Forecast the Microhardness of Ni-TiN Nanoplatings via an Artificial Neural Network Model
Abstract
:1. Introduction
2. Experiment
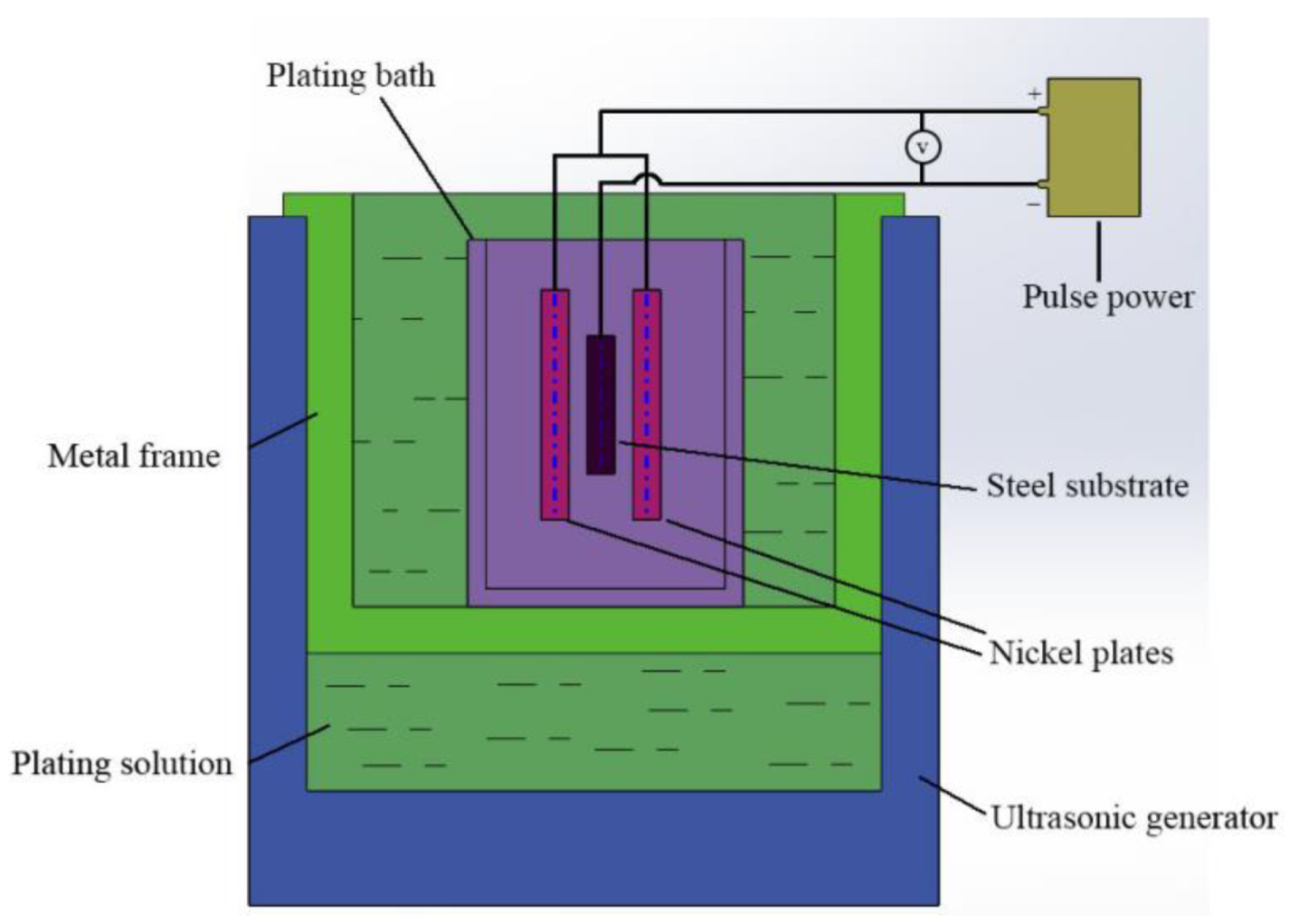
