Template Electrochemical Synthesis of Hydroxyapatite on a Titania–Silver Composite Surface for Potential Use in Implantology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Initial TiO2 Film Matrix
2.2. Electrochemical Silver Deposition
2.3. Electrochemical HAp Deposition
- (1)
- electrochemical water splitting2H2O + 2e− → H2 + 2OH−
- (2)
- hydroxide ions react with dihydrophosphate ionsH2PO4− + OH− ⇄ HPO42− + H2O
- (3)
- Ca2+ combined with HPO42−, CaHPO4·2H2O deposited on the electrodeCa2+ + HPO42− + 2H2O → CaHPO4·2H2O
- (4)
- at a high OH− concentration, reactions lead to the HAp
- (5)
- formation [47]
- (6)
- HPO42− + OH− ⇄ PO43− + H2O
- (7)
- 10Ca2+ + 6PO43− + 2OH− → Ca10(PO4)6(OH)2
- (8)
- electrochemical water splitting2H2O − 4e− ⇄ 4H+ + O2
2.4. Sample Characteristics
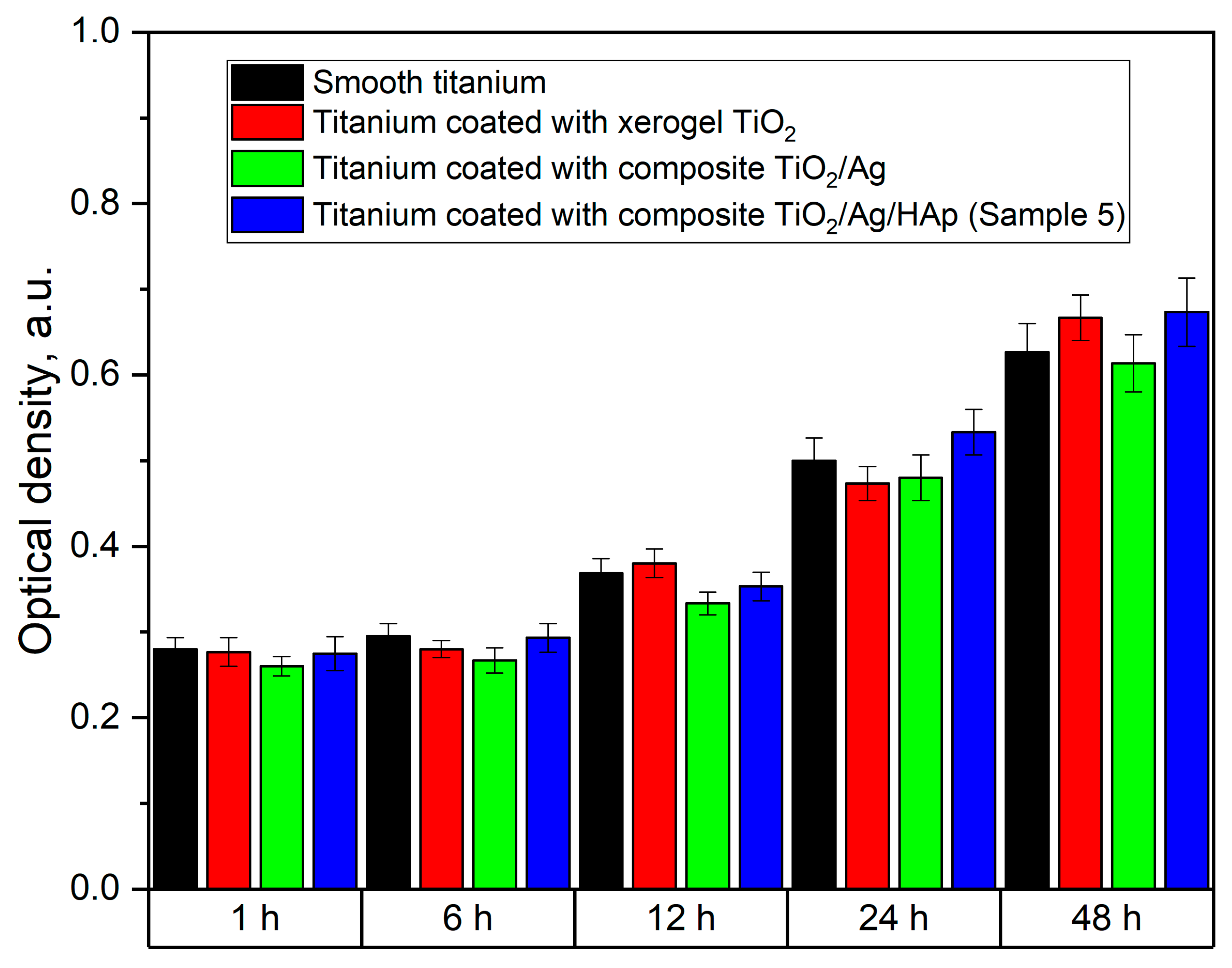
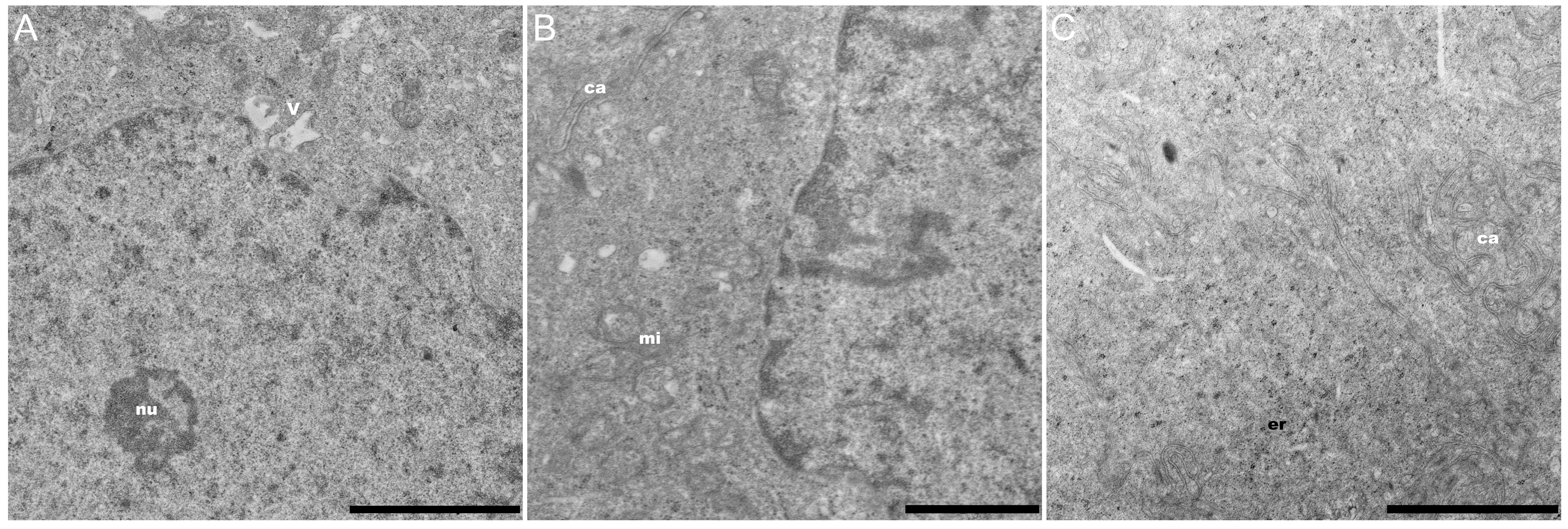
3. Results and Discussion
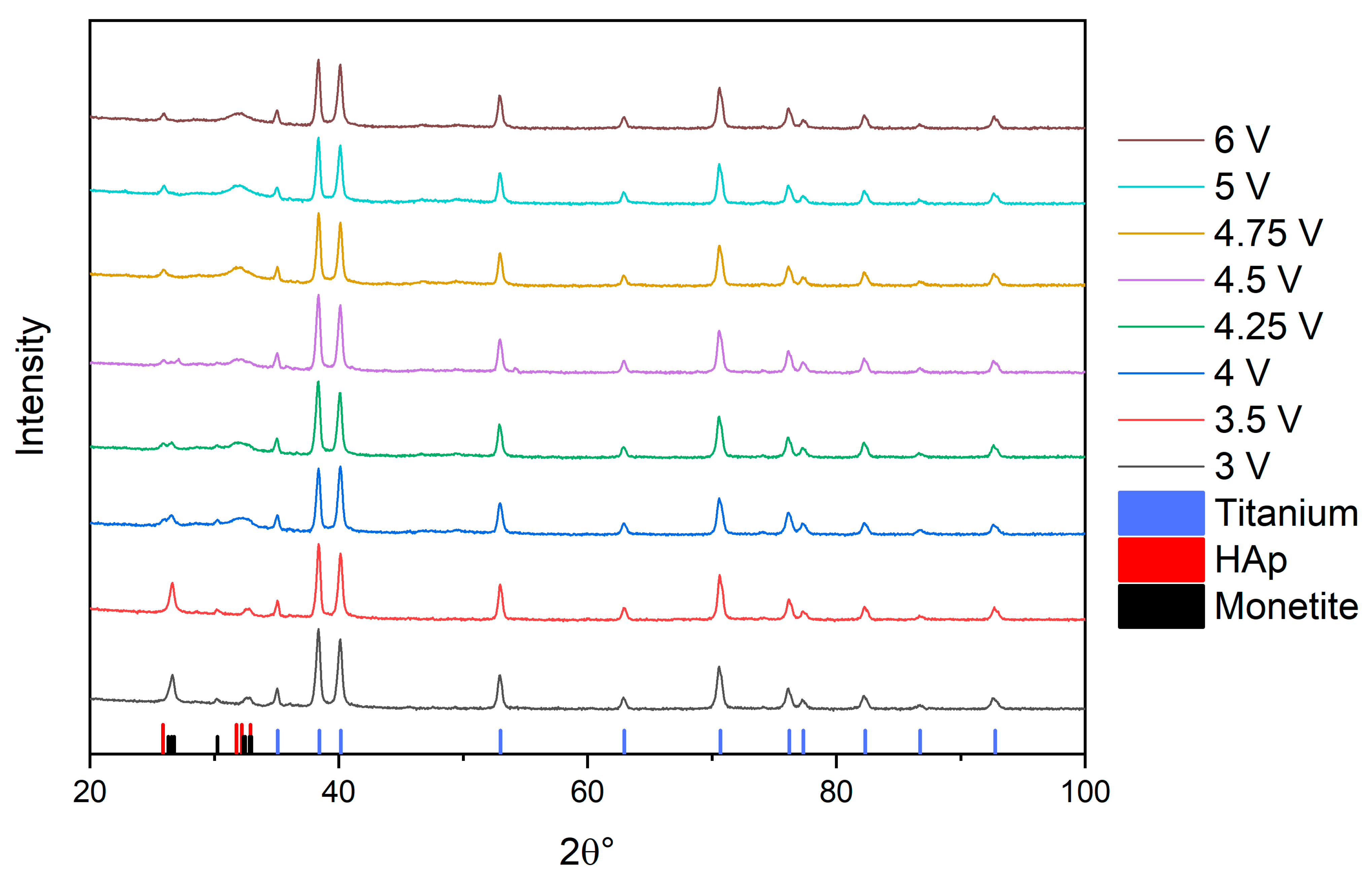
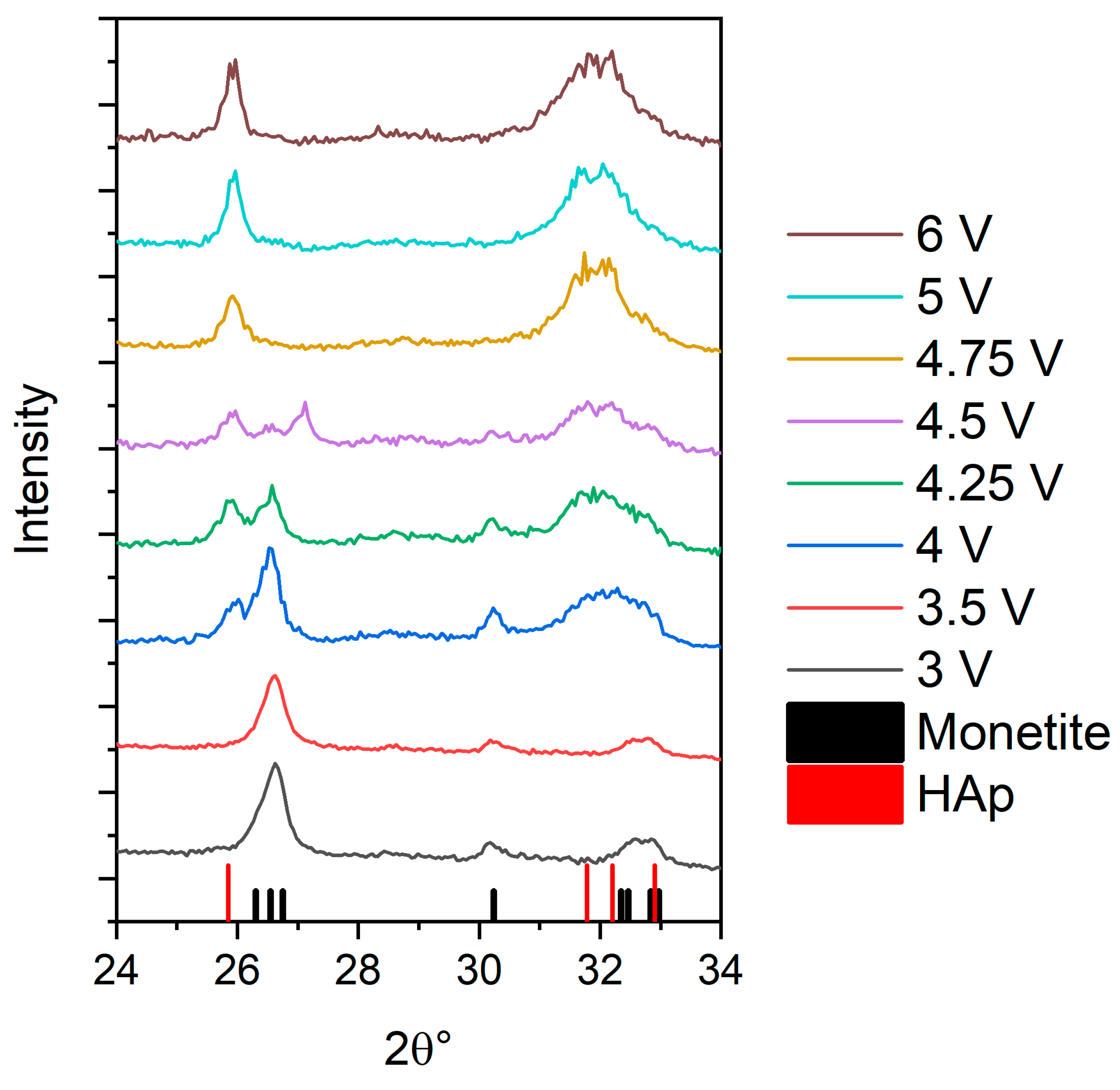
3.1. X-ray Diffraction (XRD) Determination of the Phase Composition of Electrochemically Deposited Calcium Phosphates
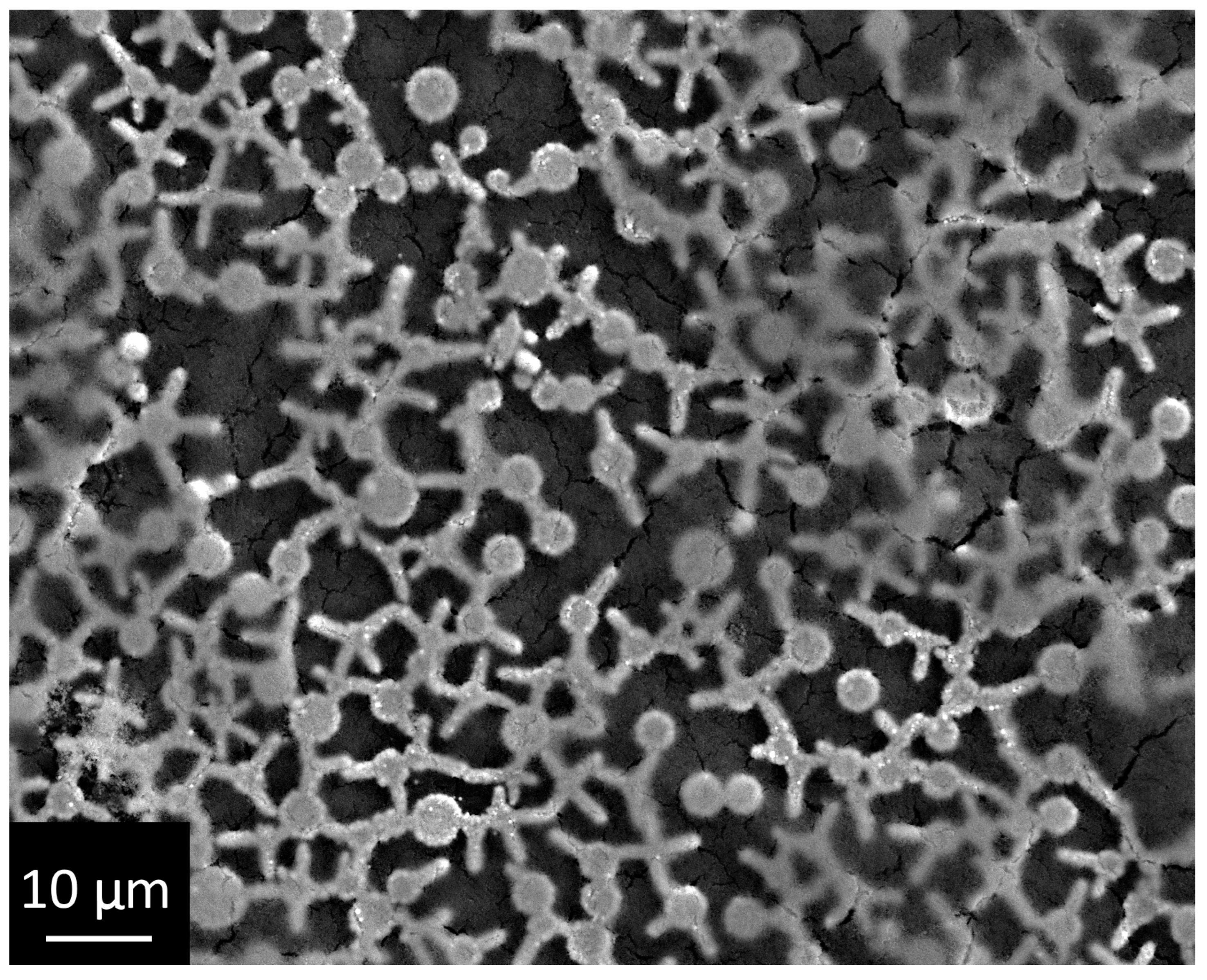
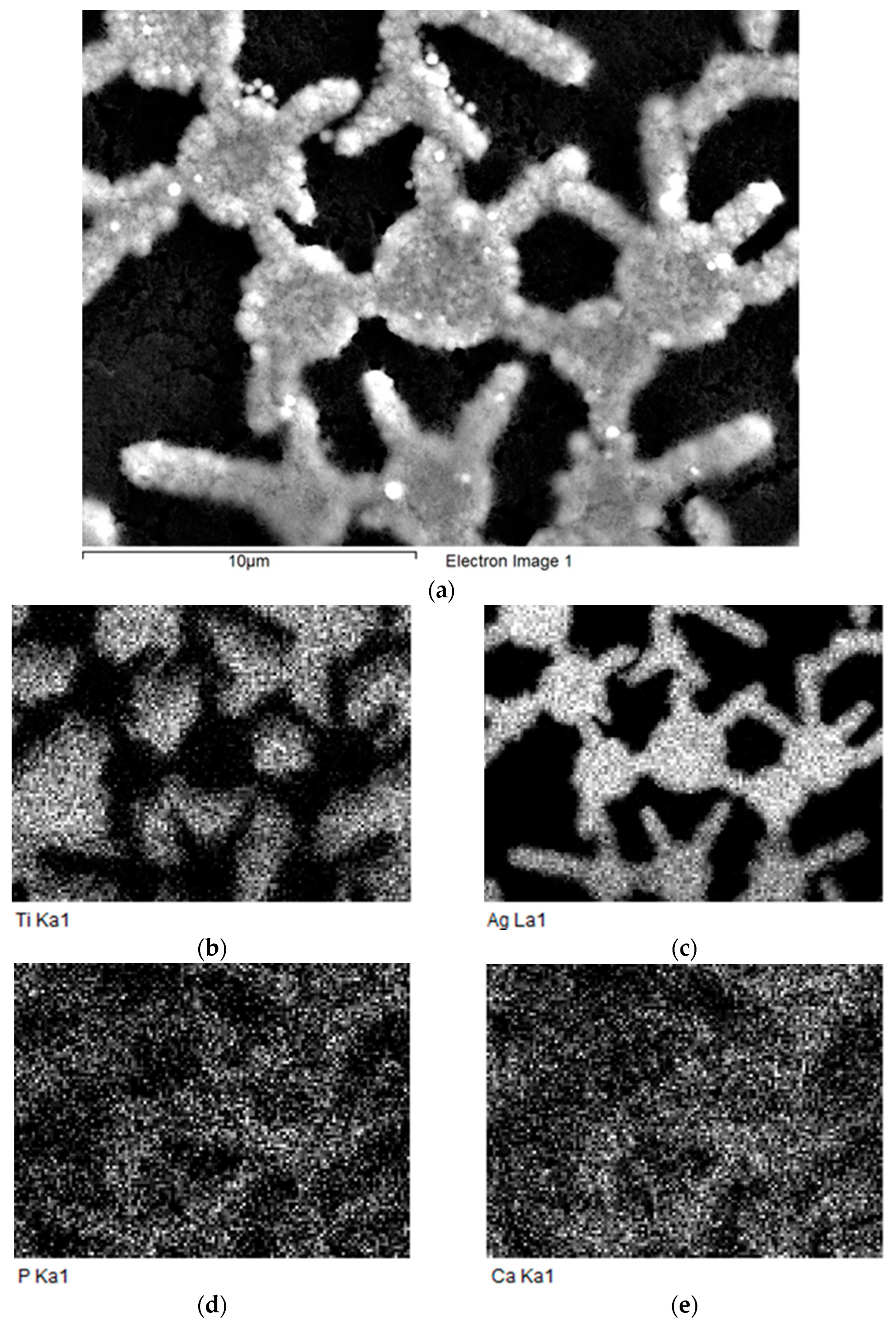
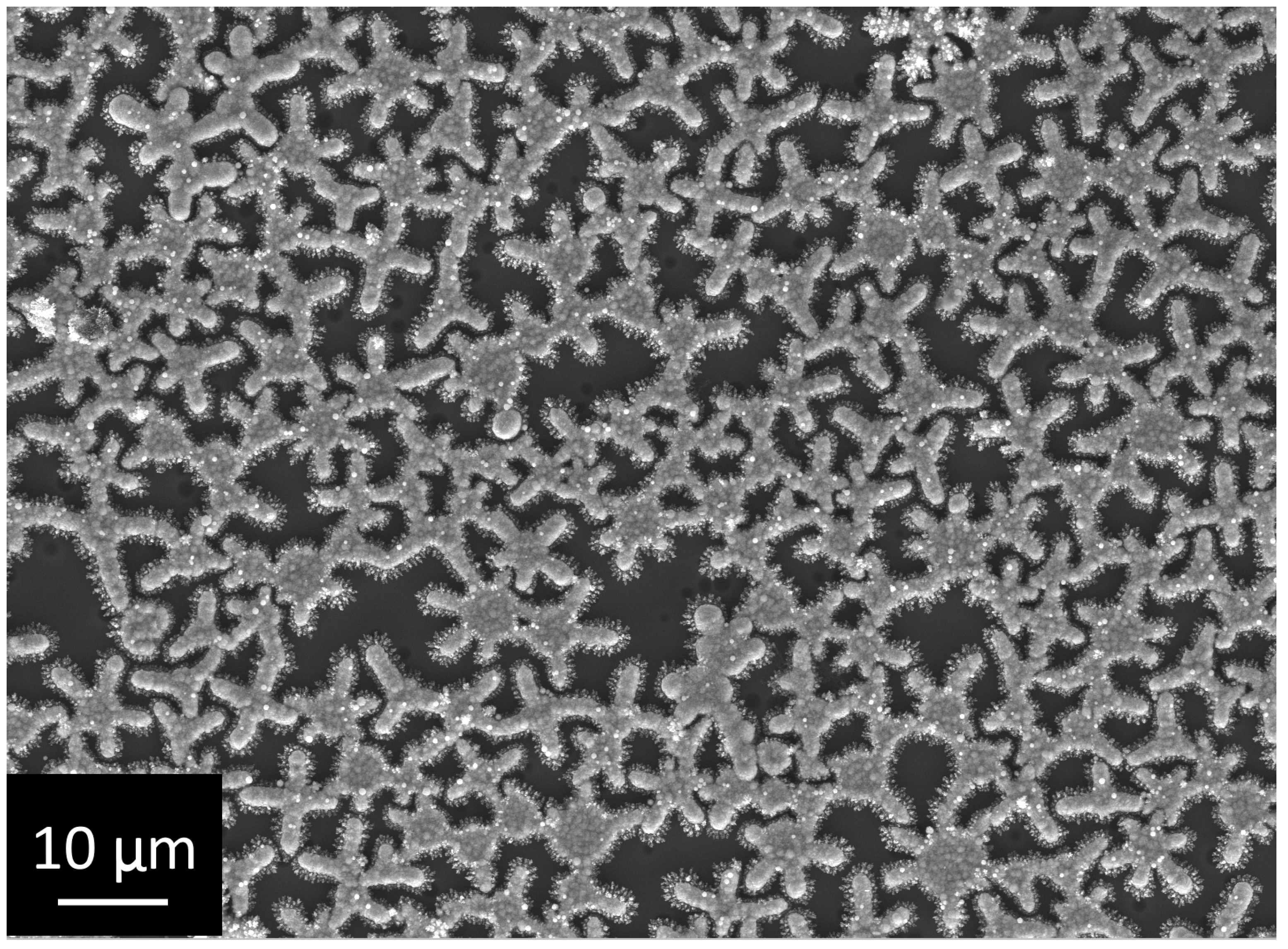
3.2. Investigation of the Structure and Elemental Composition of the TiO2/Ag/HAp Composite Coating by Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenspan, D.C. Bioactive ceramic implant materials. Current Opinion in Solid State and Materials. Science 1999, 4, 389–393. [Google Scholar]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [PubMed]
- Liang, S.X.; Feng, X.J.; Yin, L.X.; Liu, X.Y.; Ma, M.Z.; Liu, R.P. Development of a new β Ti alloy with low modulus and favorable plasticity for implant material. Mater. Sci. Eng. C 2016, 61, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Naqvi, R.A.; Abbas, N.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-High-Molecular-Weight-Polyethylene (UHMWPE) as a Promising Polymer Material for Biomedical Applications: A Concise Review. Polymers 2020, 12, 323. [Google Scholar]
