Improved Cycling Performance of Cation-Disordered Rock-Salt Li1.2Ti0.4Mn0.4O2 Cathode through Mo-Doping and Al2O3-Coating
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Materials
2.2. Characterization of Materials
2.3. Electrochemical Experiments
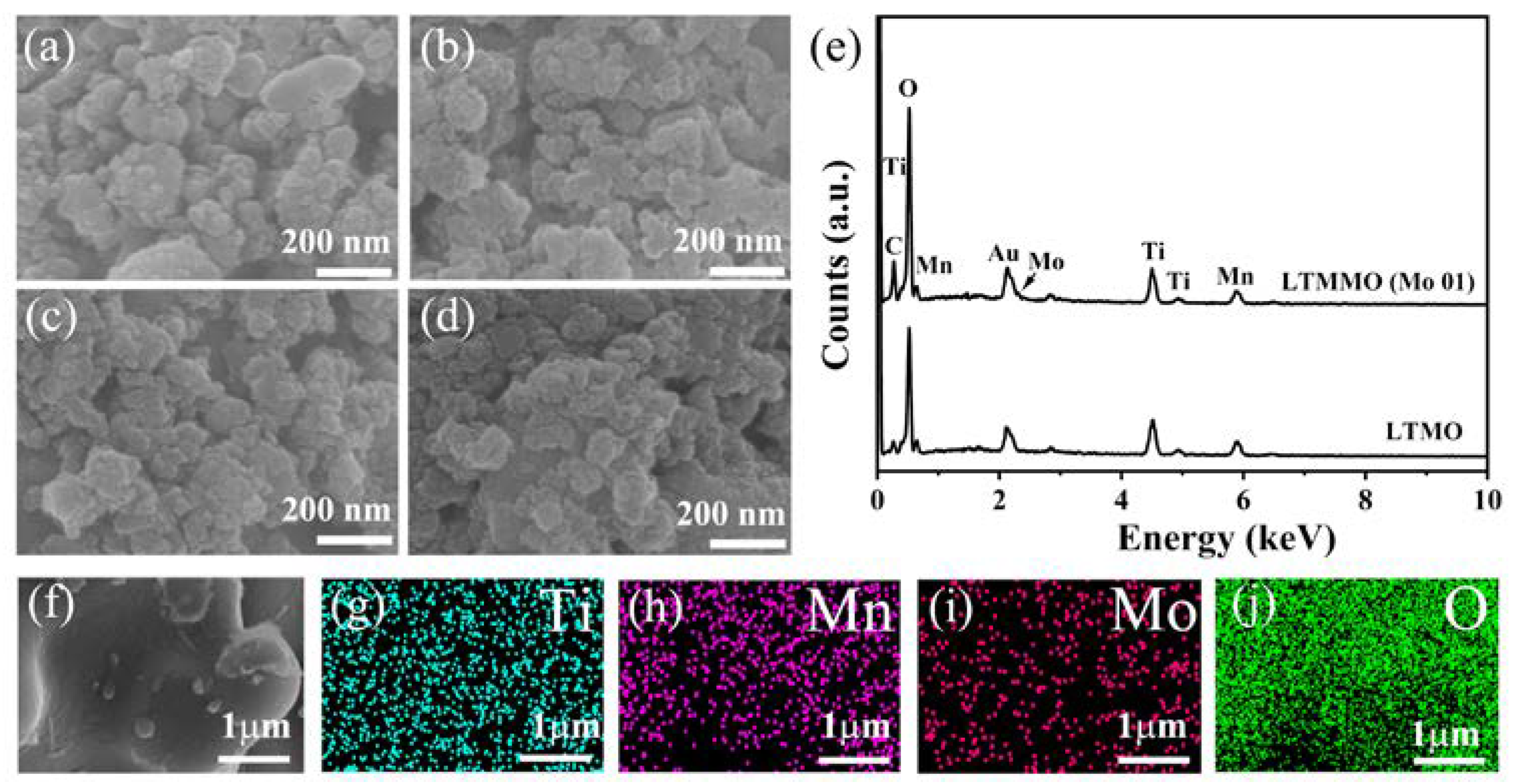
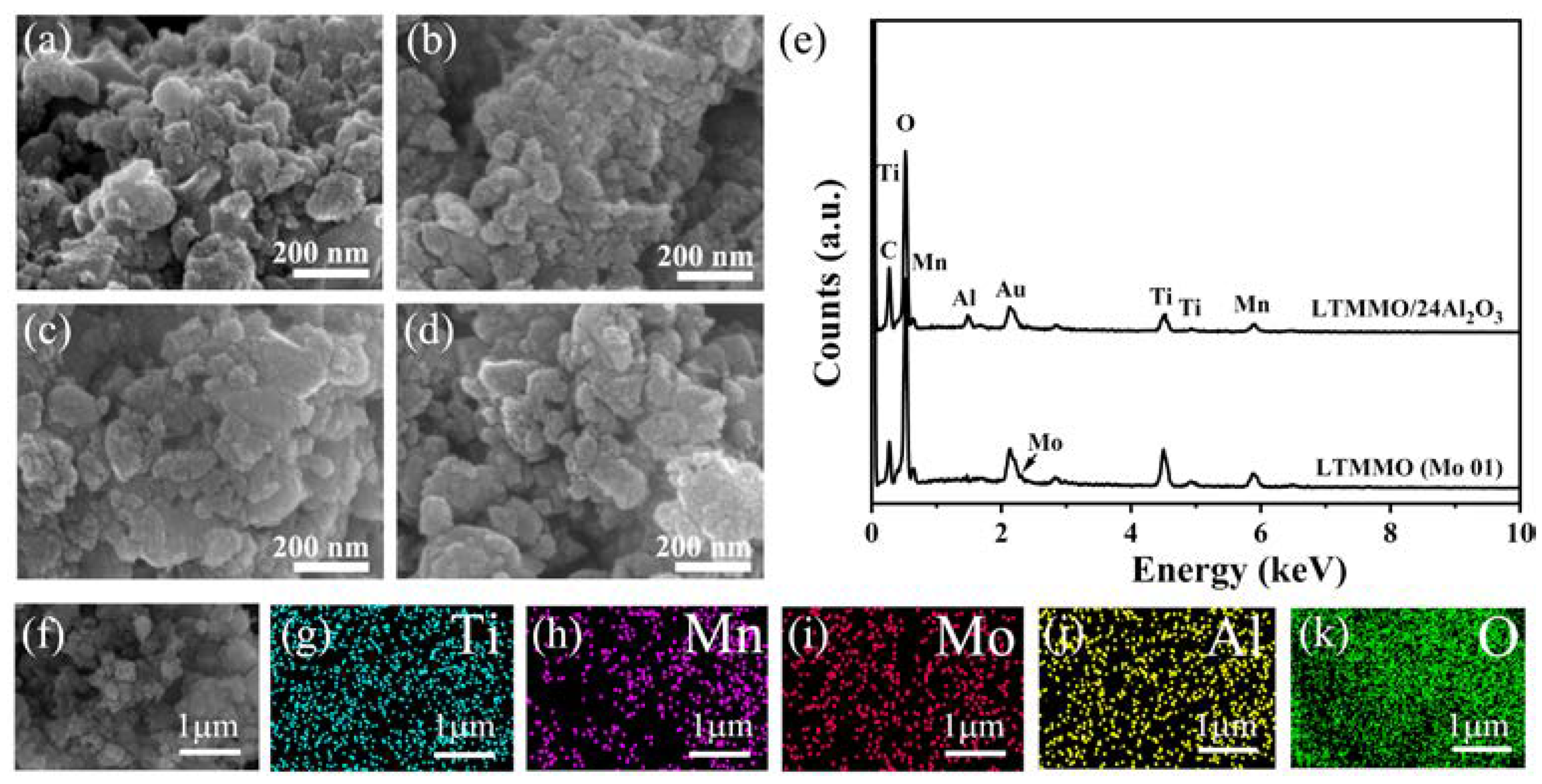
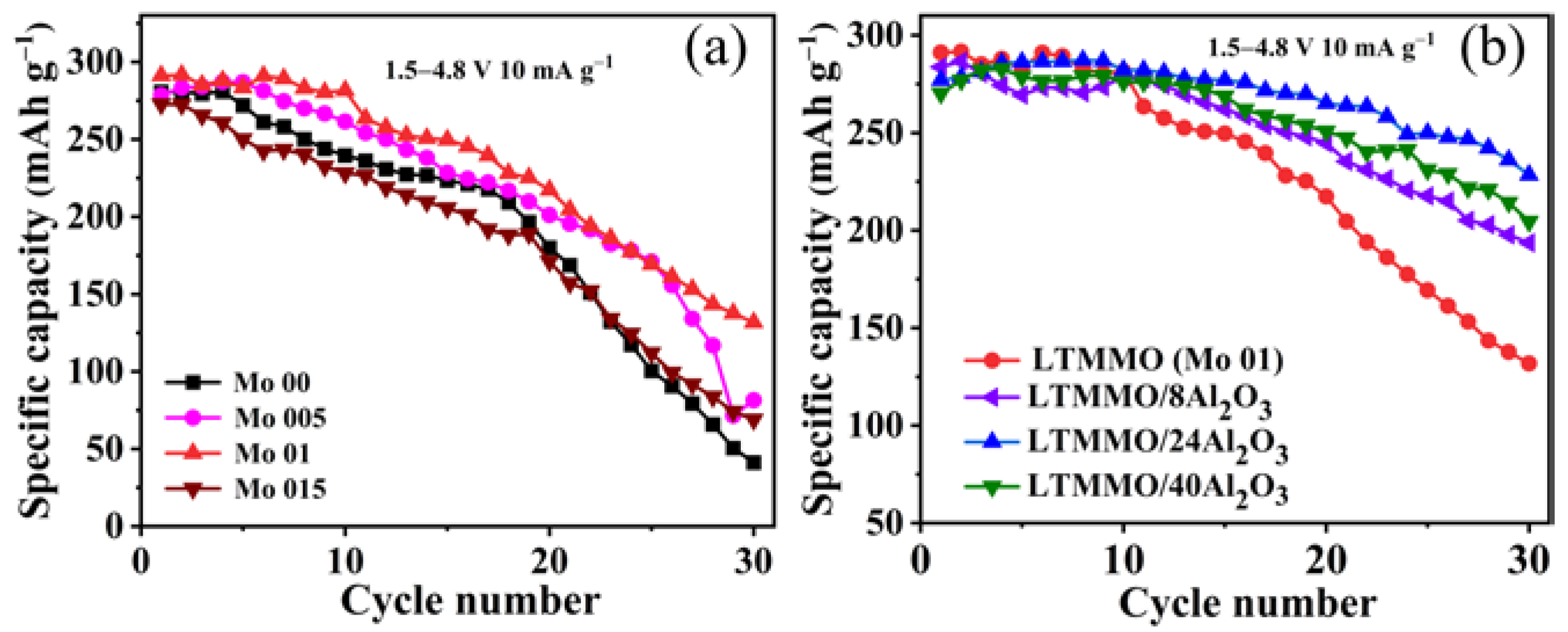
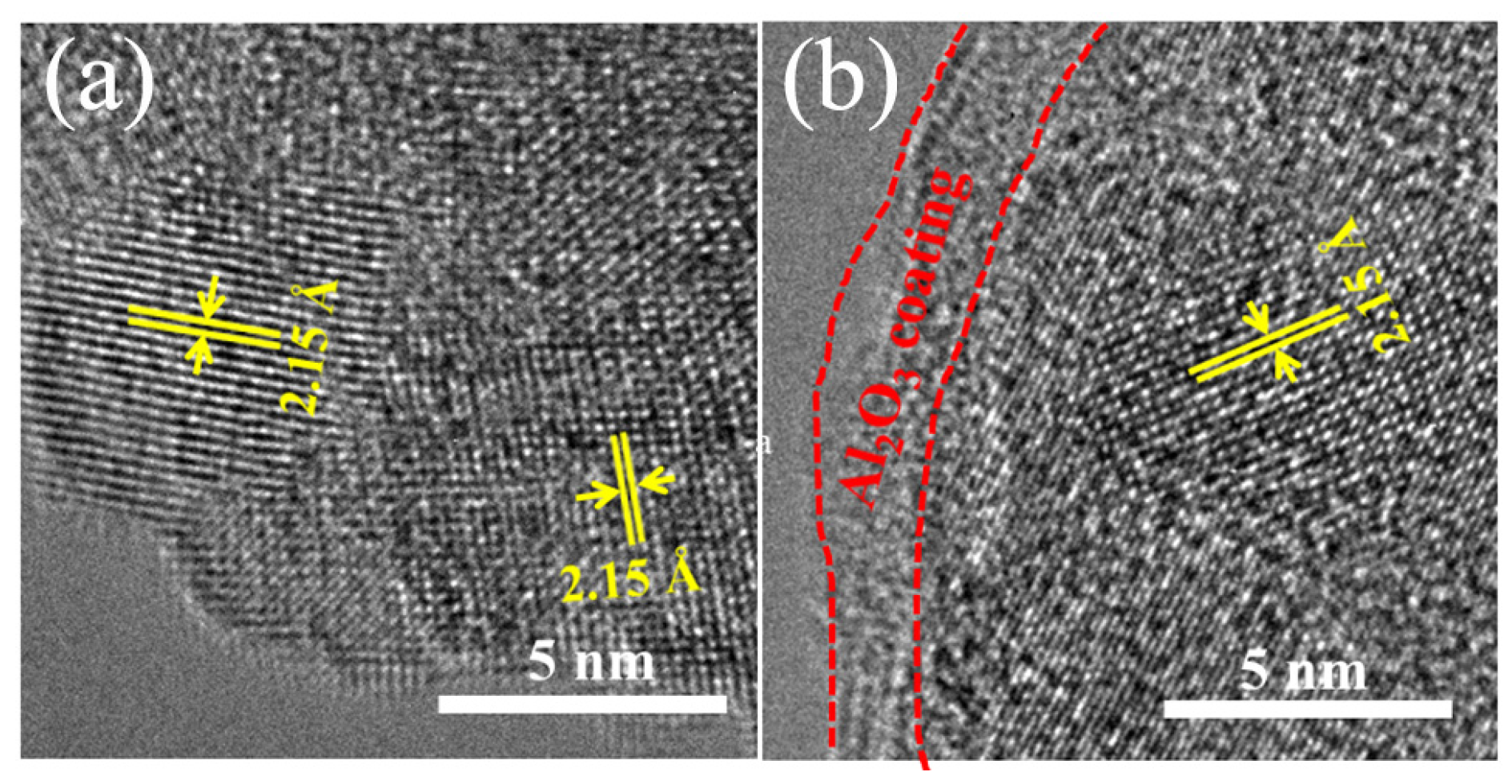
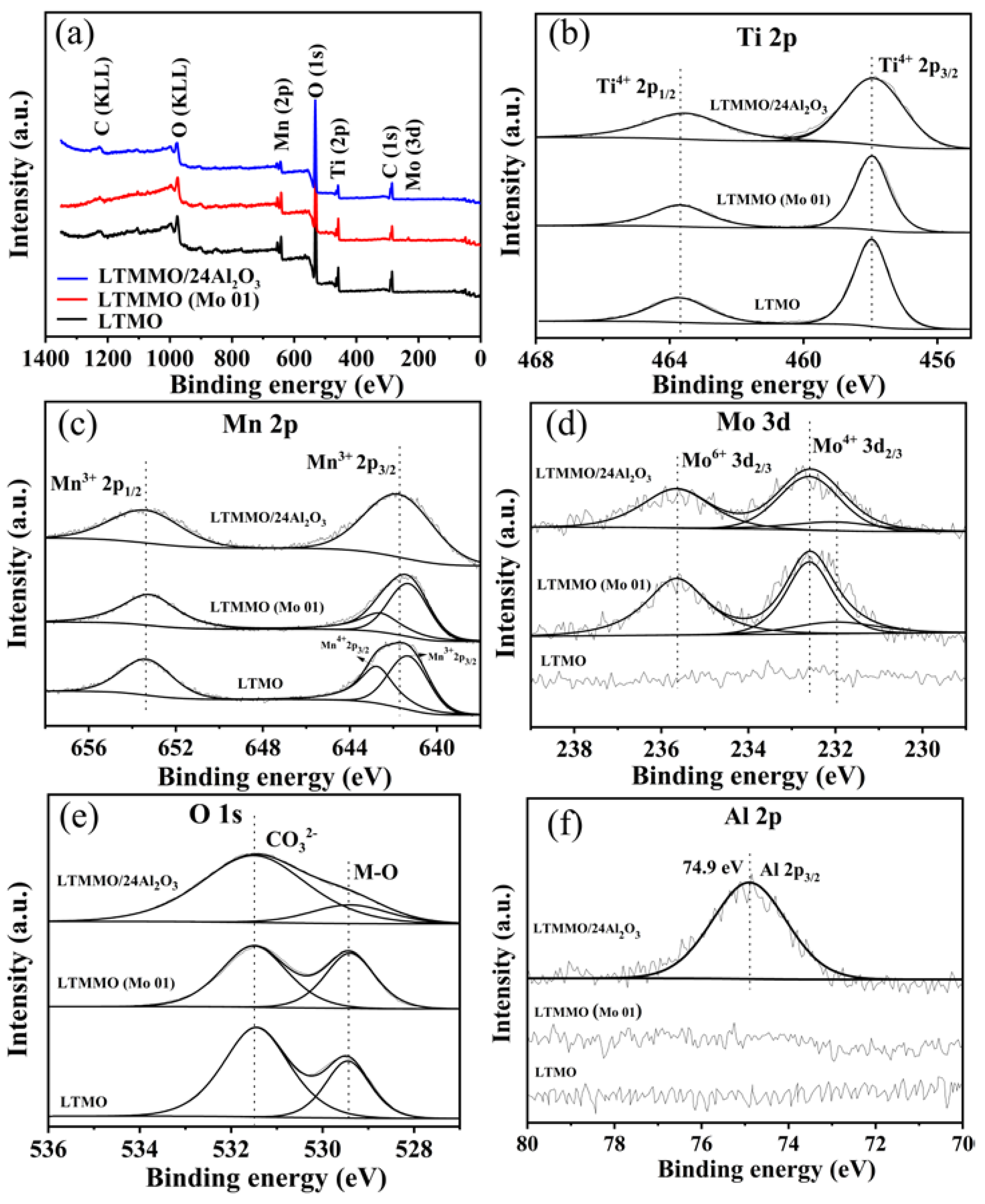
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.B. Electrochemical energy storage in a sustainable modern society. Energy Environ. Sci. 2014, 7, 14–18. [Google Scholar] [CrossRef]
- Cambaz, M.A.; Vinayan, B.P.; Euchner, H.; Johnsen, R.E.; Guda, A.A.; Mazilkin, A.; Rusalev, Y.V.; Trigub, A.L.; Gross, A.; Fichtner, M. Design of Nickel-Based Cation-Disordered Rock-Salt Oxides: The Effect of Transition Metal (M = V, Ti, Zr) Substitution in LiNi0.5Mn0.5O2 Binary Systems. ACS Appl. Mater. Interfaces 2018, 10, 21957–21964. [Google Scholar] [CrossRef]
- Han, J.; Li, H.; Kong, D.; Zhang, C.; Tao, Y.; Li, H.; Yang, Q.-H.; Chen, L. Realizing High Volumetric Lithium Storage by Compact and Mechanically Stable Anode Designs. ACS Energy Lett. 2020, 5, 1986–1995. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef]
