The LiTFSI/COFs Fiber as Separator Coating with Bifunction of Inhibition of Lithium Dendrite and Shuttle Effect for Li-SeS2 Battery
Abstract
:1. Introduction
2. Materials and Methods
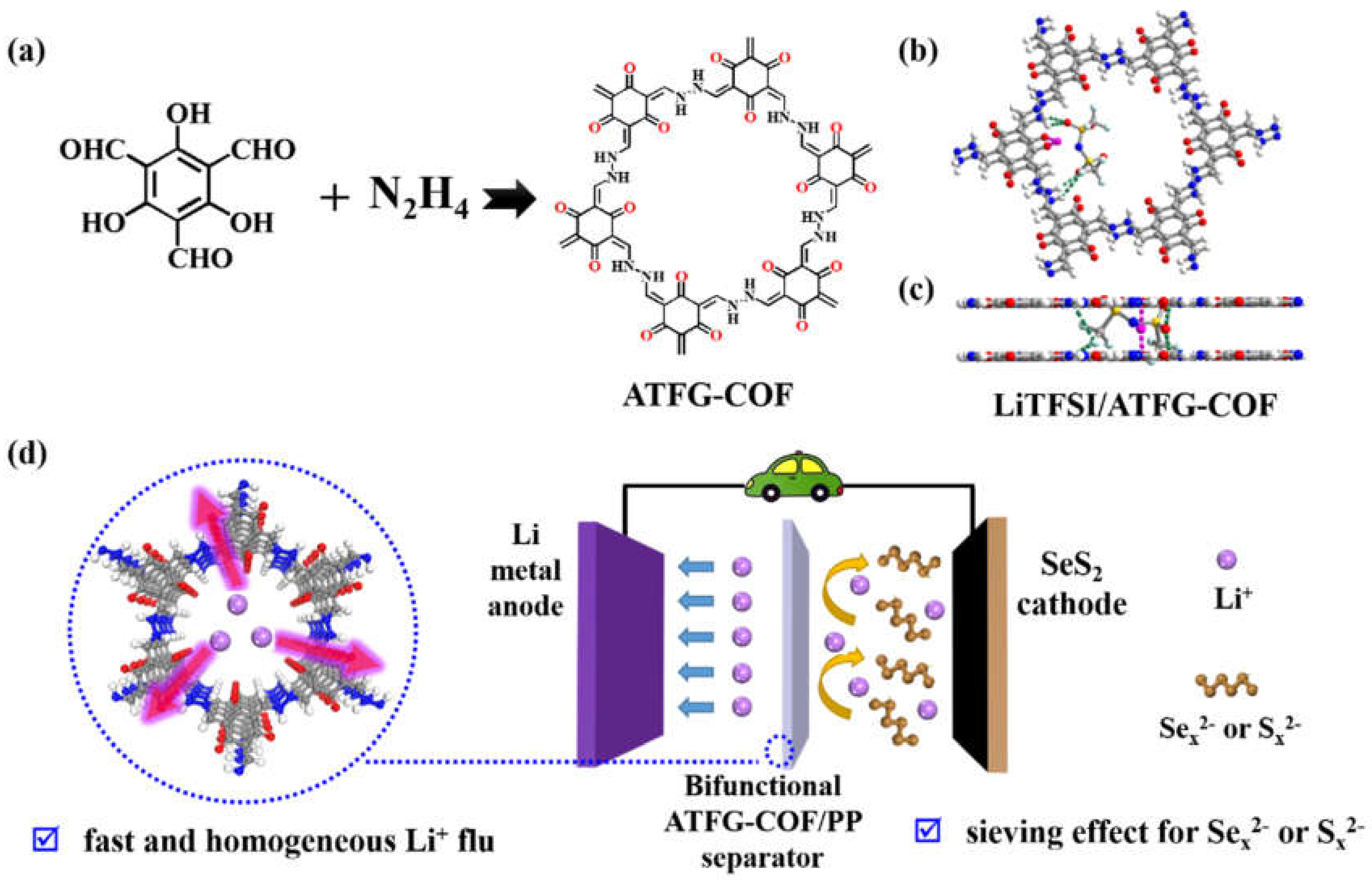
2.1. Material Selection
2.2. Preparation of ATFG-COF Fiber
2.3. Preparation of AB-COF Fiber
2.4. Preparation of ATFG-COF/PP and AB-COF/PP Separator
2.5. Preparation of SeS2 Cathode
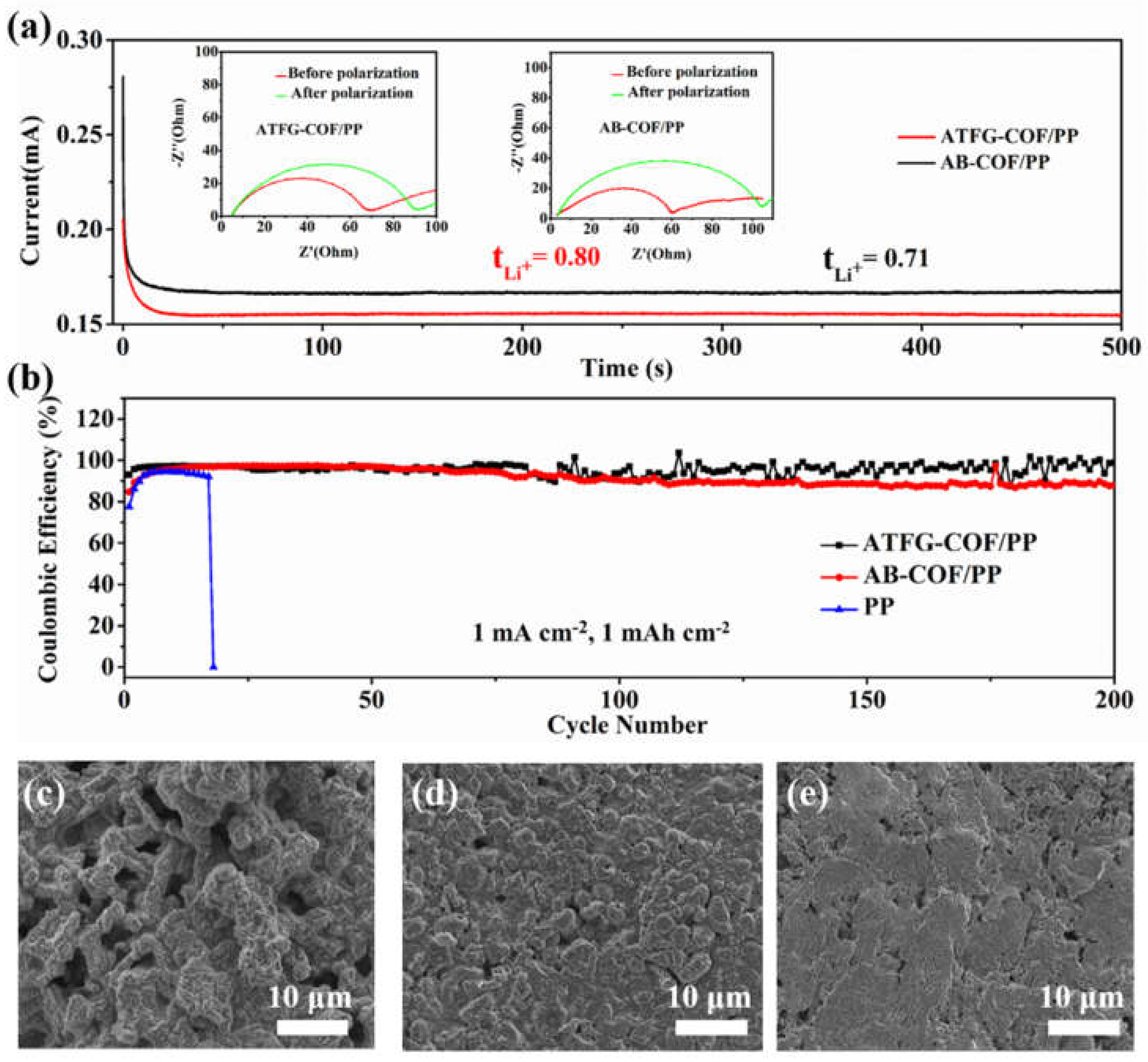
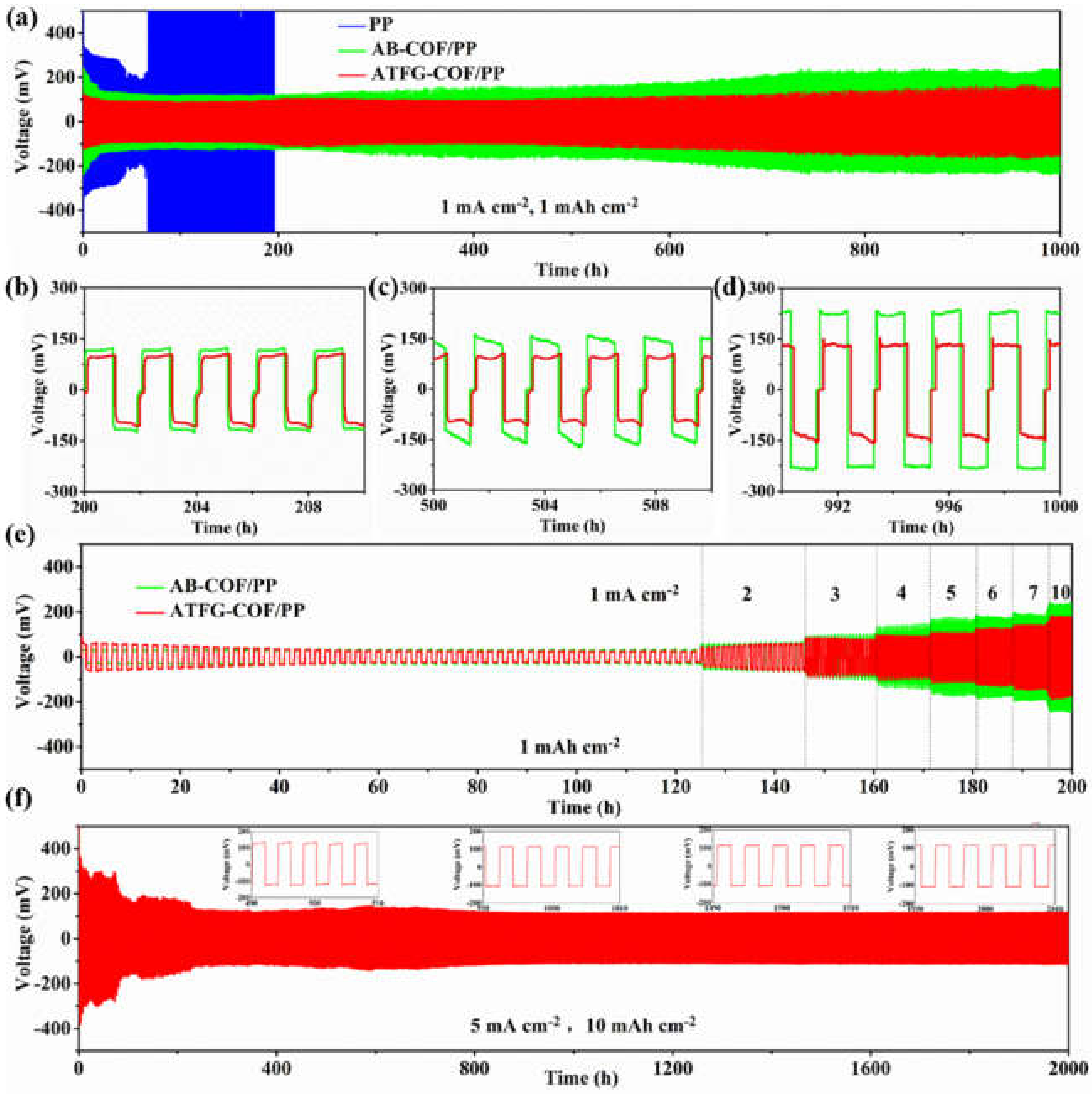
2.6. Electrochemical Measurements
2.7. Characterization
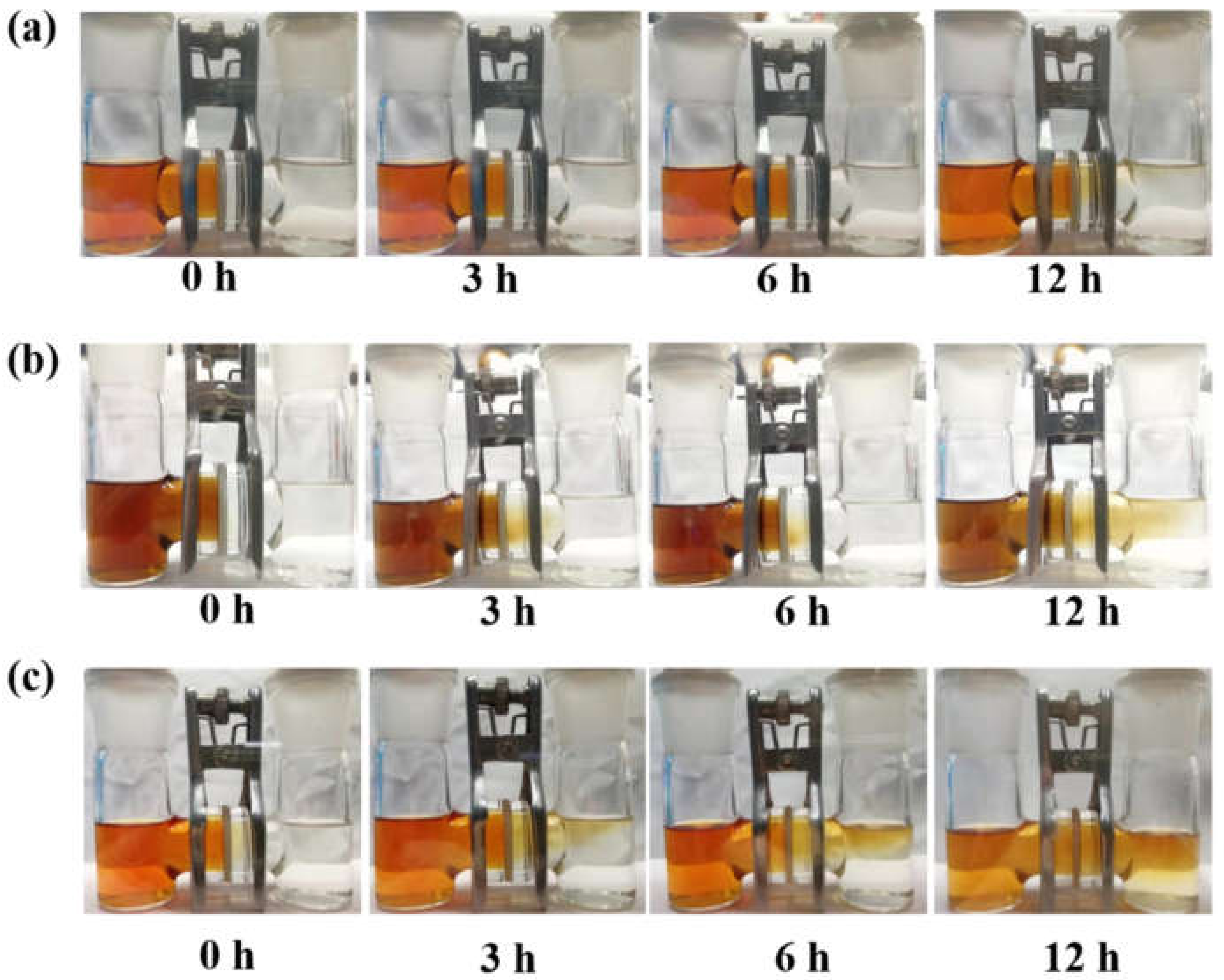
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-I.; Song, G.; Cho, S.; Han, D.-Y.; Park, S. Lithium Metal Interface Modification for High-Energy Batteries: Approaches and Characterization. Batter. Supercaps 2020, 3, 828–859. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Henzie, J.; Zhang, J.; Ha, J.; Amin, M.A.; Hossain, M.S.A.; Jun, S.C.; Yamauchi, Y. Recent Advances and Perspectives of Battery-Type Anode Materials for Potassium Ion Storage. ACS Nano 2021, 15, 18931–18973. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.-J.; Song, C.-L.; Yang, Y.; Tan, H.-C.; Li, G.-H.; Cai, Y.-P.; Wang, H. Cerium Based Metal-Organic Frameworks as an Efficient Separator Coating Catalyzing the Conversion of Polysulfides for High Performance Lithium-Sulfur Batteries. ACS Nano 2019, 13, 1923–1931. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Zhang, J.; Jun, S.C.; Yamauchi, Y. Carbonaceous Anode Materials for Non-aqueous Sodium- and Potassium-Ion Hybrid Capacitors. ACS Energy Lett. 2021, 6, 4127–4154. [Google Scholar] [CrossRef]