- Zhukova, P.A.; Senatov, F.S.; Zadorozhnyy, M.Y.; Chmelyuk, N.S.; Zaharova, V.A. Polymer Composite Materials Based on Polylactide with a Shape Memory Effect for “Self-Fitting” Bone Implants. Polymers 2021, 13, 2367. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Gavrilov, D.; Yudintceva, N.; Zemtsov, E.; Arbenin, A.; Smirnov, V.; Voronkina, I.; Adamova, P.; Blinova, M.; Mikhailova, N.; et al. Protecting the skin-implant interface with transcutaneous silver-coated skin-and-bone-integrated pylon in pig and rabbit dorsum models. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2021, 109, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Titanium for Medical Applications. Titanium in Medicine; Springer: Berlin/Heidelberg, Germany, 2001; pp. 13–24. [Google Scholar]
- Wennerberg, A.; Albrektsson, T.; Andersson, B.; Krol, J.J. A histomorghometric study of screw-shaped and removal torque titanium implants with three different surface topographies. Clin. Oral Implant. Res. 1995, 6, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Kaspar, D.; Sarkar, M.R.; Claes, L.E.; Ignatius, A.A. A scanning electron microscopy study of human osteoblast morphology on five orthopedic metals. J. Biomed. Mater. Res. Part A 2002, 63, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Dong, W.J.; He, F.M.; Wang, X.X.; Zhao, S.F.; Yang, G.L. Osteoblast response to porous titanium surfaces coated with zinc-substituted hydroxyapatite. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 313–318. [Google Scholar] [CrossRef]
