Abstract
Interest in edible coatings applications has progressively developed towards improving the quality and shelf life of climacteric fruits. This study aimed to investigate the influence of pre-harvest treatments (chitosan, chitosan nanoparticle, and CaCl2) on the physicochemical and quality attributes of Barhi date palm fruits during storage periods. Different pre-harvest treatments (control, chitosan 1, 2, and 3 g/L, CaCl2 1, 2, and 3 g/L, nano-chitosan 1, 2, and 3 cm3 /L) were applied. The results showed that all treatments were effective for enhancing the fruit quality, with increasing total soluble solids and total sugars, decreasing weight loss, discarded total acidity, and total soluble tannins compared to the control treatment. Additionally, the results revealed that the highest percentage of TSS was obtained in control fruits (35.78%). Meanwhile, the lowest mean values were obtained from chitosan nanoparticle at 3 cm3/L (33.91%). Treatments with chitosan nanoparticle at 3 cm3/L and CaCl2 at 3 g/L gave the statistically highest values of total tannins (0.225 and 0.220, respectively). The optimal treatment involved spraying the fruit with 3 cm3/L of nano-chitosan or 3 g/L of CaCl2 to increase the fruit quality and the shelf life of Barhi dates. The results indicated that weight loss was negatively linked with the moisture content and firmness, while decay had a strong positive relationship with the Rutab index and a negative correlation with the moisture content. Furthermore, the Rutab index was negatively associated with the total tannins and total chlorophyll.
1. Introduction
Date palm (Phoenix dactyllifera L.) is a monocotyledonous and dioicous plant belonging to the Arecaceae (Palmaceae) family, with one of the oldest histories among fruit around the globe. It is recognized as a “tree of life” due to its flexibility, limited-water resistance, long-term yield, and numerous uses.
Date palm is a familiar fruit tree in Arabian nations, the Middle East, North African countries, and in hot arid regions of the world [1] as the Arab Muslim world contains the main production areas, with 11 countries in these regions carrying out 94% of world production. The first place was occupied by Egypt followed successively by Iran and Saudi Arabia [2].
In Egypt, date palm is a valuable historical crop, with many cultivars such as Barhi, Zaghloul, Samany, Halaway, and Hayany grown in different regions. Date palm is climacteric and rapidly grows after harvesting, which reduces the storing, usage, and shipping options [3]. Growth and maturing of date fruit is exemplified by five various steps identified as Hababouk, Kimri, Khalal, Rutab, and Tamar, separately. The Hababouk step begins after the fruit set and ends after four to five weeks. During the Kimri phase, the fruit became green, hard, and often uneatable. At the Khalal phase, the color of the fruit changes to yellow. Moreover, the fruits of the Barhi cultivar lose the astringency and become sweet and delicious. During the Rutab phase, the fruit’s firm texture becomes mushy, dessert-like, and tasty. At the end of the maturing phase, i.e., Tamar, fruit moisture declines to <24%, body texture turns dry, and its color alters to dark brown [4,5]. The Barhi type is one of the most common date palm cultivars in the Mediterranean area and is usually collected and eaten fresh at the Khalal phase, but they are likely to rapidly ripen and enter the rutab phase under regular storing circumstances [6].
However, the economic value of Barhi dates decreases sharply as they ripen, and surplus production must be soldat lower prices. Thus, it is critical to delay maturation and extend the market-life of Barhi dates. The main aim of post-harvest technology, which seeks to utilize safe and efficient assays to keep quality management and transportation of date fruits for local market and export. Modern technology which involves methods such as application of edible coatings, cold storage, etc. is takes advantage of the synergistic impact of different treatments to enhance the post- harvest life of climacteric fruits.
Post-harvest losses of date palm fruits in Egypt can be considered as a severe problem primarily due to their fast decline during management, transportation, and storing. These losses were determined to be between 30% and 40% before delivery to the endconsumer [1] causing significant economic loss. Barhi dates, at the Khalalphase are often desired and deemed a superior product since they are physiologically ripe, solid, crunchy, light yellow in color, and have the highest moisture [6].This obviously requires the best outcome of protected post-harvest coating treatments with chitosan, calcium chloride, each alone and in combination with cold storage in delaying the fruit maturation process, keeping quality attributes, and extension of the cold storage period of Barhi date palms by managing changes of physicochemical characteristics.
Edible coatings with semipermeable emulsions can extend post-harvest fruit life due to decreasing moisture, respiration, gas exchange, and oxidative retort levels [7].
Chitosan (CH, poly-B-(1–4) N-acetyl-d-gluco amine) is an ordinary antimicrobial component. It can be extracted from crustacean skins (crabs, shrimp, and crayfishes) by both chemical and microbiological methods [8]. Chitosan is widely used as an edible coating material [9]. Earlier findings demonstrated that utilizing chitosan in various fruits was extremely useful in enhancing the quality. Zhang, et al. [10] found that chitosan preserved post-harvest quality and positively inspired strength, TSS, titratable acidity, vitamin C, and moisture content of citrus fruit after 56 d of storing at 15 °C. In a study on raspberries fruits, it was found that chitosan retained key quality factors, decreased ethylene creation and breathing level, reduced weight loss, maintained fruit quality, extended the cold storage period, and reduced decay [11]. Additionally, Shiri, et al. [12] stated that table grapes covered with 0.5 or 1% chitosan and then kept at 0 °C for 60 d revealed less weight damage, deterioration and greater levels of titratable acidity. Abd Elwahab, et al. [13] found that 1% chitosan + 4% CaCl2 reduced weight damage and postponed fluctuations in firmness, titratable acidity, TSS, ascorbic acid, anthocyanin, and respiration ratio of Crimson seedless grapes during storage periods compared to control. Also, Kamal, et al. [14] found that Zaghloul date treated with chitosan 1% as post-harvest treatments offered the smallest significant weight damage and the greatest flesh density during cold storing at the end of 90 d. However, to date, no studies are available regarding the pre-harvest treatment of Barhi date with chitosan, CaCl2, and chitosan nanoparticles. Hence, our main objective was to explore the influences of chitosan, CaCl2, and nano-chitosan as a safe pre-harvest treatment on the physicochemical and quality features of Barhi dates during the storage period.
2. Material and Methods
2.1. Preparation and Characterization of Coating Solutions
Chitosan product (90–95% chitosan) was brought from Oxford laboratory in India. In addition, all of the chemicals, assays and reagents used in this study were brought from the agent of both FLINN SCIENTIFIC Chemical Company, Batavia, the Dutch East Indies and SIGMACHEMICALS Company, Canning, Australia. To prepare chitosan solution, superior purity, low-molecular-weight chitosan food grade powder was used to prepare the solution; 10, 20, and 30 g of chitosan was mixed with 100 mL of acetic acid (CH3CO2H) liquid (1%, v/v), and lightly blended at 40 °C on a magnetic agitator. Successively, 0.75 mL/g of glycerol (C3H8O3) was mixed as the plasticizer and 0.2% of Tween 80 was mixed as an emulsifier. The pH was then modified to 5.7–6 by combining 1 mol/L NaOH, and then the liquid was shoved at 30 °C for 30 min. The ready solution was then filtrated via Whatman filter papers and autoclaved for 15 min at 121 °C [15]. While chitosan nanoparticles were prepared using the ion tropic gelatin process created by [16] and modified by Domaratzki and Ghanem [17]. The precise weight of chitosan (1 mg/mL) was dispersed in 0.175% acetic acid (v/v). Sodium tripolyphosphate, Na5P3O10, (TPP) was dispersed in d-H2O at the intensity of 2 mg/mL. Both chitosan and TPP liquids were dispersed under constant magnetic mixing at RT for 30 min at 900 rpm. Once both liquids were separately blended, they were applied via a syringe filter. A 0.45 µm syringe filter was utilized for chitosan and a 0.22 µm filter was utilized for TPP. The TPP was mixed to chitosan to make nanoparticles. A chitosan to TPP level of 5:1 was selected depending on Zhang, et al. [18] and endorsed by Domaratzki and Ghanem [17]. Chitosan–TPP nanoparticles naturally produced by the TPP initiated ionic gelatin system upon the adding of aqueous TPP to the chitosan liquids (at chitosan to TPP level rate 5:1). This was performed under moderate continuous magnetic mixing at RT for 1 min at 100 rpm. Then it was centrifugated (Beckman Coulter Ultracentrifuge, Indianapolis, IN, USA) for 30 min at 52,000× g to isolate the nanoparticles.