- Tzeng, Y.; Huang, W.-C.; Jhan, C.-Y.; Wu, Y.-H. Effects of In Situ Graphitic Nanocarbon Coatings on Cycling Performance of Silicon-Flake-Based Anode of Lithium Ion Battery. Coatings 2021, 11, 138. [Google Scholar] [CrossRef]
- Wang, W.-P.; Zhang, J.; Jia, C.; Yin, Y.-X.; You, Y.; Xin, S.; Guo, Y.-G. Solidifying Cathode-Electrolyte Interface for Lithium-Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2000791. [Google Scholar] [CrossRef]
- Hong, X.-J.; Song, C.-L.; Wu, Z.-M.; Li, Z.-H.; Cai, Y.-P.; Wang, C.-X.; Wang, H. Sulfophilic and lithophilic sites in bimetal nickel-zinc carbide with fast conversion of polysulfides for high-rate Li-S battery. Chem. Eng. J. 2021, 404, 126566. [Google Scholar] [CrossRef]
- Zhou, H.-J.; Song, C.-L.; Si, L.-P.; Hong, X.-J.; Cai, Y.-P. The development of catalyst materials for the advanced lithium-sulfur battery. Catalysts 2020, 10, 682. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Z.; Li, P.; Meng, X.; Wang, R. Ultrahigh volumetric capacity enabled by dynamic evolutions of host-guest pairs in self-supporting lithium-sulfur batteries. Nano Energy 2020, 70, 104522. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, Z.; Li, P.; Meng, X.; Wang, R. Enhanced Chemisorption and Catalytic Effects toward Polysulfides by Modulating Hollow Nanoarchitectures for Long-Life Lithium-Sulfur Batteries. Small 2020, 16, 1906114. [Google Scholar] [CrossRef]