- Zhu, X.; Son, D.W.; Ong, J.L.; Kim, K. Characterization of hydrothermally treated anodic oxides containing Ca and P on titanium. J. Mater. Sci. Mater. Med. 2003, 14, 629–634. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Induction plasma sprayed nano hydroxyapatite coatings on titanium for orthopaedic and dental implants. Surf. Coat. Technol. 2011, 205, 2785–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, J.C.; Stanford, C.M.; Wightman, J.P.; Draughn, R.A.; Zaharias, R. Characterizations of titanium implant surfaces. J. Biomed. Mater. Res. Part A 1994, 28, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Bucci-Sabattini, V.; Cassinelli, C.; Coelho, P.G.; Minnici, A.; Trani, A.; Ehrenfest, D.M.D. Effect of titanium implant surface nanoroughness and calcium phosphate low impregnation on bone cell activity in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 217–224. [Google Scholar] [PubMed]
- Juodzbalys, G.; Sapragoniene, M.; Wennerberg, A.; Baltrukonis, T. Titanium dental implant surface micromorphology optimization. J. Oral Implantol. 2007, 33, 177–185. [Google Scholar] [CrossRef]
- Zemtsova, E.G.; Yudintceva, N.M.; Morozov, P.E.; Valiev, R.Z.; Smirnov, V.M.; Shevtsov, M.A. Improved osseointegration properties of hierarchical microtopographic/nanotopographic coatings fabricated on titanium implants. Int. J. Nanomed. 2018, 13, 2175–2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemtsova, E.G.; Arbenin, A.Y.; Yudintceva, N.M.; Valiev, R.Z.; Orekhov, E.V.; Smirnov, V.M. Bioactive coating with two-layer hierarchy of relief obtained by sol-gel method with shock drying and osteoblast response of its structure. Nanomaterials 2010, 7, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetrone, F.; Variola, F.; Tambasco, P.; Zalzal, S.F.; Yi, J.H.; Sam, J.; Nanci, A. Nanoscale Oxidative Patterning of Metallic Surfaces to Modulate Cell Activity and Fate. Nano Lett. 