The creation of chitosan nanoparticles could be simply used by differing the key handling situations of chitosan dose, TPP dose, and solution PH. Within the examined variety of circumstances, and growth in the particle size demonstrated an easy direct correlation to improving TPP dose. Solution pH and chitosan dose also had a substantial impact on the nanoparticle system steadiness [19]. Figure 1 reveals characterization of chitosan a-FT-IR-b-UV-vis 44 nanoparticles.

Figure 1.
Characterization of chitosan nanoparticles. Note that Figures (a) and (b) are FTIR and UV-vis, respectively.
On the other hand, an edible grade calcium chloride (CaCl2) was purchased from an international chemical company (Wei Bang Chemical Limited Company, Jinan, China) to prepare the solution used in this study. Next, different concentrations (1, 2, 3 g (w/v)) of solution were prepared by liquefying 1, 2, and 3 g/L of CaCl2 in 1000 mL of dH2O. It was constantly stirred via a magnetic agitator for 30 min and 0.2 mL of Tween 20 was mixed to the solution to enhance wettability.
After that, ten pre-harvest treatments were carried out one month prior to harvesting; spraying was carried out twice with two-week intervals before harvest intervals of 15 d (Figure 2). Next, the chosen fruits were cleaned, air dried, and then positioned into plastic containers and separated into comparable groups (Table 1). The considered and non-treated fruits were split into several categories and directly transferred to the post-harvest laboratory after harvest.

Figure 2.
Barhi date palm fruits during the foliar spray application of some pre-harvest treatments.

Table 1.
Pre-harvest treatments of Barhi Date.
2.2. Fruits Storing Protocol
All of the treated and untreated fruits were allowed to aerobically dry; each treatment was split into two portions, I: to assess the physical features; II: to evaluate the chemical characteristics. Each portion had 3 replicates with the ratio (1.5 kg/replicate). Then, each duplicate was filled into punctured carton containers. All treated and untreated fruits were kept at 0 ± 2 °C and RH of 90–95%. The primary fruit quality features of date palm cv. Barhi after treating were determined (zero time). After 10 d intervals (i.e., at harvest, 10, 20, 30, 40, 50, 60, and 70 d), samples were taken from cold storage and fruit quality quantities were measured. Figure 3 depicts photos of Barhi date palm fruits were cold stored for 0, 10, 20, 30, 40, 50, 60, and 70 d after some pre-harvest treatments were applied via foliar spray.

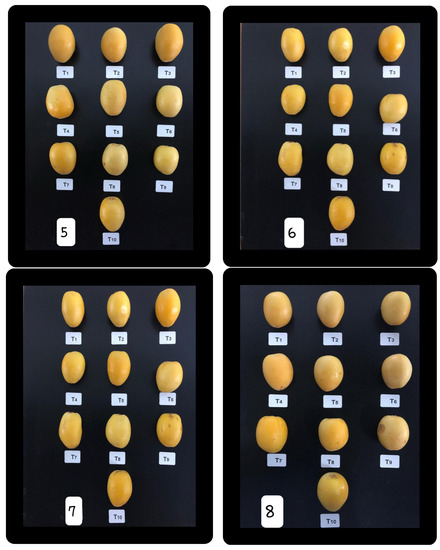
Figure 3.
Barhi date palm fruits were cold stored for 70 d divided to 8 intervals (0, 10, 20, 30, 40, 50, 60, and 70 d) after some pre-harvest treatments were applied via foliar spray.
2.3. Measurements
2.3.1. Physical and Quality Properties
Weight Loss (%)
Fruits were regularly counted, and the damage in bulk weight was noted for each repeat. Data were analyzed as a proportion from the preliminary mass. The subsequent formulation is utilized:
Decay Percentage (%)
Assessed by skin presence, withering, chilling damage, and infective rots. In every examination, rotten fruits were removed, and the fruits weight per duplicate was utilized to convey the rotting ratio. Storing was halted when decay evaluation went 25% in the kept fruits. The subsequent formulation is utilized.
Fruit Firmness
Fruit firmness was measured for three date fruits per replicate at two equatorials differing sites to measure the penetration force using a hand-held fruit firmness tester (FT-327, Rome, Italy) supported with an 8 mm cylindrical stainless steel plunger tip as it was formerly described by Sherif Fathy El-Gioushy et al. [2], EL-Gioushy and Baiea [3]. Two senses were shown on each fruit flesh after peeling. The firmness value was calculated in terms of l b/in2.
Rutab Percentage
Fruits were periodically counted and rutab fruits were noted for each repeat. Data were expressed as % of the first weight.
Fruit Moisture Percentage
It was determined by weight 100 g of fresh date palm fruits which were then oven dehydrated at 70 °C until a steady mass was reached. The moisture percentage was calculated using the following equation:
2.3.2. Chemical Properties
Fruit Total Soluble Solids Percentage (TSS)
It was quantified by digital hand refractometer (model Palette, PR-32, ATAGO, Saitama, Japan) at room temperature [20].
Overall Sugars (g/100 g “Fresh Weight” FW)
Established in kept date fruits by the assay explained by Dubois, et al. [21] as follows, 1 ml of extracted ethanol sugars was mixed with 0.5 mL phenol, C6H6O, (5%) in a test tube and immediately followed by the addition of 5 mL of concentrated sulfuric acid, H2SO4, then the mixture was shaken gently and left to cool. The blank containing all of the reagents without fruit extract which replaced with 1 mL 80% ethanol, C2H5OH. The absorbance of developed yellow-orange color was measured at 490 nm using spectrophotometer. A standard curve was carried out using pure glucose with a suitable concentration. The number of total sugars was calculated and expressed as a percentage.
Reducing Sugars
Reducing sugar content of the dates was measured by dinitrosalicylic acid assay [22].
Total Tannins (mg/100 g F.W)
Soluble tannins were determined by the assay of Taira and Ono [23]. 1 mL of tested solution was blended with 6 mL dH2O and 0.5 mL of Folin–Ciocalteu reagent (formerly reduced 10-fold with dH2O) and stirred well. After precisely 3 min, 1 mL of saturated NaCo3 was inserted and blended well. Then 1.5 mL dH2O was inserted and blended well (accumulative, 10 mL) and allowed for 1 h at RT before calculating optical density at 750 nm via a spectrophotometer. The blank includes only H2O and the reagents. Soluble tannins were counted from a standard curve taken by determining the optical density of common dose of gallic acid(C7H6O5).
Total Phenols Compounds (g/100 g F.W)
Removal of polyphenols was performed using the assay explained by Diaz and Martin [24].