- Hong, Y.-S.; Zhao, C.-Z.; Xiao, Y.; Xu, R.; Xu, J.-J.; Huang, J.-Q.; Zhang, Q.; Yu, X.; Li, H. Safe Lithium-Metal Anodes for Li-O2 Batteries: From Fundamental Chemistry to Advanced Characterization and Effective Protection. Batter. Supercaps 2019, 2, 638–658. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Guan, B.Y.; Lou, X.W. Mesoporous Carbon@Titanium Nitride Hollow Spheres as an Efficient SeS2 Host for Advanced Li-SeS2 Batteries. Angew. Chem. Int. Ed. 2017, 56, 16003–16007. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Wang, B.; Wei, Y.; Jing, P.; Wu, H.; Dai, Z.; Wang, M.; Zhang, Y. An integrated hybrid interlayer for polysulfides/selenides regulation toward advanced Li-SeS2 batteries. Carbon 2020, 161, 413–422. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, X.-J.; Song, C.-L.; Li, G.-H.; Zheng, Y.-X.; Zhou, D.-D.; Zhang, M.; Cai, Y.-P.; Wang, H. Lithium bis(trifluoromethanesulfonyl)imide assisted dual-functional separator coating materials based on covalent organic frameworks for high-performance lithium-selenium sulfide batteries. J. Mater. Chem. A 2019, 7, 16323–16329. [Google Scholar] [CrossRef]
- Guo, B.; Yang, T.; Du, W.; Ma, Q.; Zhang, L.-Z.; Bao, S.-J.; Li, X.; Chen, Y.; Xu, M. Double-walled N-doped carbon@NiCo2S4 hollow capsules as SeS2 hosts for advanced Li–SeS2 batteries. J. Mater. Chem. A 2019, 7, 12276–12282. [Google Scholar] [CrossRef]
- Chen, T.; Kong, W.; Fan, M.; Zhang, Z.; Wang, L.; Chen, R.; Hu, Y.; Ma, J.; Jin, Z. Chelation-assisted formation of multi-yolk–shell Co4N@carbon nanoboxes for self-discharge-suppressed high-performance Li–SeS2 batteries. J. Mater. Chem. A 2019, 7, 20302–20309. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Ruan, Z.-Q.; He, Q.-T.; Hong, X.-J.; Song, X.; Zheng, Q.-F.; Nie, J.-H.; Cai, Y.-P.; Wang, H. Three-Dimensional (3D) Nanostructured Skeleton Substrate Composed of Hollow Carbon Fiber/Carbon Nanosheet/ZnO for Stable Lithium Anode. ACS Appl. Mater. Interfaces 2021, 13, 3078–3088. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, R.; Shi, P.; Chen, X.; Zhang, Q. How Does External Pressure Shape Li Dendrites in Li Metal Batteries? Adv. Energy Mater. 2021, 11, 2003416. [Google Scholar] [CrossRef]
- Luo, D.; Zheng, L.; Zhang, Z.; Li, M.; Chen, Z.; Cui, R.; Shen, Y.; Li, G.; Feng, R.; Zhang, S.; et al. Constructing multifunctional solid electrolyte interface via in-situ polymerization for dendrite-free and low N/P ratio lithium metal batteries. Nat. Commun. 2021, 12, 186. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, X.-Q.; Shen, X.; Zhang, R.; Liu, H.; Zhang, Q. A Review of Composite Lithium Metal Anode for Practical Applications. Adv. Mater. Technol. 2020, 5, 1900806. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.-H.; Bai, Z.; Li, M.; Yu, A.; Luo, D.; Liu, W.; Yang, L.; Lu, J.; Amine, K.; Chen, Z. Developing high safety Li-metal anodes for future high-energy Li-metal batteries: Strategies and perspectives. Chem. Soc. Rev. 2020, 49, 5407–5445. [Google Scholar] [CrossRef]
- Ye, H.; Zheng, Z.-J.; Yao, H.-R.; Liu, S.-C.; Zuo, T.-T.; Wu, X.-W.; Yin, Y.-X.; Li, N.-W.; Gu, J.-J.; Cao, F.-F.; et al. Guiding Uniform Li Plating/Stripping through Lithium-Aluminum Alloying Medium for Long-Life Li Metal Batteries. Angew. Chem. Int. Ed. 2019, 58, 1094–1099. [Google Scholar] [CrossRef]
