Is Allergy to Titanium Bone Fixation Plates a Problem?
Abstract
1. Introduction
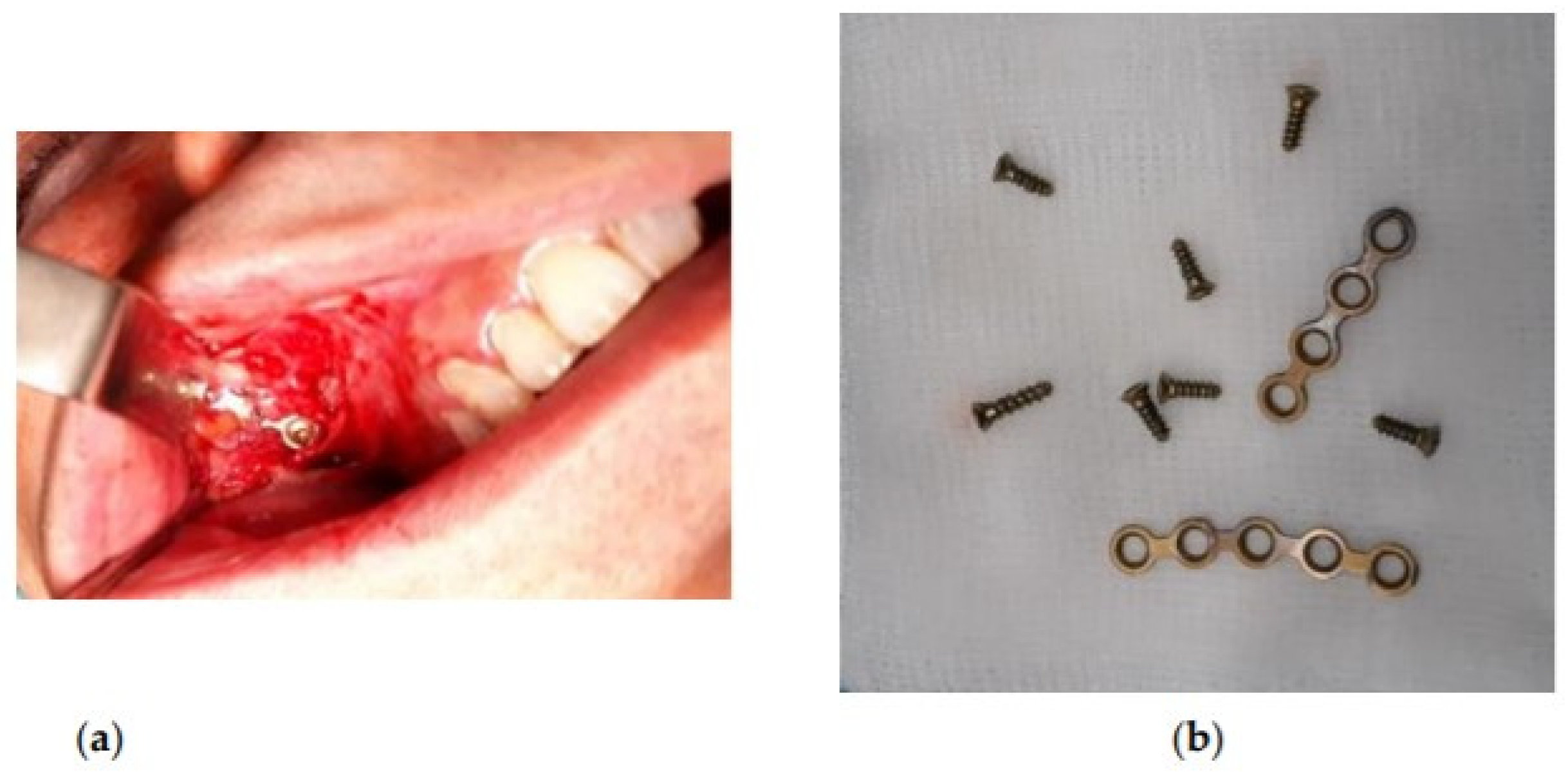
2. Materials and Methods
- The presence of titanium implants used for maxillofacial fracture fixation; or
- The presence of titanium dental implants; or
- The presence of titanium patient specific implants (PSIs) reconstructing the maxillofacial bone;
- Lack of patient’s consent;
- Malignant disease;
- Autoimmune disease;
- Tattoo or skin lesion in interscapular or suprascapular area;
- General disease.