Fruit Pigments
Chlorophyll and carotenoid contents in the pulp of three replicates were spectrophotometrically measured using the assay of Edmisten, et al. [25]. The optical density of the extract was determined at a spectrum of 663 nm for chlorophyll a, 646 nm for chlorophyll b and 470 for carotene using a spectrophotometer (UV1901PC spectrophotometer). Pigment contents were expressed by the next equations:
where E = absorbance at the specified spectrum length and findings were calculated as mg/100 g of fresh weight (FW) as follows:
Chlorophyll a (µg/mL) = 12.21 E663 − 2.81 E646
Chlorophyll b (µg/mL) = 20.13 E646 − 2.81 E663
Total Chlorophyll (µg/mL) = Chlorophyll a + Chlorophyll b
Total carotenoids (µg/mL) = (1000 E470) − (3.27 × chlorophyll a + 104 × chlorophyll b)/198
Resulting value from each equation × volume extract/(1000 × FW) × 100
2.4. Statistical Analysis
This experiment was conducted with a wholly randomized model with three replicates consisting of two factors: postharvest treatments and storing period. Measurements were performed in triplicate and their average values and standard deviations were calculated. This experiment was analyzed as a factorial experiment. Data calculated as (%) were altered to arcsine of square root before statistical analysis and non-transformed means are displayed as they are. The impact of postharvest treatments and cold-storing period on several properties was statistically analyzed by ANOVA using the MSTAT-C statistical package as per Snedecor and Cochran [26]. Comparisons among means were conducted via LSD test at 5% by Steel and Torrie [27].
3. Results and Discussion
3.1. Physical and Quality Features
3.1.1. Fruit Weight Failure Ratio
In considering the percentage of fruit weight failure, it is crucial to note that cold-stored Barhi date palm fruits (0.0 °C) had a storage life of up to 70 d. According to Figure 4, the fruit weight loss ratio of the Barhi date palm improved as the storing phase was extended in both times. As a result, fruits kept in cold storage for ten days had the smallest ratios of fruit weight failure. However, the greatest proportion of weight loss was documented after seventy days. The variations in the fresh weight damage percentage of cold kept Barhi date palm fruits owing to the impact of delaying the storing interval were obvious and achieved importance. Mentioning the exact impact of studied pre-harvest soaking treatments, Figure 4 demonstrates that all examined treatments considerably reduced the fresh fruit weight injury percentage as equated with the control. Though, chitosan nanoparticle at 3 cm3/L is likely to be the most efficient one in this regard. This tendency was significant as compared to either control or other pre-harvest treatments. Moreover, other treatments were between the two boundaries, with a comparative inclination showing that chitosan foliar spray, especially at either 1 or 2 g/L, was the minimum useful measure as compared to the other intermediary treatments during both periods of study. In relation to binding impact between storage times and checking pre-harvest treatments, data in Figure 4 shows that the connections of ten day cold storage period caused the smallest proportion of fruit weight failure, especially chitosan nanoparticle at 3 cm3/L as well as CaCl2 3 g/L. Conversely, the greatest fruit weight failure ratios were documented by the exchanges of seventy days cold storage time, mainly control fruits. The continued exchanges of the checked storing periods were reached in between.

Figure 4.
The impact of pre-harvest chitosan, chitosan nanoparticle, and CaCl2 foliar spray treatments on weight loss of Barhi date fruits under cold storing for 70 d. Note that * sign denotes the significant differences between the control and edible coatings treatments, and T1, T2, T3, T4, T5, T6, T7, T8, T9, T10 are water (control), 1 g/L chitosan, 2 g/L chitosan, 3 g/L chitosan, 1 g/L chitosan nanoparticles, 2 g/L chitosan nanoparticles, 3 g/L chitosan nanoparticles treatments, respectively.
Concerning the interface impact of dual explored factors (per-harvest treatments and length of cold storing); it is extremely apparent that both examined factors indicated their own exact impact on the weight loss (%). Hence, dissimilar mixtures after 10 d under cold storage meaningfully revealed the smallest refreshing weight loss % in Barhi dates as compared to those of late dates from one hand. On the other hand, variations among patterns of the initial testing date (after 10 d) in most incidents were insignificant and did not show anything significant in this regard. However, the minimum loss percentage was concurrent to chitosan nanoparticle at 3 cm3/L and CaCl2 at 3 g/L. Furthermore, the tendency of reply acquired the other means about as the storing period was lengthy, while changes developed more distinctly and, in most instances, reached equal significance with differences between groupings after 50, 60, and 70 d, irrespective of examined treatments. Nevertheless, three mixtures of chitosan nanoparticle at 3 cm3/L followed by those of chitosan nanoparticle at 2 cm3/L and/or CaCl2 at 3 g/L demonstrated statistically the minimum fruit loss percentage as was individually equivalent to those of other examined treatments at each of the four late testing dates.
3.1.2. Fruit Decay Percentage
Figure 5 indicates that the decay proportion steadily improved as the storing period was extended in both periods. As a result, cold storing for seventy days at 0.0 °C ± 2 decreased the preservability of Barhi date fruits, causing in the greatest fruit decline proportions compared with to ten days cold storing. The rest storing times got in between in this regard. The variations among the assessed storing cycles were large enough to be statistically important. Regarding the specific impact of tested pre-harvest treatments, Figure 5 indicates that 3 g/L CaCl2 treatment statistically recorded the lowest fruit decay proportions, observed by 3 cm3/L chitosan nanoparticle treatment.

Figure 5.
The impact of pre-harvest chitosan, chitosan nanoparticle and CaCl2 foliar spray treatments on decay percentage of Barhi date fruits under cold storing for 70 d. Note that * sign denotes the significant differences between the control and edible coatings treatments, and T1, T2, T3, T4, T5, T6, T7, T8, T9, and T10 are water (control), 1 g/L chitosan, 2 g/L chitosan, 3 g/L chitosan, 1 g/L chitosan nanoparticles, 2 g/L chitosan nanoparticles, 3 g/L chitosan nanoparticles treatments, respectively.
Conversely, the highest fruit decay ratios were combined with tap water (H2O) foliar spray “control” observed under 1 g/L chitosan treatment. Debating contact impact between storage times and checked pre-harvest treatments; it is obvious from Figure 5 that the 10 d storing period recorded the smallest fruit decay ratios in parallel with the subsequent periods of 50, 60, and 70 d cold storage times in ascending order. Furthermore, all connections of 10 d storing interval yielded the smallest fruit decay (%), especially 3 g/L CaCl2. In contrast, for the 70 d storing period, most untreated “control” fruits statistically reached the greatest fruit decay ratio. The continued exchanges resulted in in-between values in this sense.
3.1.3. Fruit Firmness
According to the data in Figure 6, extending the storing period increased the overall flexibility of Barhi date fruits. The lengthier the storing time (70 d), the softer the date fruits were compared to freshly picked Barhi dates in both periods. The differences in decreasing firmness between storing periods were pronounced and reached a level of significance in most cases. Regarding the exact impact of the examined postharvest treatments, it is obvious that the reaction of fruit inflexibility to the various explored pre-harvest treatments was statistically lacking and could be safely ignored.
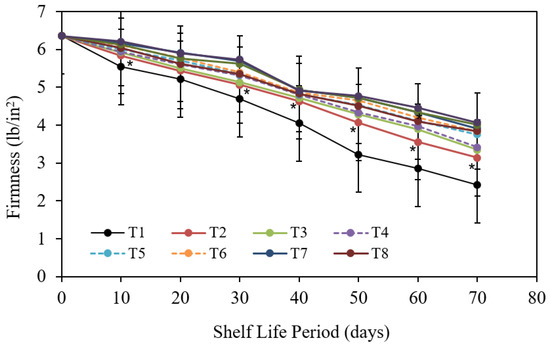
Figure 6.
The impact of pre-harvest chitosan, chitosan nanoparticle and CaCl2 foliar spray treatments on firmness of Barhi date fruits under cold storing for 70 d. Note that * sign denotes the significant differences between the control and edible coatings treatments, and T1, T2, T3, T4, T5, T6, T7, T8, T9, and T10 are water (control), 1 g/L chitosan, 2 g/L chitosan, 3 g/L chitosan, 1 g/L chitosan nanoparticles, 2 g/L chitosan nanoparticles, 3 g/L chitosan nanoparticles treatments, respectively.