2009, 9, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Yeniyol, S.; Bölükbaşi, N.; Çakir, A.F.; Bilir, A.; Özdemir, T. Effects of surface modifications with oxalic acid etching and sandblasting on surface topography and biocompatibility of cpTi surfaces. Biotechnol. Biotechnol. Equip. 2013, 27, 3995–4001. [Google Scholar] [CrossRef] [Green Version]
- Goudarzi, M.; Batmanghelich, F.; Afshar, A.; Dolati, A.; Mortazavi, G. Development of electrophoretically deposited hydroxyapatite coatings on anodized nanotubular TiO2 structures: Corrosion and sintering temperature. Appl. Surf. Sci. 2014, 301, 250–257. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F. Characterization and biological properties of TiO2/PCL hybrid layers prepared via sol–gel dip coating for surface modification of titanium implants. J. Non-Cryst. Solids 2015, 415, 9–15. [Google Scholar] [CrossRef]
- Kajihara, K.; Nakanishi, K.; Tanaka, K.; Hirao, K.; Soga, N. Preparation of Macroporous Titania Films by a Sol-Gel Dip-Coating Method from the System Containing Poly (ethylene glycol). J. Am. Ceram. Soc. 1998, 81, 2670–2676. [Google Scholar] [CrossRef]
- Tian, B.; Chen, W.; Yu, D.; Lei, Y.; Ke, Q.; Guo, Y.; Zhu, Z. Fabrication of silver nanoparticle-doped hydroxyapatite coatings with oriented block arrays for enhancing bactericidal effect and osteoinductivity. J. Mech. Behav. Biomed. Mater. 2016, 6, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.; Wang, D.; Zhang, S.; Xie, Y.; Huang, C.; Zhang, Z. Synthesis and bactericidal ability of Ag/TiO2 composite films deposited on titanium plate. Appl. Surf. Sci. 2010, 257, 974–978. [Google Scholar] [CrossRef]
- Yu, B.; Leung, K.M.; Guo, Q.; Lau, W.M.; Yang, J. Synthesis of Ag–TiO2 composite nano thin film for antimicrobial application. Nanotechnology 2011, 22, 115603. [Google Scholar] [CrossRef] [Green Version]