- Chen, D.; Wu, J.; Papp, J.K.; McCloskey, B.D.; Yang, W.; Chen, G. Role of Redox-Inactive Transition-Metals in the Behavior of Cation-Disordered Rocksalt Cathodes. Small 2020, 16, e2000656. [Google Scholar] [CrossRef]
- Lee, J.; Urban, A.; Li, X.; Su, D.; Hautier, G. Unlocking the Potential of Cation Disordered Oxides for Rechargeable Lithium Batteries. Science 2014, 343, 519–522. [Google Scholar] [CrossRef]
- Kitchaev, D.A.; Lun, Z.; Richards, W.D.; Ji, H.; Clément, R.J.; Balasubramanian, M.; Kwon, D.-H.; Dai, K.; Papp, J.K.; Lei, T.; et al. Design principles for high transition metal capacity in disordered rocksalt Li-ion cathodes. Energy Environ. Sci. 2018, 11, 2159–2171. [Google Scholar] [CrossRef]
- Cai, Z.; Ji, H.; Ha, Y.; Liu, J.; Kwon, D.-H.; Zhang, Y.; Urban, A.; Foley, E.E.; Giovine, R.; Kim, H.; et al. Realizing continuous cation order-to-disorder tuning in a class of high-energy spinel-type Li-ion cathodes. Matter 2021, 4, 3897–3916. [Google Scholar] [CrossRef]
- Lee, H.; Choi, W.; Lee, W.; Shim, J.H.; Kim, Y.M.; Yoon, W.S. Impact of Local Separation on the Structural and Electrochemical Behaviors in Li2MoO3-LiCrO2 Disordered Rock-Salt Cathode Material. Adv. Energy Mater. 2020, 11, 2002958. [Google Scholar] [CrossRef]
- Kazda, T.; Vondrák, J.; Visintin, A.; Sedlaříková, M.; Tichý, J.; Čudek, P. Electrochemical performance of Mo doped high voltage spinel cathode material for lithium-ion battery. J. Energy Storage 2018, 15, 329–335. [Google Scholar] [CrossRef]