Figure 6 displays that the alterations among numerous blends (storing period postharvest actions) were thus minor and that they were not significant in most cases, except when comparing those of control treated fruits after 70 d storage with the majority of those of two former sampling dates (after 10, 20, and 30 d) regardless of postharvest treatments. This trend for the contact impact of two studied factors (storing period and pre-harvest treatments) was observed and it could be clarified on that the variance in degree of reply to both examined issues was comparatively advanced with storing period and too minor to be observed with pre-harvest treatments.
3.1.4. Rutab Fruit (%)
Figure 7 shows that the percentage of rutab fruit increased steadily as the storing period was extended. Following that, cold storing for seventy d at 0 °C ± 2 decreased the preservability of Barhi date fruits, resulting in the highest rutab fruit proportions when compared to the conforming ones of 10 d cold storing. The alterations among the assessed storing periods were significant. In terms of the exact consequence of the tried pre-harvest conducts, Figure 7 shows that chitosan nanoparticle at 3 cm3/L treated fruits statistically saw the lowest rutab fruit proportions, monitored by CaCl2 at 3 g/L treatment. Furthermore, chitosan nanoparticle at 2 cm3/L treatment had similar and lower rutab proportions. On the other hand, the uppermost rutab fruit proportions were seen in tap water dipped “control” fruits, followed by the chitosan at 1 g/L treatment. Determining contact impact among storing periods and pre-harvest treatments it is evident from Figure 7 that the relations of the 10 d storing period recorded the smallest fruit rutab proportions compared with the matching ones at 10, 20, 30, 40, 50, 60, and 70 d of cold storage ascendingly. Thus, all 10 d storage durations created the lowest rutab proportions, mainly 3 cm3/L chitosan nanoparticle treatments. In contrast, of all 70 d storage duration samples, control fruits achieved the greatest rutab proportion. The other test sets reached in-between rates of rutab fruit in this regard.
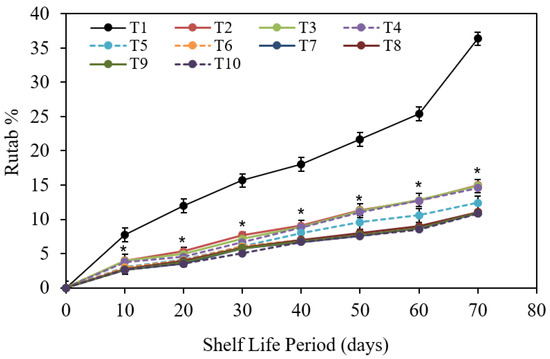
Figure 7.
The impact of pre-harvest chitosan, chitosan nanoparticle, and CaCl2 foliar spray treatments on rutab proportion of Barhi date fruits under cold storage for 70 d. Note that * sign denotes the significant differences between the control and edible coatings treatments, and T1, T2, T3, T4, T5, T6, T7, T8, T9, and T10 are water (control), 1 g/L chitosan, 2 g/L chitosan, 3 g/L chitosan, 1 g/L chitosan nanoparticles, 2 g/L chitosan nanoparticles, 3 g/L chitosan nanoparticles treatments, respectively.
3.1.5. Fruit Moisture Content (%)
The decrease in moisture content of Barhi date fruits is proportional to the development of the storage period, as shown in Figure 8. Thus, fruits that had been cold stored for 70 d had the lowest fruit moisture content values. In contrast, newly collected fruits (zero-days of storage) received the greatest scores in this category. Other cold storage period rates fell somewhere in the middle of the two formerly stated types. The disparities among the storing periods studied were apparent and significant. In terms of the exact impact of pre-harvest treatments, statistical analysis of data in Figure 8 shows that none of the treatments studied had a significant impact on fruit moisture content. Nevertheless, the comparative decrease rate of moisture content was usually coupled by control and chitosan at 1 g/L treatment corresponding to relative higher content of chitosan nanoparticles at 1 & 2 cm3/L as well as the CaCl2 treatment at 3 g/L. Additionally, other examined treatments were in between the aforementioned two. Acquired data as taken from Figure 8 indicates that each studied treatment had its own certain impact. Consequently, seven mixtures with 10 d storing period generally kept the greatest fruit moisture content, but variations were so slight as to be insignificant as they matched to the corresponding ones of 20 d storing. As cold storage continued, the significance of variations become more evident, mostly in terms of moisture content of seven mixtures after 10 d storage compared to either those of 50, 60, and 70 d cold storage. In other terms, the reduction rate in fruit moisture content developed more severely after 50 d of cold storing. The serious decrease was also continued to the final storage date (after 70 d). The least moisture content was identified in 7 mixtures after 70 d of storing as matched to (10, 20, and 30 d), irrespective of pre-harvest treatments utilized.
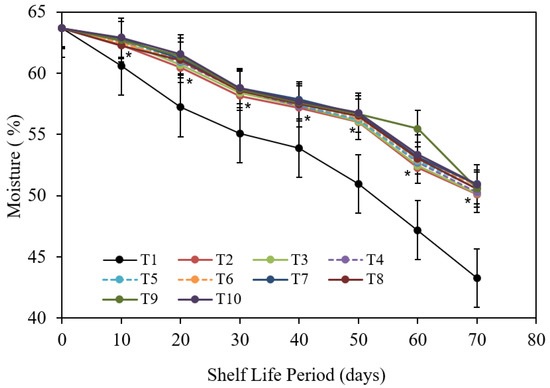
Figure 8.
The impact of pre-harvest chitosan, chitosan nanoparticle, and CaCl2 foliar spray treatments on moisture of Barhi date fruits under cold storing for 70 d. Note that * sign denotes the significant differences between the control and edible coatings treatments, and T1, T2, T3, T4, T5, T6, T7, T8, T9, and T10 are water (control), 1 g/L chitosan, 2 g/L chitosan, 3 g/L chitosan, 1 g/L chitosan nanoparticles, 2 g/L chitosan nanoparticles, 3 g/L chitosan nanoparticles treatments, respectively.
3.2. Chemical Characteristics
3.2.1. Total Soluble Solids (TSS)
Data showed the impact of pre-harvest treatments on TSS percentage of Barhi date fruits during cold storing at 0 °C and 90–95% RH for 70 d are presented in Figure 9 TSS enhanced with expansion of the storing interval achieving the highest rates at a storing period of (70 d) for the Barhi date cultivar. Generally, it could be mentioned that all safe post-harvest treatments caused significantly lower TSS values than the untreated fruits compared with control. At the end of the storing period, it showed that the highest percentage of TSS was obtained for control fruits (35.78%). Meanwhile, the lowest mean values were obtained from chitosan nanoparticle at 3 cm3/L (33.91%); followed by chitosan nanoparticle at 2 cm3/L (33.92%), followed by chitosan nanoparticle at 1 cm3/L (34.08%), and then CaCl2 3 g/L at (34.02%) and CaCl2 2 g/L (34.09%); followed by CaCl2 at 1 g/L (34.13%) and then chitosan at 3 g/L; 2 g/L and 1 g/L (34.30%); (34.36%) and (34.48%) in downward order gave the deepest values of TSS compared with the untreated fruits (35.22%).

Figure 9.
The impact of pre-harvest chitosan, chitosan nanoparticle and CaCl2 foliar spray treatments on TSS of Barhi date fruits under cold storing for 70 d. Note that * sign denotes the significant differences between the control and edible coatings treatments, and T1, T2, T3, T4, T5, T6, T7, T8, T9, and T10 are water (control), 1 g/L chitosan, 2 g/L chitosan, 3 g/L chitosan, 1 g/L chitosan nanoparticles, 2 g/L chitosan nanoparticles, 3 g/L chitosan nanoparticles treatments, respectively.
Assessing the impact of storing periods and safe post-harvest treatments, data showed that the 70 d cold storing period saw the greatest levels of fruit TSS proportion in untreated fruits (control). All pre-harvest coating treatments showed the lowest increase in TSS. The failure of a considerable section of H2O improves the TSS concentration. This concern makes the fruit much sweeter [5]. The lower TSS is owing to the gentler switch from carbohydrates to sugars [28].