- Arbenin, A.Y.; Zemtsova, E.G.; Orekhov, E.V.; Smirnov, V.M. Features of the sol-gel synthesis of TiO2 nanolayers with calcium phosphate structures on titanium surface. Russ. J. Gen. Chem. 2017, 87, 340–341. [Google Scholar] [CrossRef]
- Fu, C. Synthesis and Characterization of Hydroxyapatite Coatings for Medical Applications; University of Rochester: Rochester, NY, USA, 2016; pp. 46–70. [Google Scholar]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. Part A 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Li, T.; Lee, J.; Kobayashi, T.; Aoki, H. Hydroxyapatite coating by dipping method, and bone bonding strength. J. Mater. Sci. Mater. Med. 1996, 7, 355–357. [Google Scholar] [CrossRef]
- Yang, G.L.; He, F.M.; Hu, J.A.; Wang, X.X.; Zhao, S.F. Biomechanical comparison of biomimetically and electrochemically deposited hydroxyapatite–coated porous titanium implants. J. Oral Maxillofac. Surg. 2010, 68, 420–427. [Google Scholar] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 2000, 21, 1803–1810. [Google Scholar] [CrossRef]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009, 5, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Linner-Krčmar, B.; Friess, W.; Greil, P. Mechanical properties and in vitro cell compatibility of hydroxyapatite ceramics with graded pore structure. Biomaterials 2002, 23, 4285–4294. [Google Scholar] [CrossRef]
- Wenisch, S.; Stahl, J.P.; Horas, U.; Heiss, C.; Kilian, O.; Trinkaus, K.; Hild, A.; Schnettler, R. In vivo mechanisms of hydroxyapatite ceramic degradation by osteoclasts: Fine structural microscopy. J. Biomed. Mater. Res. Part A 2003, 67, 713–718. [Google Scholar] [CrossRef]