- Active infectious disease;
- Antiallergic drugs usage;
- Steroid therapy;
- Extensive sunbathing;
- Cryotherapy;
- Vaccination.
- Group 1 (research group) (n = 50)—including the patients treated for maxillofacial fractures with the aid of titanium hardware.
- Group 2 (control group) (n = 20)—comprising healthy subjects with no history of medical titanium usage.
- Thorough medical history evaluation regarding prior orthopedic or dental procedures, in which titanium devices were used, and their occupational and environmental exposure to titanium or its compounds;
- Clinical examination with emphasis on skin and oral mucosa screening for lesions of ACD or ACS type;
- Skin allergy test with the aid of patch test.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.F. The post–anaphylaxis dilemma: How long is long enough to observe a patient after resolution of symptoms? Curr. Allergy Asthma Rep. 2008, 8, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.B. Allergy and Allergic Diseases. N. Engl. J. Med. 2001, 344, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Wood, N.H.; Khammissa, R.A.G.; Lemmer, J. Review: Allergic contact stomatitis. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Kalboussi, H.; Kacem, I.; Aroui, H.; El Maalel, O.; Maoua, M.; Brahem, A.; El Guedri, S.; Chatti, S.; Ghariani, N.; Mrizak, N. Impact of Allergic Contact Dermatitis on the Quality of Life and Work Productivity. Dermatol. Res. Pr. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Czajkowski, R.; Kowaliszyn, B.; Żbikowska-Gotz, M.; Bartuzi, Z. Analysis of skin patch test results and metalloproteinase–2 levels in a patient with contact dermatitis. Adv. Dermatol. Allergol. 2015, 32, 154–161. [Google Scholar] [CrossRef]
- Garau, V.; Masala, M.G.; Cortis, M.C.; Pittau, R. Contact stomatitis due to palladium in dental alloys: A clinical report. J. Prosthet. Dent. 2005, 93, 318–320. [Google Scholar] [CrossRef]
- Hosoki, M.; Nishigawa, K.; Miyamoto, Y.; Ohe, G.; Matsuka, Y. Allergic contact dermatitis caused by titanium screws and dental implants. J. Prosthodont. Res. 2016, 60, 213–219. [Google Scholar] [CrossRef]
- DE Rossi, S.S.; Greenberg, M.S. Intraoral Contact Allergy: A Literature Review and Case Reports. J. Am. Dent. Assoc. 1998, 129, 1435–1441. [Google Scholar] [CrossRef]
- Ditrichova, D.; Kapralova, S.; Tichy, M.; Ticha, V.; Dobesova, J.; Justova, E.; Eber, M.; Pirek, P. Oral lichenoid lesions and allergy to dental materials. Biomed. Pap. 2007, 151, 333–339. [Google Scholar] [CrossRef]
- Ricciardi, L.; Minciullo, P.L.; Saitta, S.; Trombetta, D.; Saija, A.; Gangemi, S. Increased serum levels of IL–22 in patients with nickel contact dermatitis. Contact Dermat. 2009, 60, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Minciullo, P.L.; Paolino, G.; Vacca, M.; Gangemi, S.; Nettis, E. Unmet diagnostic needs in contact oral mucosal allergies. Clin. Mol. Allergy 2016, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Dinakar, D.; Kurian, S.S.; Bindoo, Y. Investigation of contact allergy to dental materials by patch testing. Indian Dermatol. Online J. 2014, 5, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Ghomi, E.R.; Alipour, S.; Ramakrishna, S.; Sukiman, N.L. A state–of–the–art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Bio–Design Manuf. 2021, 1, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, L. Metals used in maxillofacial surgery. Oral Implant. 2016, 9, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.; McFadden, R.; Chan, C.-W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef]