- Liu, X.; Huang, T.; Yu, A. Fe doped Li1.2Mn0.6-x/2Ni0.2-x/2FexO2 (x ≤ 0.1) as cathode materials for lithium-ion batteries. Electrochim. Acta 2014, 133, 555–563. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, M.; Hu, X.; Zhao, Z.; Wang, Y.; Lv, Z. Effects of Sn doping on the structural and electrochemical properties of Li1.2Ni0.2Mn0.8O2 Li-rich cathode materials. Electrochim. Acta 2015, 174, 1167–1174. [Google Scholar] [CrossRef]
- Huang, B.; Wang, R.; Gong, Y.; He, B.; Wang, H. Enhanced Cycling Stability of Cation Disordered Rock-Salt Li1.2Ti0.4Mn0.4O2 Material by Surface Modification With Al2O3. Front. Chem. 2019, 7, 107. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, B.; Liu, J.; Kaliyappan, K.; Sun, Q.; Liu, Y.; Dadheech, G.; Balogh, M.P.; Yang, L.; Sham, T.-K.; et al. Highly stable Li1.2Mn0.54Co0.13Ni0.13O2 enabled by novel atomic layer deposited AlPO4 coating. Nano Energy 2017, 34, 120–130. [Google Scholar] [CrossRef]
- Zhang, X.; Belharouak, I.; Li, L.; Lei, Y.; Elam, J.W.; Nie, A.; Chen, X.; Yassar, R.S.; Axelbaum, R.L. Structural and Electrochemical Study of Al2O3 and TiO2Coated Li1.2Ni0.13Mn0.54Co0.13O2 Cathode Material Using ALD. Adv. Energy Mater. 2013, 3, 1299–1307. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y. Atomic layer deposition of epitaxial ZrO2 coating on LiMn2O4 nanoparticles for high-rate lithium ion batteries at elevated temperature. Nano Energy 2013, 2, 882–889. [Google Scholar] [CrossRef]
