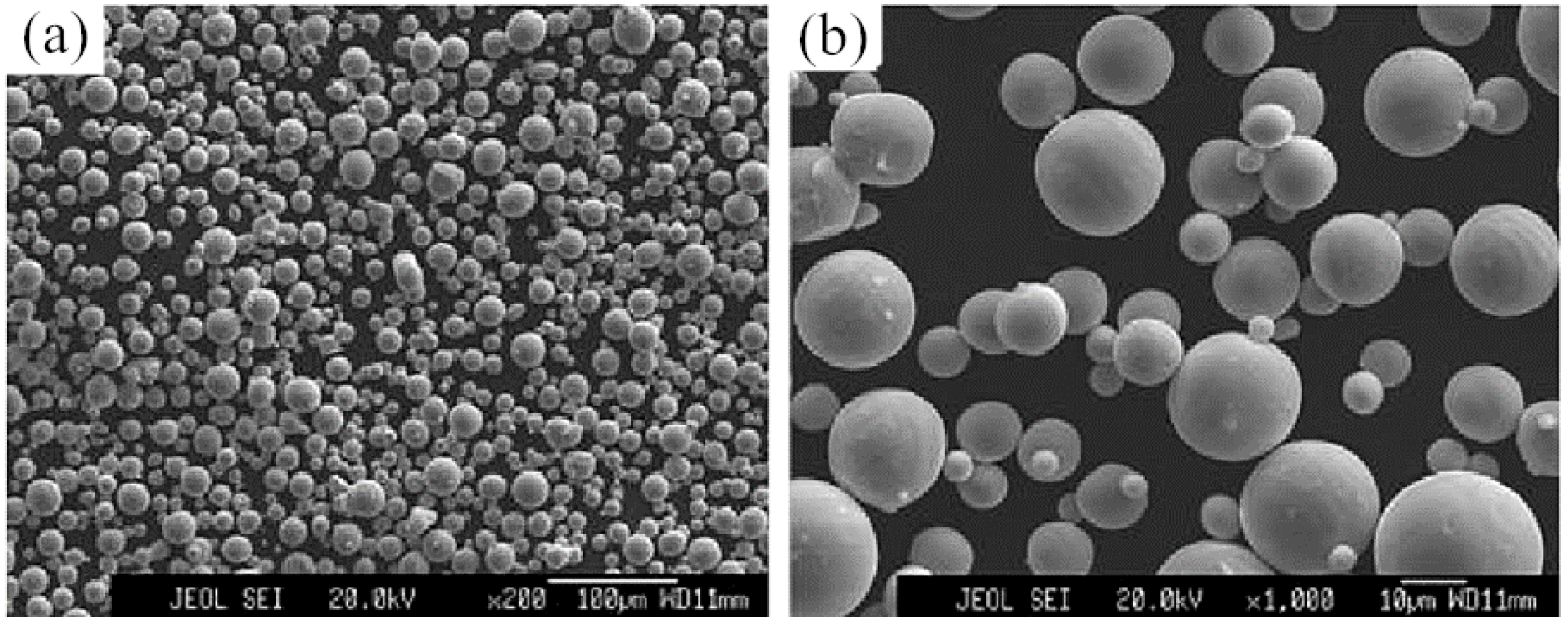




| Element | Ti6Al4V Powder | ASTM F75 Standard | Element | Ti6Al4V Powder | ASTM F75 Standard |
|---|---|---|---|---|---|
| Al | 5.5–6.5% | 5.5–6.5% | N | 0.03% | <0.05% |
| V | 3.5–4.5% | 3.5–4.5% | H | 0.012% | <0.012% |
| Fe | 0.25% | <0.3% | O | <0.08% | <0.13% |
| C | 0.08% | <0.08% | Ti | Balance | Balance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Li, J.; Zhang, C.; Wang, A. Design and Molding of Thyroid Cartilage Prosthesis Based on 3D Printing Technology. Coatings 2022, 12, 336. https://doi.org/10.3390/coatings12030336
Zhang G, Li J, Zhang C, Wang A. Design and Molding of Thyroid Cartilage Prosthesis Based on 3D Printing Technology. Coatings. 2022; 12(3):336. https://doi.org/10.3390/coatings12030336
Chicago/Turabian StyleZhang, Guoqing, Junxin Li, Chengguang Zhang, and Anmin Wang. 2022. "Design and Molding of Thyroid Cartilage Prosthesis Based on 3D Printing Technology" Coatings 12, no. 3: 336. https://doi.org/10.3390/coatings12030336
APA StyleZhang, G., Li, J., Zhang, C., & Wang, A. (2022). Design and Molding of Thyroid Cartilage Prosthesis Based on 3D Printing Technology. Coatings, 12(3), 336. https://doi.org/10.3390/coatings12030336