- Wang, S.-H.; Yue, J.; Dong, W.; Zuo, T.-T.; Li, J.-Y.; Liu, X.; Zhang, X.-D.; Liu, L.; Shi, J.-L.; Yin, Y.-X.; et al. Tuning wettability of molten lithium via a chemical strategy for lithium metal anodes. Nat. Commun. 2019, 10, 4930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.-Y.; Zeng, X.-X.; Zhang, X.-D.; Zuo, T.-T.; Yan, M.; Yin, Y.-X.; Shi, J.-L.; Wu, X.-W.; Guo, Y.-G.; Wan, L.-J. Engineering Janus Interfaces of Ceramic Electrolyte via Distinct Functional Polymers for Stable High-Voltage Li-Metal Batteries. J. Am. Chem. Soc. 2019, 141, 9165–9169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Eshetu, G.G.; Judez, X.; Li, C.; Armand, M. Electrolyte Additives for Lithium Metal Anodes and Rechargeable Lithium Metal Batteries: Progress and Perspectives. Angew. Chem. 2018, 57, 15002–15027. [Google Scholar] [CrossRef] [PubMed]
- Zachman, M.J.; Tu, Z.; Choudhury, S.; Archer, L.A.; Kourkoutis, L.F. Cryo-STEM mapping of solid–liquid interfaces and dendrites in lithium-metal batteries. Nature 2018, 560, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Pei, A.; Oh, J.Y.; Wang, G.-J.N.; Cui, Y.; Bao, Z. Effects of Polymer Coatings on Electrodeposited Lithium Metal. J. Am. Chem. Soc. 2018, 140, 11735–11744. [Google Scholar] [CrossRef]