- Zhao, J.; Qu, G.; Flake, J.C.; Wang, Y. Low temperature preparation of crystalline ZrO2 coatings for improved elevated-temperature performances of Li-ion battery cathodes. Chem Commun. (Camb) 2012, 48, 8108–8110. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y. Surface modifications of Li-ion battery electrodes with various ultrathin amphoteric oxide coatings for enhanced cycleability. J. Solid State Electrochem. 2012, 17, 1049–1058. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Shi, B.; Ding, F.; Liu, X.; Wu, H.; Shi, C.; Zhao, N. Enhanced Cyclability of Cr8O21 Cathode for PEO-Based All-Solid-State Lithium-Ion Batteries by Atomic Layer Deposition of Al2O3. Materials 2021, 14, 5380. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Liu, L.; Lee, J.; Seo, D.-H.; Bo, S.-H.; Urban, A.; Ceder, G. A disordered rock-salt Li-excess cathode material with high capacity and substantial oxygen redox activity: Li1.25Nb0.25Mn0.5O2. Electrochem. Commun. 2015, 60, 70–73. [Google Scholar] [CrossRef]
- Lee, J.; Wang, C.; Malik, R.; Dong, Y.; Huang, Y.; Seo, D.H.; Li, J. Determining the Criticality of Li-Excess for Disordered-Rocksalt Li-Ion Battery Cathodes. Adv. Energy Mater. 2021, 11, 2100204. [Google Scholar] [CrossRef]