- Poli, P.P.; de Miranda, F.V.; Polo, T.O.B.; Júnior, J.F.S.; Neto, T.J.L.; Rios, B.R.; Assunção, W.G.; Ervolino, E.; Maiorana, C.; Faverani, L.P. Titanium Allergy Caused by Dental Implants: A Systematic Literature Review and Case Report. Mater. 2021, 14, 5239. [Google Scholar] [CrossRef]
- Tanwar, N.; Prakash, C.; Chaudhary, K.; Tewari, S.; Bhagavatheeswaran, S. Titanium allergy in dentistry: A new allergen in rapidly evolving implant dentistry. Contemp. Clin. Dent. 2021, 12, 317. [Google Scholar] [CrossRef]
- Singh, R.; Lehl, G.; Hussain, A.B.; Abhang, T.N.; Kulkarni, M.M.; Elagib, M.F.A.; Tiwari, R.V.C. Prevalence of Titanium Hypersensitivity in Patients with Titanium Implants: A Systematic Review and Meta–Analysis. J. Pharm. Bioallied Sci. 2021, 13, S1345. [Google Scholar] [CrossRef]
- Comino-Garayoa, R.; Brinkmann, J.C.-B.; Peláez, J.; López-Suárez, C.; Martínez-González, J.M.; Suárez, M.J. Allergies to Titanium Dental Implants: What Do We Really Know about Them? A Scoping Review. Biology 2020, 9, 404. [Google Scholar] [CrossRef]
- de Viteri, V.S.; Fuentes, E. Titanium and Titanium Alloys as Biomaterials. In Tribology: Fundamentals and Advancements; Bod–Books on Demand: Norderstedt, Germany, 2013; pp. 155–181. [Google Scholar] [CrossRef]
- Rack, H.; Qazi, J. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Capucha, T.; Shilo, D.; Aslan, R.A.; Blanc, O.; Ginini, J.G.; Semel, G.; Emodi, O.; Rachmiel, A. Is Open Reduction Internal Fixation Using Titanium Plates in the Mandible as Successful as We Think? J. Craniofacial Surg. 2021. Publish ahead. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, C.; Bhoj, M. Evaluation of postoperative complications of open reduction and internal fixation in the management of mandibular fractures: A retrospective study. Indian J. Dent. Res. 2019, 30, 94–96. [Google Scholar] [PubMed]
- Chrcanovic, B.R. Open versus closed reduction: Comminuted mandibular fractures. Oral Maxillofac. Surg. 2012, 17, 95–104. [Google Scholar] [CrossRef]
- Richards, L.J.; Streifel, A.; Rodrigues, J.M. Utility of Patch Testing and Lymphocyte Transformation Testing in the Evaluation of Metal Allergy in Patients with Orthopedic Implants. Cureus 2019, 11, e5761. [Google Scholar] [CrossRef]
- Thomas, P.; Bandl, W.-D.; Maier, S.; Summer, B.; Przybilla, B. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T–cell hyperresponsiveness in vitro: Case report and review of the literature. Contact Dermat. 2006, 55, 199–202. [Google Scholar] [CrossRef]
- Lalor, P.; Revell, P.; Gray, A.; Wright, S.; Railton, G.; Freeman, M. Sensitivity to titanium. A cause of implant failure? J. Bone Jt. Surgery. Br. Vol. 1991, 73-B, 25–28. [Google Scholar] [CrossRef]
- Müller, K.; Valentine-Thon, E. Hypersensitivity to titanium: Clinical and laboratory evidence. Neuro Endocrinol. Lett. 2006, 27 (Suppl. 1), 31–35. [Google Scholar]
- Chaturvedi, T. Allergy related to dental implant and its clinical significance. Clin. Cosmet. Investig. Dent. 2013, 5, 57–61. [Google Scholar] [CrossRef]
- Lane, W. On the Advantage of the Steel Screw in the Treatment of Ununited Fractures. Lancet 1893, 142, 1500–1501. [Google Scholar] [CrossRef][Green Version]
- Yaremchuk, M.J.; Gruss, J.S.; Manson, P.N.; Vasconez, H.C. Rigid Fixation of the Craniomaxillofacial Skeleton. Plast. Reconstr. Surg. 1993, 92, 168. [Google Scholar] [CrossRef]