- Liu, Y.; Tzeng, Y.-K.; Lin, D.; Pei, A.; Lu, H.; Melosh, N.A.; Shen, Z.-X.; Chu, S.; Cui, Y. An Ultrastrong Double-Layer Nanodiamond Interface for Stable Lithium Metal Anodes. Joule 2018, 2, 1595–1609. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.; Zhang, J.; Chen, X.; Zhang, X.-D.; Li, J.-Y.; Huang, L.-B.; Zhang, X.; Shi, J.-L.; Yin, Y.-X.; Zhang, Q.; et al. Uniform Nucleation of Lithium in 3D Current Collectors via Bromide Intermediates for Stable Cycling Lithium Metal Batteries. J. Am. Chem. Soc. 2018, 140, 18051–18057. [Google Scholar] [CrossRef]
- Song, C.-L.; Li, Z.-H.; Ma, L.-Y.; Li, M.-Z.; Huang, S.; Hong, X.-J.; Cai, Y.-P.; Lan, Y.-Q. Single-Atom Zinc and Anionic Framework as Janus Separator Coatings for Efficient Inhibition of Lithium Dendrites and Shuttle Effect. ACS Nano 2021, 15, 13436–13443. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Lou, X.W. A Freestanding Selenium Disulfide Cathode Based on Cobalt Disulfide-Decorated Multichannel Carbon Fibers with Enhanced Lithium Storage Performance. Angew. Chem. Int. Ed. 2017, 56, 14107–14112. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Wu, H.B.; Lou, X.W. An Improved Li–SeS2 Battery with High Energy Density and Long Cycle Life. Adv. Energy Mater. 2017, 7, 1700281. [Google Scholar] [CrossRef]
- Zhong, H.; Sa, R.; Lv, H.; Yang, S.; Yuan, D.; Wang, X.; Wang, R. Covalent Organic Framework Hosting Metalloporphyrin-Based Carbon Dots for Visible-Light-Driven Selective CO2 Reduction. Adv. Funct. Mater. 2020, 30, 2002654. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef]
- Haug, W.K.; Moscarello, E.M.; Wolfson, E.R.; McGrier, P.L. The luminescent and photophysical properties of covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 839–864. [Google Scholar] [CrossRef]