- Thanh, D.T.M.; Nam, P.T.; Phuong, N.T.; Van Anh, N.; Hoang, T.; Dai Lam, T. Controlling the electrodeposition, morphology and structure of hydroxyapatite coating on 316L stainless steel. Mater. Sci. Eng. C 2013, 33, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.C.; Yen, S.K. The process of electrochemical deposited hydroxyapatite coatings on biomedical titanium at room temperature. Mater. Sci. Eng. C 2002, 20, 153–160. [Google Scholar] [CrossRef]
- Eliaz, N.S.T.M.; Sridhar, T.M.; Kamachi Mudali, U.; Raj, B. Electrochemical and electrophoretic deposition of hydroxyapatite for orthopaedic applications. Surf. Eng. 2005, 21, 238–242. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, B.; Wang, Y.; Zhou, X.; Weng, J.; Qu, S.; Feng, B.; Watari, F.; Ding, Y.; Leng, Y. Nano-Ag-loaded hydroxyapatite coatings on titanium surfaces by electrochemical deposition. J. R. Soc. Interface 2011, 8, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.M.; Venugopal, A.; Subrahmanyam, M. Hydroxyapatite-supported Ag–TiO2 as Escherichia coli disinfection photocatalyst. Water Res. 2007, 41, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, X.; Huang, Y.; Ding, Q.; Pang, X. Antibacterial and bioactivity of silver substituted hydroxyapatite/TiO2 nanotube composite coatings on titanium. Appl. Surf. Sci. 2014, 314, 348–357. [Google Scholar] [CrossRef]
- Mo, A.; Liao, J.; Xu, W.; Xian, S.; Li, Y.; Bai, S. Preparation and antibacterial effect of silver–hydroxyapatite/titania nanocomposite thin film on titanium. Appl. Surf. Sci. 2008, 255, 435–438. [Google Scholar] [CrossRef]
- Arbenin, A.; Zemtsova, E.G.; Ermakov, S.S.; Gas’kov, A.M.; Baburova, P.I.; Sokolova, D.N.; Smirnov, V.M. Three-component working electrode micron-sized Ag particles/TiO2 layer/Ti: Template electrochemical synthesis and potential use as electrochemical sensor for glutathione detection. Mater. Res. Express 2020, 7, 035401. [Google Scholar] [CrossRef]