- Simonsen, A.; Johansen, J.; Deleuran, M.; Mortz, C.; Skov, L.; Sommerlund, M. Children with atopic dermatitis may have unacknowledged contact allergies contributing to their skin symptoms. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 428–436. [Google Scholar] [CrossRef]
- Yoshihisa, Y.; Shimizu, T. Metal Allergy and Systemic Contact Dermatitis: An Overview. Dermatol. Res. Pract. 2012, 2012, 749561. [Google Scholar] [CrossRef]
- Roach, M. Base Metal Alloys Used for Dental Restorations and Implants. Dent. Clin. N. Am. 2007, 51, 603–627. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Linneberg, A.; Menné, T.; Johansen, J.D. The epidemiology of contact allergy in the general population—Prevalence and main findings. Contact Dermat. 2007, 57, 287–299. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant. Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Shetty, V.H.; Eram, H.; Babu, A.M. Study of the clinical pattern of contact dermatitis over the face and its correlation with patch testing. Int. J. Res. Dermatol. 2019, 5, 350–356. [Google Scholar] [CrossRef]
- Spiewak, R.; Pietowska, J.; Curzytek, K. Nickel: A unique allergen–from molecular structure to European legislation. Expert Rev. Clin. Immunol. 2007, 3, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Watchmaker, J.; Collins, R.; Chaney, K. Allergic Contact Dermatitis to Manganese in Metallic Implant. Dermat. 2015, 26, 149–150. [Google Scholar] [CrossRef]
- Calnan, C.D. Nickel Dermatitis. Br. J. Dermatol. 1956, 68, 229–236. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, A.W.; Aitken, C.V.; Ridsdill–Smith, R. Urticaria after insertion of Smith–Petersen Vitallium nail. BMJ 1967, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, J. Nickel sensitivity as a cause of infusion reactions. Lancet 1960, 276, 741–742. [Google Scholar] [CrossRef]
- Schuh, M.A.; Thomas, P.; Kachler, W.; Göske, J.; Wagner, L.; Holzwarth, U.; Forst, R. Das Allergiepotenzial von Implantatwerkstoffen auf Titanbasis. Der Orthopäde 2005, 34, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, M.; Nishigaw, K. Dental Metal Allergy. Contact Dermatitis 2011, 1, 89–108. [Google Scholar] [CrossRef]
- Goutam, M.; Giriyapura, C.; Mishra, S.K.; Gupta, S. Titanium allergy: A literature review. Indian J. Dermatol. 2014, 59, 630. [Google Scholar] [CrossRef]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Implant. Res. 2008, 19, 823–835. [Google Scholar] [CrossRef]
- Haug, R.H. Retention of asymptomatic bone plates used for orthognathic surgery and facial fractures. J. Oral Maxillofac. Surg. 1996, 54, 611–617. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S.; Baker, D.L. Basic Immunology: Functions and Disorders of the Immune System, 6th ed.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Wahlberg, J.E.; Lindberg, M. Patch Testing. In Contact Dermatitis; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Pigatto, P.D.; Brambilla, L.; Ferrucci, S.; Zerboni, R.; Somalvico, F.; Guzzi, G. Systemic allergic contact dermatitis associated with allergy to intraoral metals. Dermatol. Online J. 2014, 20, 20. [Google Scholar] [CrossRef]
- Hallab, N.; Merritt, K.; Jacobs, J.J. Metal Sensitivity in Patients with Orthopaedic Implants. J. Bone Jt. Surg. 2001, 83, 428–436. [Google Scholar] [CrossRef]
- Hallab, N.; Mikecz, K.; Jacobs, J. A Triple Assay Technique for the Evaluation of Metal-induced, Delayed-type Hypersensitivity Responses in Patients with or Receiving Total Joint Arthroplasty. J. Biomed. Mater. Res. 2000, 53, 480–489. [Google Scholar] [CrossRef]