- Banerjee, T.; Gottschling, K.; Savasci, G.; Ochsenfeld, C.; Lotsch, B.V.; Gottschling, K.; Savasci, G.; Ochsenfeld, C.; Lotsch, B.V.; Gottschling, K.; et al. H2 Evolution with Covalent Organic Framework Photocatalysts. ACS Energy Lett. 2018, 3, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Zhang, M.; Liu, C.-G.; Liu, J.; Shang, L.-J.; Wang, M.; Chang, J.-N.; Li, S.-L.; Lan, Y.-Q. Stable Dioxin-Linked Metallophthalocyanine Covalent Organic Frameworks (COFs) as Photo-Coupled Electrocatalysts for CO2 Reduction. Angew. Chem. Int. Ed. 2021, 133, 4914–4921. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, M.; Lang, Z.-L.; Liu, J.; Liu, M.; Chang, J.-N.; Li, L.-Y.; Shang, L.-J.; Wang, M.; Li, S.-L.; et al. semiconductor/covalent-organic-framework z-scheme heterojunctions for artificial photosynthesis. Angew. Chem. Int. Ed. 2020, 59, 6500–6506. [Google Scholar] [CrossRef]
- Chen, D.; Huang, S.; Zhong, L.; Wang, S.; Xiao, M.; Han, D.; Meng, Y. In Situ Preparation of Thin and Rigid COF Film on Li Anode as Artificial Solid Electrolyte Interphase Layer Resisting Li Dendrite Puncture. Adv. Funct. Mater. 2020, 30, 1907717. [Google Scholar] [CrossRef]
- Peng, P.; Shi, L.; Huo, F.; Zhang, S.; Mi, C.; Cheng, Y.; Xiang, Z. In Situ Charge Exfoliated Soluble Covalent Organic Framework Directly Used for Zn-Air Flow Battery. ACS Nano 2019, 13, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tu, H.; Hu, C.; Liu, Y.; Dong, D.; Sun, Y.; Dai, Y.; Wang, S.; Qian, H.; Lin, Z.; et al. Cationic Covalent Organic