- Xiao, Z.; Meng, J.; Li, Q.; Wang, X.; Huang, M.; Liu, Z.; Han, C.; Mai, L. Novel MOF shell-derived surface modification of Li-rich layered oxide cathode for enhanced lithium storage. Sci. Bull. 2018, 53, 46–53. [Google Scholar] [CrossRef]
- Kim, D.; Quang, N.D.; Hien, T.T.; Chinh, N.D.; Kim, C.; Kim, D. 3D inverse-opal structured Li4Ti5O12 Anode for fast Li-Ion storage capabilities. Electron. Mater. Lett. 2017, 13, 505–511. [Google Scholar] [CrossRef]
- Wei, H.-X.; Huang, Y.-D.; Tang, L.-B.; Yan, C.; He, Z.-J.; Mao, J.; Dai, K.; Wu, X.-W.; Jiang, J.-B.; Zheng, J.-C. Lithium-rich manganese-based cathode materials with highly stable lattice and surface enabled by perovskite-type phase-compatible layer. Nano Energy 2021, 88, 106288. [Google Scholar] [CrossRef]
- Das, R.; Jaiswal, A.; Adyanthaya, S.; Poddar, P. Effect of particle size and annealing on spin and phonon behavior in TbMnO3. J. Appl. Phys. 2011, 109, 064309. [Google Scholar] [CrossRef]
- Yu, C.; Li, G.; Guan, X.; Zheng, J.; Li, L. Composite Li[Li0.11Mn0.57Ni0.32]O2: Two-step molten-salt synthesis, oxidation state stabilization, and uses as high-voltage cathode for lithium-ion batteries. J. Alloy. Compd. 2012, 528, 121–125. [Google Scholar] [CrossRef]