TSS demonstrated an expanding tendency during fruit growth during cold storage. Elevated TSS rates were characterized by the greater proportion of sugars; fruit sweetener in the last phases of improvement is found in most fruits and can be ascribed to the hydrolytic transfer of insoluble carbohydrates into soluble sugars [29]. But in the case of date fruit, the failure of a significant part of the water improves the soluble solids concentration. This concern impacts both the taste and the texture of date fruit and makes the fruit much sweeter [5].
Similar results in mango fruit coated with chitosan had less soluble solids than untreated fruits. Also, in papaya, chitosan supplied an efficient control in prolonged variations in soluble solids concentration during five weeks of storing [30]. A comparable impact was noted for chitosan, with decreases in respiration rates, delayed ripening [31], and slow rise in TSS [10]. Meanwhile, the impact of calcium in reducing the TSS content of fruits, reducing the senescence rate and fruit maturation has been observed [32]. Chitosan coating mixed with Ca reduced papaya ripening as indicated by their increased retention of insoluble solids.
3.2.2. Total Sugars
Data collected indicating the impact of various pre-harvest treatments on total soluble sugars content of collected “Barhi” date fruits is displayed in Figure 10.
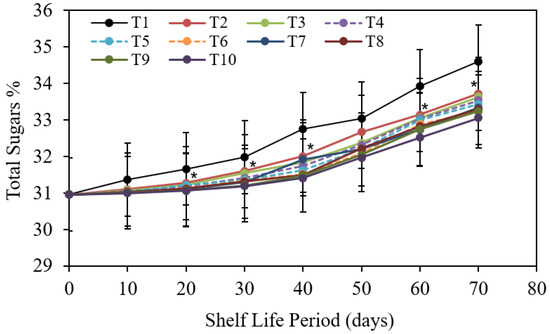
Figure 10.
The impact of pre-harvest chitosan, chitosan nanoparticle, and CaCl2 foliar spray treatments on total sugars of Barhi date fruits under cold storing for 70 d. Note that * sign denotes the significant differences between the control and edible coatings treatments, and T1, T2, T3, T4, T5, T6, T7, T8, T9, and T10 are water (control), 1 g/L chitosan, 2 g/L chitosan, 3 g/L chitosan, 1 g/L chitosan nanoparticles, 2 g/L chitosan nanoparticles, 3 g/L chitosan nanoparticles treatments, respectively.
It is obvious that total soluble sugars gradually and significantly improved with extension of the storing period.
The control treatment demonstrated greater and faster expansion in total soluble sugars during cold storage than observed in fruits with pre-harvest treatments. In this regard: chitosan nanoparticle at 3 cm3 (31.52%) followed by chitosan nanoparticle at 2 cm3 (31.26 %) followed by CaCl2 at 3 g/L and 2 g/L (31.76%) and then CaCl2 at 2 g/L and chitosan nanoparticle at 1 cm3 treatments (31.78%) and then chitosan nanoparticle at 1 cm3 and CaCl2 at 3 g/L (31.81%) followed by CaCl2 at 1 g/L recorded (31.85%); chitosan alone at 3 g/L; 2 g/L and 1 g/L (31.90%); (31.96%) and (32.05%) treatments in descendant order provided the lowest rates of total sugars as compared with the control treatment which saw the highest rates of total sugars (32.53%).
Furthermore, the impact of contact showed that at the ending of the storing period (70 d), fruits treated with the pre-harvest treatments showed the smallest values of total sugars at initial periods.
It could be stated that rising total soluble sugars may be owing to improving hydrolysis of polysaccharides and starch to soluble sugars during the cold storage period.
All pre-harvest treatments decrease improves in total soluble sugars, while the control handed the maximum content of total sugars in both periods. This may be due to the fact that the elevated breathing of control fruit transforms stored sugars or starch into energy and improves maturation.
The growth in sugars content of fruits could be owing to the ageing procedure that led to the conversion of some carbohydrates as starch to sugars by the enzymatic behaviors [33]. The greater total sugar content as “Barhi” date fruits given from the Khalal to Rutab (complete grow fruits or lessening) phase.
Fruit sweetening in the last phases of growth is realized in highly fruits and can be ascribed to the hydrolytic alteration of unsolvable carbohydrate polymers into low-intensity soluble sugars [29].
3.2.3. Reducing Sugars
Data in Figure 11 refer to the reducing sugars content increased significantly with delaying cold storing times. The control treatment showed the maximum rates of reducing sugars content. Chitosan nanoparticle at 3 cm3/L recorded the highest values (29.47) followed by chitosan nanoparticle at 3 cm3/L and CaCl2 1 g/L (29.52); CaCl2 at 3 g/L and chitosan nanoparticle at 2 cm3/L (29.53) and then chitosan nanoparticle at 1 cm3/L and CaCl2 at 3 g/L (29.54); and CaCl2 at 2 g/L (29.58) and followed by CaCl2 1 g/L and chitosan nanoparticle 1 cm3 (29.60) and then chitosan 3 g/L (29.86), chitosan 2 g/L (29.86) and chitosan 1 g/L (29.90) treatments (in descending order) provided the last rates of reducing sugars as related with the control treatment which noted the maximum values of reducing sugars (30.18%).

Figure 11.
The impact of pre-harvest chitosan, chitosan nanoparticle, and CaCl2 foliar spray treatments on reducing sugars of Barhi date fruits under cold storing for 70 d. Note that * sign denotes the significant differences between the control and edible coatings treatments, and T1, T2, T3, T4, T5, T6, T7, T8, T9, T10 are water (control), 1 g/L chitosan, 2 g/L chitosan, 3 g/L chitosan, 1 g/L chitosan nanoparticles, 2 g/L chitosan nanoparticles, and 3 g/L chitosan nanoparticles treatments, respectively.
Data showed that interaction of the treatments and cold storing period recorded the highest values percentage of reducing sugars are in the control treatment (untreated fruits). All pre-harvest safe treatments showed the lowest increase in reducing sugars. The higher reducing sugar in Barhi date fruits during pass fruit from the Khalal stage to rutab stage.
3.2.4. Total Tannins
The impacts of pre-harvest treatments on Barhi date total tannins content were discovered to be statistically significant Figure 12. At the ending of the 70 d storing period, the fruit total tannins content was reduced.
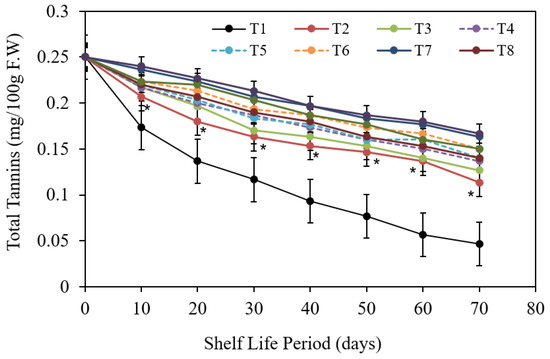
Figure 12.
The impact of pre-harvest chitosan, chitosan nanoparticle and CaCl2 foliar spray treatments on total tannins of Barhi date fruits under cold storing for 70 d. Note that * sign denotes the significant differences between the control and edible coatings treatments, and T1, T2, T3, T4, T5, T6, T7, T8, T9, T10 are water (control), 1 g/L chitosan, 2 g/L chitosan, 3 g/L chitosan, 1 g/L chitosan nanoparticles, 2 g/L chitosan nanoparticles, and 3 g/L chitosan nanoparticles treatments, respectively.