Framework Nanosheets for Fast Li-Ion Conduction. J. Am. Chem. Soc. 2018, 140, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Stegbauer, L.; Hahn, M.W.; Jentys, A.; Savasci, G.; Ochsenfeld, C.; Lercher, J.A.; Lotsch, B.V. Tunable Water and CO2 Sorption Properties in Isostructural Azine-Based Covalent Organic Frameworks through Polarity Engineering. Chem. Mater. 2015, 27, 7874–7881. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Chen, J.-H.; Chen, Z.-C.; Wu, Z.-Y.; Zhong, X.-N.; Ke, J.-P. The LiTFSI/COFs Fiber as Separator Coating with Bifunction of Inhibition of Lithium Dendrite and Shuttle Effect for Li-SeS2 Battery. Coatings 2022, 12, 289. https://doi.org/10.3390/coatings12020289
Wang J, Chen J-H, Chen Z-C, Wu Z-Y, Zhong X-N, Ke J-P. The LiTFSI/COFs Fiber as Separator Coating with Bifunction of Inhibition of Lithium Dendrite and Shuttle Effect for Li-SeS2 Battery. Coatings. 2022; 12(2):289. https://doi.org/10.3390/coatings12020289
Chicago/Turabian StyleWang, Jun, Jia-He Chen, Zhen-Chong Chen, Zhen-Yi Wu, Xiao-Na Zhong, and Jing-Ping Ke. 2022. "The LiTFSI/COFs Fiber as Separator Coating with Bifunction of Inhibition of Lithium Dendrite and Shuttle Effect for Li-SeS2 Battery" Coatings 12, no. 2: 289. https://doi.org/10.3390/coatings12020289
APA StyleWang, J., Chen, J.-H., Chen, Z.-C., Wu, Z.-Y., Zhong, X.-N., & Ke, J.-P. (2022). The LiTFSI/COFs Fiber as Separator Coating with Bifunction of Inhibition of Lithium Dendrite and Shuttle Effect for Li-SeS2 Battery. Coatings, 12(2), 289. https://doi.org/10.3390/coatings12020289