2.1. Preparation of Sample
2.2. Characterization of Sample
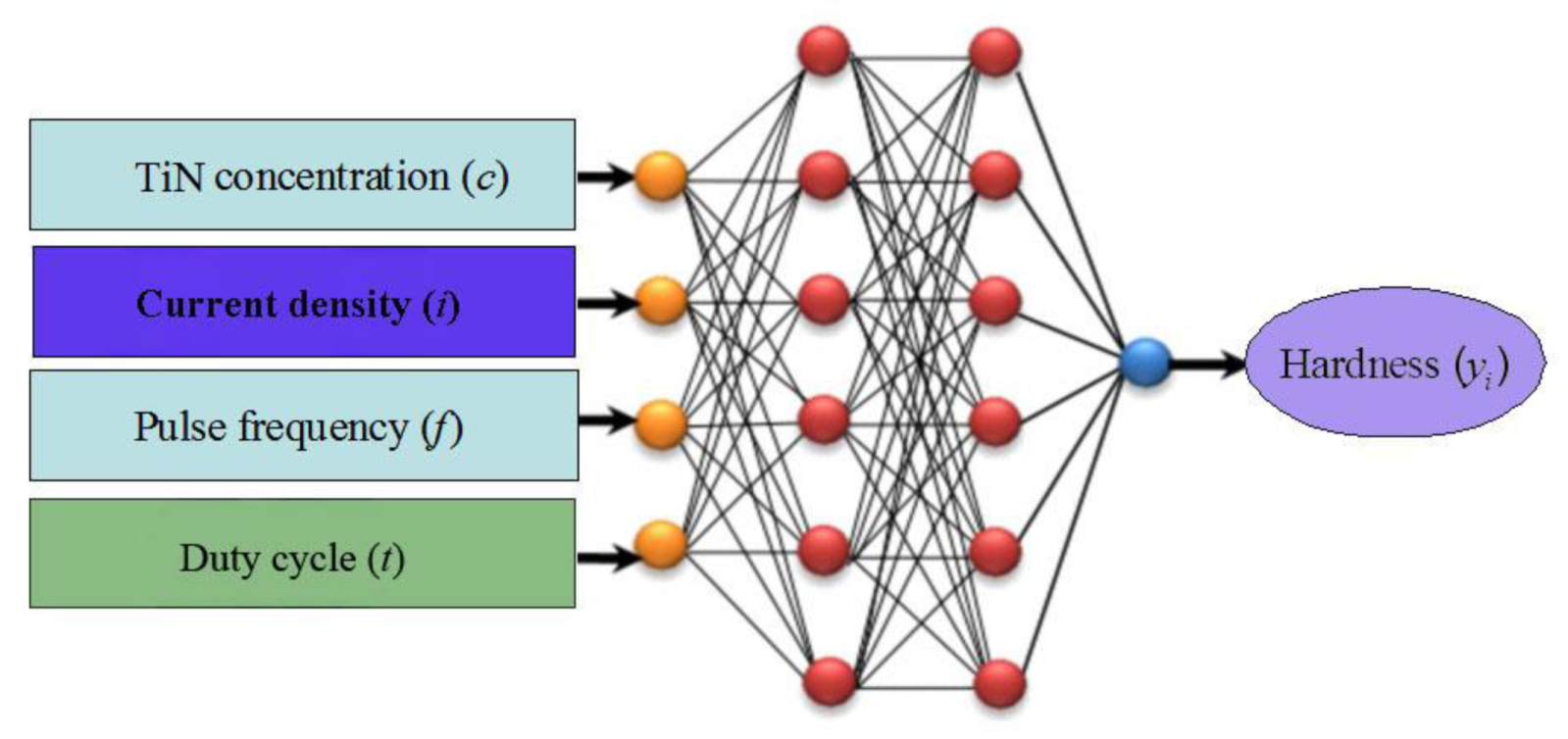
2.3. BP Model and Structure