While failure was much greater in control; all postharvest coating treatments specially prevented the decrease of total tannins (chitosan nanoparticle at 3 cm3/L; CaCl2 at 3 g/L; chitosan nanoparticle at 2 cm3/L and CaCl2 at 3 g/L) and (chitosan nanoparticle at 3 cm3/L; CaCl2 at 3 g/L) gave the highest statistically values (0.225; 0.220; 0.219 and 0.214) in total tannins, respectively. Interaction data show significant differences among numerous treatments and storing periods, the uppermost total tannins content was obtained from Barhi date fruits covered with chitosan nanoparticle at 3 cm3/L and CaCl2 at 3 g/L treatments compared to control fruits which recorded the highest decline of means total tannins.
The lowest reduction of total tannins during storing were obtained from different pre-harvest edible coatings of Barhi date fruits (mainly with chitosan only).
This could be due to pre-harvest treatments which reduced tannin deterioration by decreasing the respiration level and produced an adapted atmosphere within the fruit that affect its metabolism [34] as expand the Khalal phase and postponed the opening in rutab phase thus, aided to postpone the maturation and protected quality of Barhi date fruits.
Al-Redhaiman [35] stated that total tannins content reduced as Barhi dates developed from the Khalal phase (Bisr or complete mature stage of development) to the mature phase (rutab). Tannin components are described as a cover under the skin of the date and mainly contain polyphenols and flavones, which are cracked down during maturing and transferred to unsolvable components that have blandness [36]. In this study, soluble tannins concentrations in fruits by safe postharvest treatment application might be due to their influence in the delayed fruit ripening process.
The current results are supported by proof that chitosan alone or blended with CaCl2 covered grape [14], aloe vera coated sweet cherry and papaya [37,38], and propolis extract coated sweet cherries [39] has aided in prolonging maturation, maintenance of fruit quality, and extension the shelf life.
3.2.5. Fruit Total Phenols Content
The findings in Figure 13 showed that all pre-harvest treatments were impactive in lowering total phenols content. Nevertheless, untreated fruits had the lowest total phenolic content, followed by chitosan at 1 g/L. and chitosan at 2 g/L. in descending order, whereas chitosan nanoparticle at 3 cm3/L, CaCl2 at 3 g/L and chitosan nanoparticle at 2 cm3/L had the highest total phenolic content.
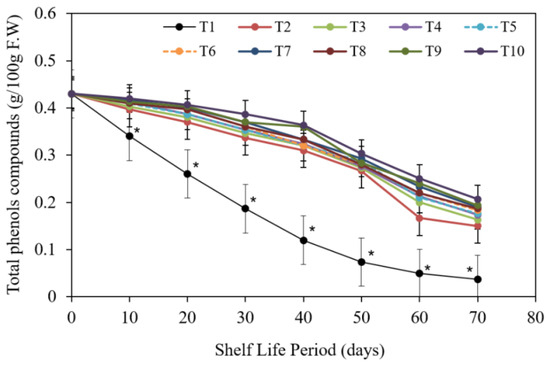
Figure 13.
The impact of pre-harvest chitosan, chitosan nanoparticle, and CaCl2 foliar spray treatments on total phenols compounds of Barhi date fruits under cold storing for 70 d. Note that * sign denotes the significant differences between the control and edible coatings treatments, and T1, T2, T3, T4, T5, T6, T7, T8, T9, T10 are water (control), 1 g/L chitosan, 2 g/L chitosan, 3 g/L chitosan, 1 g/L chitosan nanoparticles, 2 g/L chitosan nanoparticles, and 3 g/L chitosan nanoparticles treatments, respectively.
In terms of the impact of storing periods, Figure 13 shows that, irrespective of the primary reading, total phenolic content gradually declined as the cold storing period was improved from 10 d to 70 d. Nevertheless, stored fruits for 70 d gained the lowest rates equated with storing periods for 10 d.
In terms of the contact impact between the tested pre-harvest treatments and storing periods regardless of the early data (zero storing period), the combination of 70 d storing periods produced the lowest values of total phenolic content, particularly with untreated fruits and chitosan at 1 g/L. and chitosan at 2 g/L. treatments. The combination of 10 d and 20 d storing periods, on the other hand, produced the greatest rates of this parameter, particularly for untreated fruits.
3.2.6. Fruit Total Chlorophyll Content
The reduction in total chlorophyll content is proportional to the progress of storing period, as shown by Figure 14. Thus, after 70 d of cold storing, fruits had the lowest total chlorophyll content. In contrast, recently harvested fruits (zero-day storing) received the greatest ratings in this category. Other storing period rates fell somewhere in the middle of the two previously mentioned categories. The conflicts among the examined storing periods were apparent and significant. Regarding the exact impact of pre-harvest treatments, statistical analysis of data in Figure 14 shows that none of the treatments studied had a significant impact on total chlorophyll content reduction.

Figure 14.
The impact of pre-harvest chitosan, chitosan nanoparticle, and CaCl2 foliar spray treatments on total chlorophyll content of Barhi date fruits under cold storing for 70 d. Note that * sign denotes the significant differences between the control and edible coatings treatments, and T1, T2, T3, T4, T5, T6, T7, T8, T9, T10 are water (control), 1 g/L chitosan, 2 g/L chitosan, 3 g/L chitosan, 1 g/L chitosan nanoparticles, 2 g/L chitosan nanoparticles, and 3 g/L chitosan nanoparticles treatments, respectively.
As indicated in Figure 14, it was revealed that each examined treatment had a distinct impact. Following this, seven mixtures of 70 d storing periods had the lowest total chlorophyll content values. Temporarily, as the cold storing was expanded, the variations turned out to be more noticeable in reach degree of significance, mainly in terms of evaluating total chlorophyll content levels of seven mixtures after 10 d storing to those of either 60 d or 70 d cold storing. In other terms, the ratio of decrease in total chlorophyll content levels became inferior after 60 d of cold storing, where changes between seven blends of such date (60 d) were significant when equating them to the fourteen mixtures of the two former dates (10 d and 20 d), irrespective of pre-harvest treatments beneficial.
3.2.7. Fruit Total Carotenoids Content
The data in Figure 15 show that extending the cold storing period of Barhi date fruits increased the total carotenoids content of the fruit. The preliminary readings (i.e., before cold storing (zero-day storing)) had the smallest levels, whereas expanding the cold storing period up to 70 d had the greatest levels. The variations between the storing periods examined were large enough to be statistically significant.

Figure 15.
The impact of pre-harvest chitosan, chitosan nanoparticle, and CaCl2 foliar spray treatments on total carotenoids content of Barhi date fruits under cold storing for 70 d. Note that * sign denotes the significant differences between the control and edible coatings treatments, and T1, T2, T3, T4, T5, T6, T7, T8, T9, T10 are water (control), 1 g/L chitosan, 2 g/L chitosan, 3 g/L chitosan, 1 g/L chitosan nanoparticles, 2 g/L chitosan nanoparticles, and 3 g/L chitosan nanoparticles treatments, respectively.
In terms of the impact of the analyzed pre-harvest treatments, statistical analysis shows that the findings follow the same pattern in terms of the rejoinder of fruit total carotenoids content to the examined treatments. However, the greatest fruit total carotenoids content was created by the control and chitosan at 1 g/L and 2 g/L. Meanwhile, the lowest fruit total carotenoids content was generated by the chitosan nanoparticle at 3 cm3/l, chitosan nanoparticle at 2 cm3/L, and CaCl2 at 3 g/L treatments.
In terms of the interaction impact between storing periods and checked pre-harvest treatments, the data in Figure 15 show that the interactions of seventy days cold storing period, particularly untreated fruits, had the greatest carotenoids content values. In contrast, irrespective of zero-day storing, the mixtures of cold-stored Barhi date fruits for ten days, particularly those treated with chitosan nanoparticle at 3 cm3/L and CaCl2 at 3 g/L had the smallest fruit total carotenoids content.