- Fage, S.W.; Muris, J.; Jakobsen, S.S.; Thyssen, J.P. Titanium: A review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Dermat. 2016, 74, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Verbov, J. Pacemaker contact sensitivity. Contact Dermat. 1985, 12, 173. [Google Scholar] [CrossRef]
- Peters, M.S.; Schroeter, A.L.; Van Hale, H.M.; Broadbent, J.C. Pacemaker contact sensitivity. Contact Dermat. 1984, 11, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.L.; Synnott, S.A.; Van Dercreek, J.A. Tissue reaction involving an intraoral skin graft and CP titanium abutments: A clinical report. Int. J. Oral Maxillofac. Implant. 1990, 5, 79–84. [Google Scholar]
- Holgers, K.-M.; Thomsen, P.; Tjellström, A. Persistent Irritation of the Soft Tissue Around an Osseointegrated Titanium Implant: Case Report. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1994, 28, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Darlenski, R.B.; Demerdjieva, Z.; Kazandjieva, J.S.; Tsankov, N.K. Systemic Contact Dermatitis to Nickel. OA Dermatol. 2014, 2, 7. [Google Scholar]
- Suwarsa, O.; Rahardjo, R.M.; Sutedja, E.; Dharmadji, H.P.; Hindritiani, R.; Gunawan, H. Systemic contact dermatitis due to corrosion of titanium–coated nickel and cobalt bone plate fixation. Medicine 2017, 96, e9120. [Google Scholar] [CrossRef]

| Inclusion Criteria | |
| Titanium Hardware Used for Maxillofacial Fracture Fixation Titanium Dental Implants Titanium Patient Specific Implants (PSIs) | |
| Exclusion Criteria | |
| Permanent | Temporary |
| Lack of consent Malignant disease Autoimmune disease Tattoo/skin lesion in interscapular area General disease | Active infectious disease Antiallergic drugs Steroid therapy Extensive sunbathing Cryotherapy Vaccination |
| Symbol | Relevance | Clinical Appearance |
|---|---|---|
| ?+ | Doubtful reaction | Faint, nonpalpable erythema |
| + | Weak reaction | Erythema, infiltration, possible papules |
| ++ | Strong reaction | Erythema, infiltration, papules, vesicles |
| +++ | Extreme reaction | Erythema, infiltration, coalescing vesicles; bullae or ulceration |
| − | Negative reaction | No changes |
| IR | Irritant reaction | No induration |
| NT | Not tested |
| Clinical Symptom | Number of Patients | Percentage (%) |
|---|---|---|
| Surgical complications | ||
| Paresthesia | 23 | 46 |
| Wound dehiscence | 4 | 2 |
| Loss of hardware | 0 | 0 |
| Swelling | 19 | 38 |
| Allergic manifestations | ||
| Eczema on skin | 3 | 6 |
| Mucosal lesions | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedzielska, I.; Sitek-Ignac, N.; Bąk, M.; Niedzielski, D. Is Allergy to Titanium Bone Fixation Plates a Problem? Coatings 2022, 12, 214. https://doi.org/10.3390/coatings12020214
Niedzielska I, Sitek-Ignac N, Bąk M, Niedzielski D. Is Allergy to Titanium Bone Fixation Plates a Problem? Coatings. 2022; 12(2):214. https://doi.org/10.3390/coatings12020214
Chicago/Turabian StyleNiedzielska, Iwona, Natalia Sitek-Ignac, Michał Bąk, and Damian Niedzielski. 2022. "Is Allergy to Titanium Bone Fixation Plates a Problem?" Coatings 12, no. 2: 214. https://doi.org/10.3390/coatings12020214
APA StyleNiedzielska, I., Sitek-Ignac, N., Bąk, M., & Niedzielski, D. (2022). Is Allergy to Titanium Bone Fixation Plates a Problem? Coatings, 12(2), 214. https://doi.org/10.3390/coatings12020214