- Kuppan, S.; Shukla, A.K.; Membreno, D.; Nordlund, D.; Chen, G. Revealing Anisotropic Spinel Formation on Pristine Li- and Mn-Rich Layered Oxide Surface and Its Impact on Cathode Performance. Adv. Energy Mater. 2017, 7, 1374–1384. [Google Scholar]
- Chen, K.; Zhang, X.-M.; Yang, X.-F.; Jiao, M.-G.; Zhou, Z.; Zhang, M.-H.; Wang, D.-H.; Bu, X.-H. Electronic structure of heterojunction MoO2/g-C3N4 catalyst for oxidative desulfurization. Appl. Catal. B Environ. 2018, 238, 263–273. [Google Scholar] [CrossRef]
- Li, J.S.; Wang, Y.; Liu, C.H.; Li, S.L.; Wang, Y.G.; Dong, L.Z.; Dai, Z.H.; Li, Y.F.; Lan, Y.Q. Coupled molybdenum carbide and reduced graphene oxide electrocatalysts for efficient hydrogen evolution. Nat. Commun. 2016, 7, 11204. [Google Scholar] [CrossRef]
- Bravo-Sanchez, M.; Romero-Galarza, A.; Ramírez, J.; Gutiérrez-Alejandre, A.; Solís-Casados, D.A. Quantification of the sulfidation extent of Mo in CoMo HDS catalyst through XPS. Appl. Surf. Sci. 2019, 493, 587–592. [Google Scholar] [CrossRef]
- Bauer, D. TiO2/MoO2 Nanocomposite as Anode Materials for High Power Li-ion Batteries with Exceptional Capacity. Int. J. Electrochem. Sci. 2018, 13, 5120–5140. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, J.; Huang, J.; Zeng, R.; Zhang, J.; Chen, H.; Shi, G. Improving the Ni-rich LiNi0.5Co0.2Mn0.3O2 cathode properties at high operating voltage by double coating layer of Al2O3 and AlPO4. J. Alloy. Compd. 2017, 724, 1109–1116. [Google Scholar] [CrossRef]