3.3. Correlation Matrix among the Different Properties
Correlation analysis was applied for investigating the interdependence of the Barhi date characteristics (Table 2). The results indicated that weight loss was negatively linked with the moisture content and firmness, while decay had a strong positive relationship with the Rutab index and a negative correlation with the moisture content. Furthermore, the Rutab index was negatively associated with the total tannins and total chlorophyll.

Table 2.
Correlation matrix among the different properties of the stored Barhi date.
4. Discussion
Post-harvest losses of date palm fruits are a serious issue and are a result of rapid deterioration during handling, transportation, and storage. Barhi dates, fruits at the khalal stage, are frequently preferred and regarded as a premium product due to their physiological maturity, hardness, crispness, and bright yellow color [6]. Moreover, Abd Elwahab, et al. [40] reported that as storage time increased, so did weight loss and total sugars content. Firmness, total phenols, and total tannins decreased as storage time increased. The combination of 1.5 percent chitosan and 4 percent ethanol extracted propolis, followed by 1.5 percent chitosan and aloe vera gel, resulted in the least weight loss, the greatest firmness, and the slowest compositional changes in total phenols, total sugars, and total tannins. Thus, the chitosan + propolis extract or chitosan + aloe vera gel were proven to be the most effective treatments for preserving the quality of Barhi date palm during cold storage at 0 °C. Also, AbdElwahab, et al. [13] found that a post-harvest application of 1% chitosan + 4% calcium chloride reduced weight loss and maintained quality parameters in crimson seedless grapes during storage periods when compared to a control. Nanoparticles have distinct properties due to their small size [41,42]. The nano chitosan and silicon coatings increased vitamin C content, possibly since the coating reduces the gas exchange rate with the environment, preventing ascorbic acid exposure to O2 and concentrating it in the fruit. This finding is consistent with the findings of Han, et al. [43], who discovered delayed vitamin C degradation in chitosan-coated luffa fruits (Luffa cylindrica). On the other hand, Shi, et al. [44] found a clear decline in the Vitamin C value of the coated fruit with nano chitosan over the storage period.
The concentration of soluble solids rises as a result of significant water loss. The flavor of the fruit is enhanced by this phenomenon [5]. TSS is reduced since carbohydrates are converted to sugars more slowly [28]. TSS increased during the development of the fruit in cold storage. Fruit sweetening in the final stages of development is seen in most fruits and can be attributed to the hydrolytic conversion of insoluble carbohydrates into soluble sugars [29]. However, in the case of date fruit, the loss of a significant amount of water increases the concentration of soluble solids. This problem affects both the taste and texture of date fruit, making it much sweeter [5]. Chitosan has a similar effect in that it reduces respiration rates, delays ripening [31], and causes a slow rise in TSS [10]. Meanwhile, calcium has been shown to reduce the TSS content of fruits as well as the rate of senescence and fruit ripening [32]. The retention delays’ insoluble solid increase demonstrated that the chitosan coating combined with calcium slowed papaya ripening.
The the total sugar content of Barhi date palm fruits was higher as they progressed from the Khalal to Rutab (fully ripened fruits or softening) stage [45]. However, calcium is a major component of the total reducing and non-reusing parameters, and it plays an important role in the strengthening of cell walls and membrane structure as well as the retardation of these parameters [46]. According to Al-Redhaiman [35], the total tannins content of Barhi dates decreased as they matured from the Khalalstage (Bisr, or fully mature stage of development) to the ripe stage (rutab). Furthermore, the presence of soluble tannins in fruits after safe postharvest treatment application may be due to their influence on the delayed fruit ripening process. The current findings are supported by evidence from the use of chitosan, alone or in combination with calcium chloride-coated grapes [47].
5. Conclusions
Edible coatings have been progressively used for enhancing the quality and shelf life of food products during cold storage. In this study, the Barhi date has grabbed our attention. We attempted to extend its shelf life during cold storage utilizing edible coatings (since it is an economical product in the Arab world). So, in this work the main aim was to explore the influences of different concentrations of chitosan, CaCl2, and nano-chitosan as a safe pre-harvest treatment on the physicochemical and quality features of Barhi dates fruits during their storing period. The results revealed that the highest percentage of TSS was obtained in control fruits (35.78%). Meanwhile, the lowest means values were obtained from chitosan nanoparticle at 3 cm3/L (33.91%). Treatments with chitosan nanoparticle at 3 cm3/L and CaCl2 at 3 g/L gave the highest statistically values of total tannins (0.225 and 0.220, respectively). Overall, it can be concluded that all edible coatings treatments were very impactful in improving fruit quality and shelf life compared with the control treatment. The optimal treatment involved spraying the fruit with either 3 cm3/L of nano-chitosan or 3 g/L of CaCl2 to increase the fruit quality and the shelf-life of Barhi dates. Furthermore, the results showed that weight loss was negatively linked with moisture content and firmness, while decay had a strong positive relationship with the Rutab index and had a negative correlation with the moisture content. Furthermore, the Rutab index was negatively associated with the total tannins and total chlorophyll.
Author Contributions
Conceptualization, S.F.E.-G., A.M.E.-M., A.A.M.A.-M., M.F. and H.E.E.-B.; methodology, A.M.E.-M., S.F.E.-G., H.E.E.-B., M.F., M.F.E.-K. and A.E.S.; software, E.A., G.A., R.M.A. and H.A.; validation, R.M.A., M.F., R.S. and M.F.E.-K.; formal analysis, S.F.E.-G., A.M.E.-M., A.A.M.A.-M., M.F. and H.E.E.-B.; investigation, S.F.E.-G., A.M.E.-M., A.A.M.A.-M., M.F. and H.E.E.-B.; resources, S.F.E.-G., A.M.E.-M., A.A.M.A.-M., M.F., R.S. and R.M.A.; data curation, S.F.E.-G., A.M.E.-M., A.A.M.A.-M., M.F. and H.E.E.-B.; writing—original draft preparation, S.F.E.-G., A.M.E.-M., A.A.M.A.-M., M.F. and H.E.E.-B.; writing—review and editing, M.F.E.-K., G.A., R.M.A., H.A., R.S., A.A.M.A.-M. and A.M.E.-M.; visualization, S.F.E.-G., A.M.E.-M., A.A.M.A.-M., M.F. and H.E.E.-B.; supervision, S.F.E.-G., N.B. and H.E.E.-B.; funding acquisition, R.M.A., E.A., G.A., H.A. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available upon request from the corresponding author.
Acknowledgments
Taif University Researchers Supporting Project Number (TURSP-2020/140), Taif University, Taif, Saudi Arabia. Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2022R43), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Also, the authors thank Prince Sattam Bin Abdulaziz University, Al-Kharj for their scientific contributions.
Conflicts of Interest
The authors declared no conflict of interest.