- Li, G.; Cao, H.; Zhang, W.; Ding, X.; Yang, G.; Qiao, Y.; Liu, X.; Jiang, X. Enhanced Osseointegration of Hierarchical Micro/Nanotopographic Titanium Fabricated by Microarc Oxidation and Electrochemical Treatment. ACS Appl. Mater. Interfaces 2016, 8, 3840–3852. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Ma, L.; Zhang, B.; Sun, J.; Sun, Y.; Fan, Y.; Gou, Z.; Jou, C.; Zhang, X. Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication 2017, 9, 045008. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Zhang, C.; Liu, Y.; Wang, J.; Deng, F. Improved bioactivity of selective laser melting titanium: Surface modification with micro-/nano-textured hierarchical topography and bone regeneration performance evaluation. Mater. Sci. Eng. C 2016, 68, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Zemtsova, E.G.; Orehhov, E.V.; Arbenin, A.Y.; Valiev, R.Z.; Smirnov, V.M. The creation of nanocoatings of various morphology on the basis of titanium dioxide on a titanium matrix for bone implant. Mater. Phys. Mech. 2016, 29, 138–144. [Google Scholar]
- Kar, A.; Raja, K.S.; Misra, M. Electrodeposition of hydroxyapatite onto nanotubular TiO2 for implant applications. Surf. Coat. Technol. 2006, 201, 3723–3731. [Google Scholar] [CrossRef]
- Qiu, X.; Wan, P.; Tan, L.; Fan, X.; Yang, K. Preliminary research on a novel bioactive silicon doped calcium phosphate coating on AZ31 magnesium alloy via electrodeposition. Mater. Sci. Eng. C 2014, 36, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, E.; Velten, D.; Schenk-Meuser, K.; Linez, P.; Biehl, V.; Duschner, H.; Hildebrand, H. Interactions between cells and titanium surfaces. Biomol. Eng. 2002, 19, 243–249. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. Electrodeposited hydroxyapatite-based biocoatings: Recent progress and future challenges. Coatings 2021, 11, 110. [Google Scholar]