- Xiao, B.; Liu, H.; Liu, J.; Sun, Q.; Wang, B.; Kaliyappan, K.; Zhao, Y.; Banis, M.N.; Liu, Y.; Li, R.; et al. Nanoscale Manipulation of Spinel Lithium Nickel Manganese Oxide Surface by Multisite Ti Occupation as High-Performance Cathode. Adv. Mater. 2017, 29, 1703764. [Google Scholar] [CrossRef]
- Ma, Q.; Li, R.; Zheng, R.; Liu, Y.; Huo, H.; Dai, C. Improving rate capability and decelerating voltage decay of Li-rich layered oxide cathodes via selenium doping to stabilize oxygen. J. Power Sources 2016, 331, 112–121. [Google Scholar] [CrossRef]
- Kong, J.-Z.; Zhai, H.-F.; Qian, X.; Wang, M.; Wang, Q.-Z.; Li, A.-D.; Li, H.; Zhou, F. Improved electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material coated with ultrathin ZnO. J. Alloy. Compd. 2017, 694, 848–856. [Google Scholar] [CrossRef]
- Jiang, K.C.; Wu, X.L.; Yin, Y.X.; Lee, J.S.; Kim, J.; Guo, Y.G. Superior hybrid cathode material containing lithium-excess layered material and graphene for lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 4858–4863. [Google Scholar] [CrossRef]



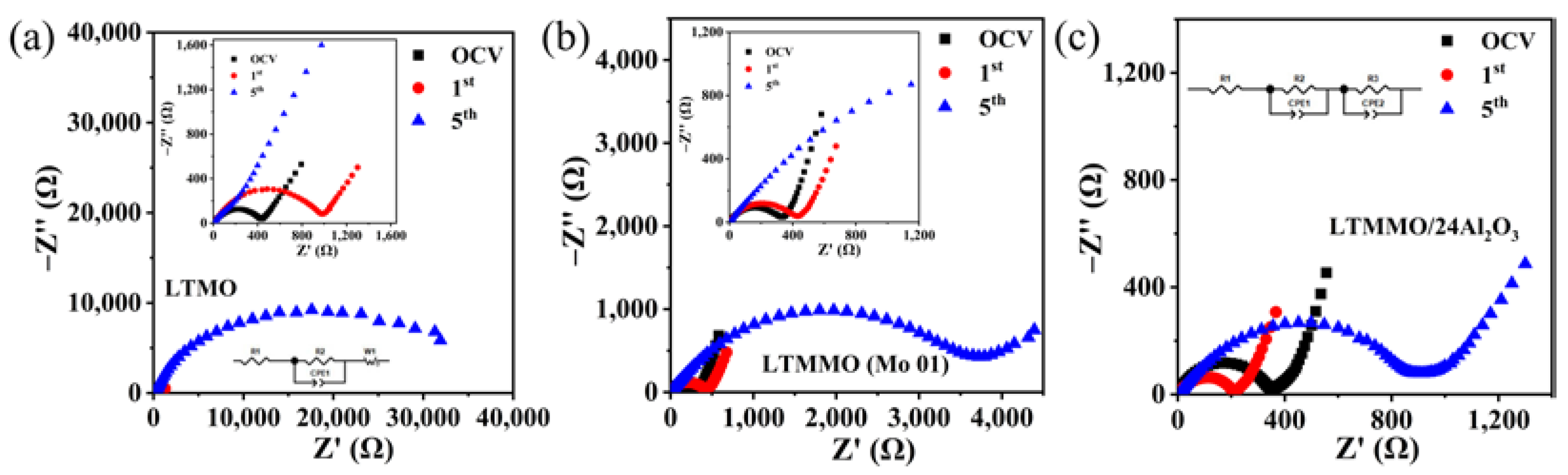
| Samples | R2 | R3 | ||||
|---|---|---|---|---|---|---|
| Battery State | OCV (Ω) | 1st 4.8V (Ω) | 5th 4.8V (Ω) | OCV (Ω) | 1st 4.8V (Ω) | 5th 4.8V (Ω) |