References
- Chao, C.T.; Krueger, R.R. The date palm (Phoenix dactylifera L.): Overview of biology, uses, and cultivation. HortScience 2007, 42, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Boufersaoui, A.E.-K. The date in all its forms. In Proceedings of the 2nd International Conference for Date Palm (ICDP 2016), Qassim, Saudi Arabia, 10–12 October 2016. [Google Scholar]
- Mitra, S.K. Postharvest Physiology and Storage of Tropical and Subtropical Fruits; CAB international: New York, NY, USA, 1997. [Google Scholar]
- Baliga, M.S.; Baliga, B.R.V.; Kandathil, S.M.; Bhat, H.P.; Vayalil, P.K. A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.). Food Res. Int. 2011, 44, 1812–1822. [Google Scholar] [CrossRef]
- Mortazavi, S.; Arzani, K.; Barzegar, M. Analysis of sugars and organic acids contents of date palm (Phoenix dactylifera L.) ‘Barhee’during fruit development. In Proceedings of the IV International Date Palm Conference 882, Abu Dhabi, United Arab Emirates, 15–17 March 2010; pp. 793–801. [Google Scholar]
- Ismail, B.; Haffar, I.; Baalbaki, R.; Mechref, Y.; Henry, J. Physico-chemical characteristics and total quality of five date varieties grown in the United Arab Emirates. Int. J. Food Sci. Technol. 2006, 41, 919–926. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Elgammal, R.E.; Alhaithloul, H.A.S.; Alghanem, S.M.; Fikry, M.; Abdein, M.A.; Hikal, D.M. Enhance Fruit Ripening Uniformity and Accelerate the Rutab Stage by Using ATP in ‘Zaghloul’ Dates during the Shelf Life. Foods 2021, 10, 2641. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [Green Version]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Jiang, T.; Deng, M.; James, R.; Nair, L.S.; Laurencin, C.T. Micro-and nanofabrication of chitosan structures for regenerative engineering. Acta Biomater. 2014, 10, 1632–1645. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Liu, W. Effects of chitin and its derivative chitosan on postharvest decay of fruits: A review. Int. J. Mol. Sci. 2011, 12, 917–934. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT-Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Shiri, M.A.; Bakhshi, D.; Ghasemnezhad, M.; Dadi, M.; Papachatzis, A.; Kalorizou, H. Chitosan coating improves the shelf life and postharvest quality of table grape (Vitis vinifera) cultivar Shahroudi. Turk. J. Agric. For. 2013, 37, 148–156. [Google Scholar]
- Abd Elwahab, W.; Abd Elwahab, S.; Kamel, O. Using Chitosan Ethanol, Bergamot Oil, Acetic Acid and Calcium Chloride as Safe Alternatives to Sulfur Dioxide for Control Postharvest Decay, Maintain Quality of Crimson Grape. Master’s Thesis, Faculty of Agriculture, Cairo University, Cairo, Egypt, 2014. [Google Scholar]
- Kamal, H.M.; El-Wahab, S.M.; Farrag, A.H.; Zainhoum, A.A. Improving fruit quality and Storability of Zaghloul Date Palm Fruits By using Safe Pre and Post-harvest Substance. Biol. Chem. Environ. Sci. J. 2014, 10, 2243–2265. [Google Scholar]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Domaratzki, R.E.; Ghanem, A. Encapsulation and release of cladribine from chitosan nanoparticles. J. Appl. Polym. Sci. 2013, 128, 2173–2179. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Xing, R.; Hu, X.Y. A green hydrothermal route to copper nanocrystallites. J. Cryst. Growth 2004, 273, 280–284. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Qian, J.-Q.; Shi, L.-E. Preparation of chitosan nanoparticles as carrier for immobilized enzyme. Appl. Biochem. Biotechnol. 2007, 136, 77–96. [Google Scholar] [CrossRef]
- El-Gioushy, S.F.; Abdelkader, M.F.M.; Mahmoud, M.H.; El Ghit, H.M.A.; Fikry, M.; Bahloul, A.M.E.; Morsy, A.R.; Lo’ay, A.A.; Abdelaziz, A.M.R.A.; Alhaithloul, H.A.S.; et al. The Effects of a Gum Arabic-Based Edible Coating on Guava Fruit Characteristics during Storage. Coatings 2022, 12, 90. [Google Scholar] [CrossRef]
- EL-Gioushy, S.F.; Baiea, M.H.M. Impact of gelatin, lemongrass oil and peppermint oil on storability and fruit quality of Samany date palm under cold storage. Bull. Natl. Res. Cent. 2020, 44, 1–13. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; AOAC: Washington, DC, USA, 1995; Volume 7. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Taira, S.; Ono, M. Reduction of astringency in persimmon caused by adhesion of tannins to cell wall fragments. In Proceedings of the I International Persimmon Symposium 436, Chang Mai City, Thailand, 1 January 1997; pp. 235–242. [Google Scholar]
- Diaz, D.H.; Martin, G.C. Peach seed dormancy in relation to endogenous inhibitors and applied growth substances. Am. Soc. Hort. Sci. J. 1972, 651–654. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201302322820 (accessed on 3 January 2022).
- Edmisten, K.; Wood, C.; Reeves, D.; Tracy, P. Determination of cotton nitrogen status with a hand-held chlorophyll meter in Alabama and Missouri. In Proceedings of the Beltwide Cotton Conferences (USA), Nashville, TN, USA, 6–10 January 1992. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 6th ed.; Oxford and IBH Publishing Co.: New Delhi, India, 1980. [Google Scholar]
- Steel, R.; Torrie, J. Reproduced from Principles and Procedures of Statistics; Printed with the permission of CI Bliss; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1980; pp. 448–449. [Google Scholar]
- Rohani, M.; Zaipun, M.; Norhayati, M. Effect of modified atmosphere on the storage life and quality of Eksotika papaya. J. Trop. Agric. Food Sci. 1997, 25, 103–114. [Google Scholar]
- Saleem, S.A.; Baloch, A.K.; Baloch, M.K.; Baloch, W.A.; Ghaffoor, A. Accelerated ripening of Dhakki dates by artificial means: Ripening by acetic acid and sodium chloride. J. Food Eng. 2005, 70, 61–66. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Du, J.; Gemma, H.; Iwahori, S. Effects of chitosan coating on the storage of peach, Japanese pear, and kiwifruit. J. Jpn. Soc. Hortic. Sci. 1997, 66, 15–22. [Google Scholar] [CrossRef]
- Mahajan, B.; Dhatt, A. Studies on postharvest calcium chloride application on storage behaviour and quality of Asian pear during cold storage. J. Food Agric. Environ. 2004, 2, 157–159. [Google Scholar]
- Karemera, N.U.; Habimana, S. Effect of pre-harvest calcium chloride on post-harvest behavior of Mango fruits (Mangifera indica L.) cv. Alphonso. Univers. J. Agric. Res. 2014, 2, 119–125. [Google Scholar] [CrossRef]
- Guilbert, S.; Gontard, N.; Gorris, L.G. Prolongation of the shelf-life of perishable food products using biodegradable films and coatings. LWT-Food Sci. Technol. 1996, 29, 10–17. [Google Scholar] [CrossRef]
- Al-Redhaiman, K. Modified atmosphere improves storage ability, controls decay, and maintains quality and antioxidant contents of Barhi date fruits. J. Food Agric. Environ. 2004, 2, 25–32. [Google Scholar]
- Tafti, A.G.; Fooladi, M. Changes in physical and chemical characteristics of Mozafati date fruit during development. J. Biol. Sci 2005, 5, 319–322. [Google Scholar]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest. Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Marpudi, S.L.; Abirami, L.; Srividya, N. Enhancement of Storage Life and Quality Maintenance of Papaya Fruits Using Aloe Vera Based Antimicrobial Coating; NISCAIR-CSIR: New Delhi, India, 2011; pp. 83–89. [Google Scholar]
- Candir, E.E.; Ozdemir, A.; Soylu, E.M.; Sahinler, N.; Gul, A. Effects of propolis on storage of sweet cherry cultivar Aksehir Napolyon. Asian J. Chem. 2009, 21, 2659–2666. [Google Scholar]
- Abd Elwahab, S.M.; Abd Allatif, A.M.; Farid, M.A.; Soliman, S.M. Effect of safe post-harvest alternatives on quality and storage life of “barhi” date palm. Plant Arch. 2019, 19, 3937–3945. [Google Scholar]
- Monica, R.C.; Cremonini, R. Nanoparticles and higher plants. Caryologia 2009, 62, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Roller, S. Natural Antimicrobials for the Minimal Processing of Foods; Woodhead Publishing: Sawston, UK, 2003. [Google Scholar]
- Han, C.; Zuo, J.; Wang, Q.; Xu, L.; Zhai, B.; Wang, Z.; Dong, H.; Gao, L. Effects of chitosan coating on postharvest quality and shelf life of sponge gourd (Luffa cylindrica) during storage. Sci. Hortic. 2014, 166, 1–8. [Google Scholar] [CrossRef]
- Shi, S.; Wang, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125–131. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).