- Lin, W.C.; Chuang, C.C.; Wang, P.T.; Tang, C.M. A comparative study on the direct and pulsed current electrodeposition of cobalt-substituted hydroxyapatite for magnetic resonance imaging application. Materials 2019, 12, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Sample No. | Deposition Mode | |||
|---|---|---|---|---|
| 1 | Constant Current | 6 V (τ = 15 min) | ||
| 2 | 1200 cycles | 12 V | −2 V | 0 V |
| = 3 ms | = 2 ms | = 85 ms | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orekhov, E.V.; Arbenin, A.Y.; Zemtsova, E.G.; Sokolova, D.N.; Ponomareva, A.N.; Shevtsov, M.A.; Yudintceva, N.M.; Smirnov, V.M. Template Electrochemical Synthesis of Hydroxyapatite on a Titania–Silver Composite Surface for Potential Use in Implantology. Coatings 2022, 12, 266. https://doi.org/10.3390/coatings12020266
Orekhov EV, Arbenin AY, Zemtsova EG, Sokolova DN, Ponomareva AN, Shevtsov MA, Yudintceva NM, Smirnov VM. Template Electrochemical Synthesis of Hydroxyapatite on a Titania–Silver Composite Surface for Potential Use in Implantology. Coatings. 2022; 12(2):266. https://doi.org/10.3390/coatings12020266
Chicago/Turabian StyleOrekhov, Evgeniy V., Andrey Yu. Arbenin, Elena G. Zemtsova, Darya N. Sokolova, Alexandra N. Ponomareva, Maxim A. Shevtsov, Natalia M. Yudintceva, and Vladimir M. Smirnov. 2022. "Template Electrochemical Synthesis of Hydroxyapatite on a Titania–Silver Composite Surface for Potential Use in Implantology" Coatings 12, no. 2: 266. https://doi.org/10.3390/coatings12020266
APA StyleOrekhov, E. V., Arbenin, A. Y., Zemtsova, E. G., Sokolova, D. N., Ponomareva, A. N., Shevtsov, M. A., Yudintceva, N. M., & Smirnov, V. M. (2022). Template Electrochemical Synthesis of Hydroxyapatite on a Titania–Silver Composite Surface for Potential Use in Implantology. Coatings, 12(2), 266. https://doi.org/10.3390/coatings12020266