| LTMO | 423.5 | 963.8 | 34547 | - | - | - |
| LTMO (Mo01) | - | - | - | 291.0 | 384.2 | 3762 |
| LTMMO/24Al2O3 | - | - | - | 330.1 | 209.3 | 920.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, Z.; Huang, B.; Wang, H.; He, B.; Gong, Y.; Jin, J.; Wang, R. Improved Cycling Performance of Cation-Disordered Rock-Salt Li1.2Ti0.4Mn0.4O2 Cathode through Mo-Doping and Al2O3-Coating. Coatings 2022, 12, 1613. https://doi.org/10.3390/coatings12111613
Li Z, Zhang Z, Huang B, Wang H, He B, Gong Y, Jin J, Wang R. Improved Cycling Performance of Cation-Disordered Rock-Salt Li1.2Ti0.4Mn0.4O2 Cathode through Mo-Doping and Al2O3-Coating. Coatings. 2022; 12(11):1613. https://doi.org/10.3390/coatings12111613
Chicago/Turabian StyleLi, Zongchang, Zhihao Zhang, Baojun Huang, Huanwen Wang, Beibei He, Yansheng Gong, Jun Jin, and Rui Wang. 2022. "Improved Cycling Performance of Cation-Disordered Rock-Salt Li1.2Ti0.4Mn0.4O2 Cathode through Mo-Doping and Al2O3-Coating" Coatings 12, no. 11: 1613. https://doi.org/10.3390/coatings12111613
APA StyleLi, Z., Zhang, Z., Huang, B., Wang, H., He, B., Gong, Y., Jin, J., & Wang, R. (2022). Improved Cycling Performance of Cation-Disordered Rock-Salt Li1.2Ti0.4Mn0.4O2 Cathode through Mo-Doping and Al2O3-Coating. Coatings, 12(11), 1613. https://doi.org/10.3390/coatings12111613