3. Results and Discussion
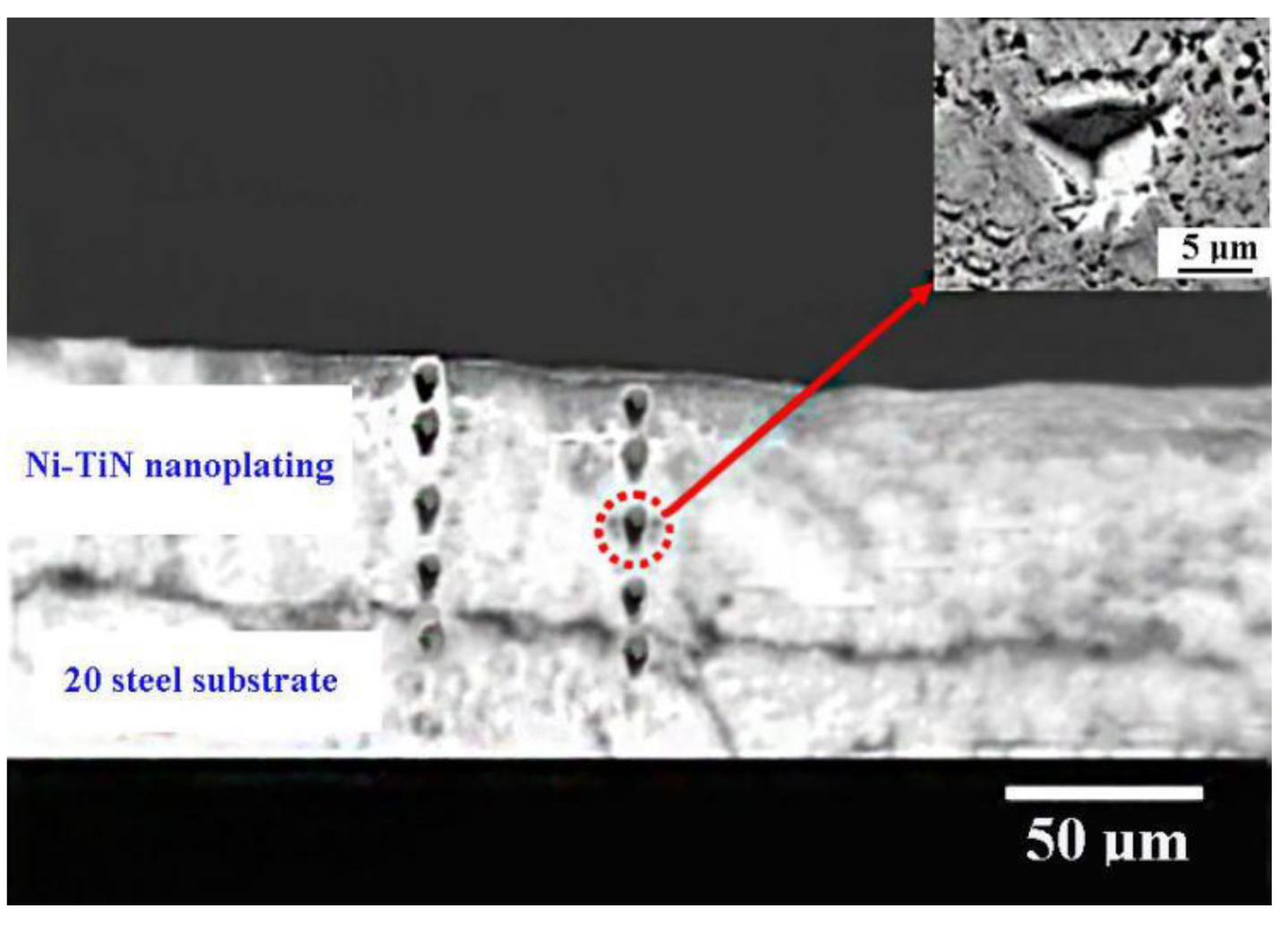
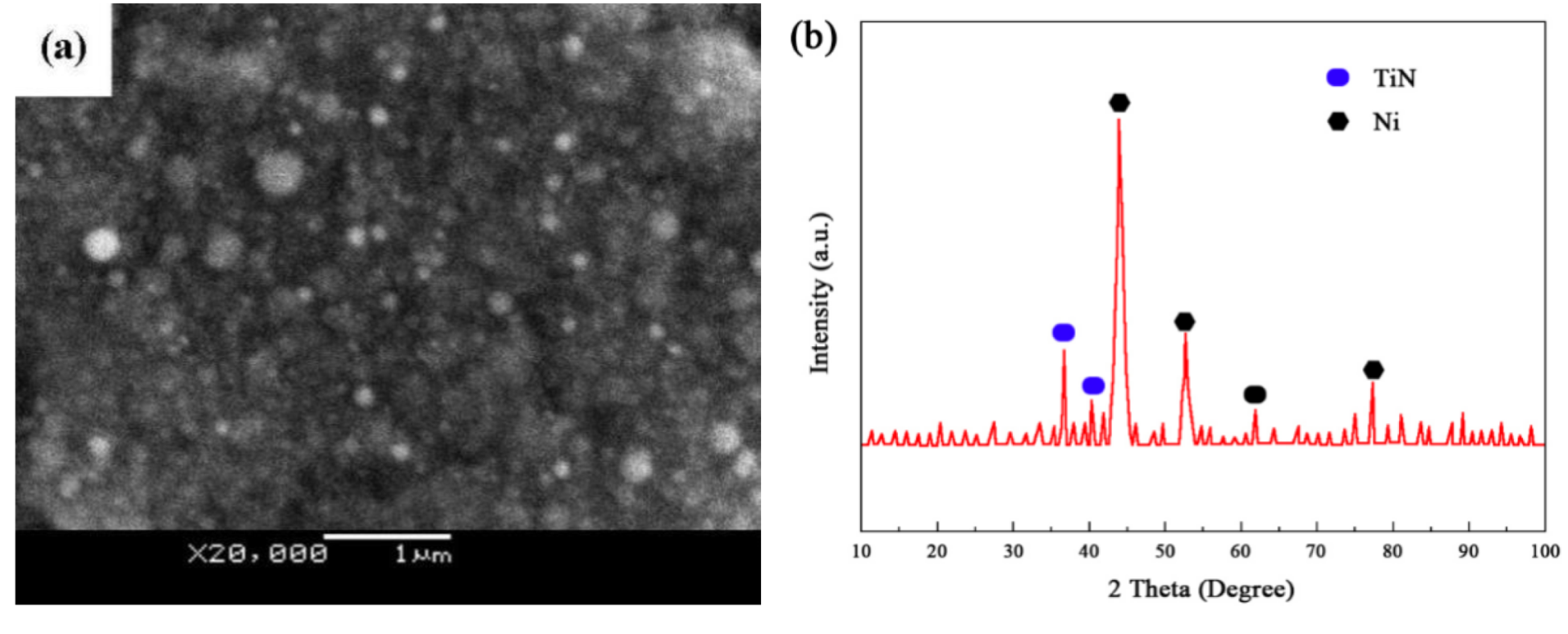
3.1. Ni-TiN Nanoplatings Microstructure Analysis
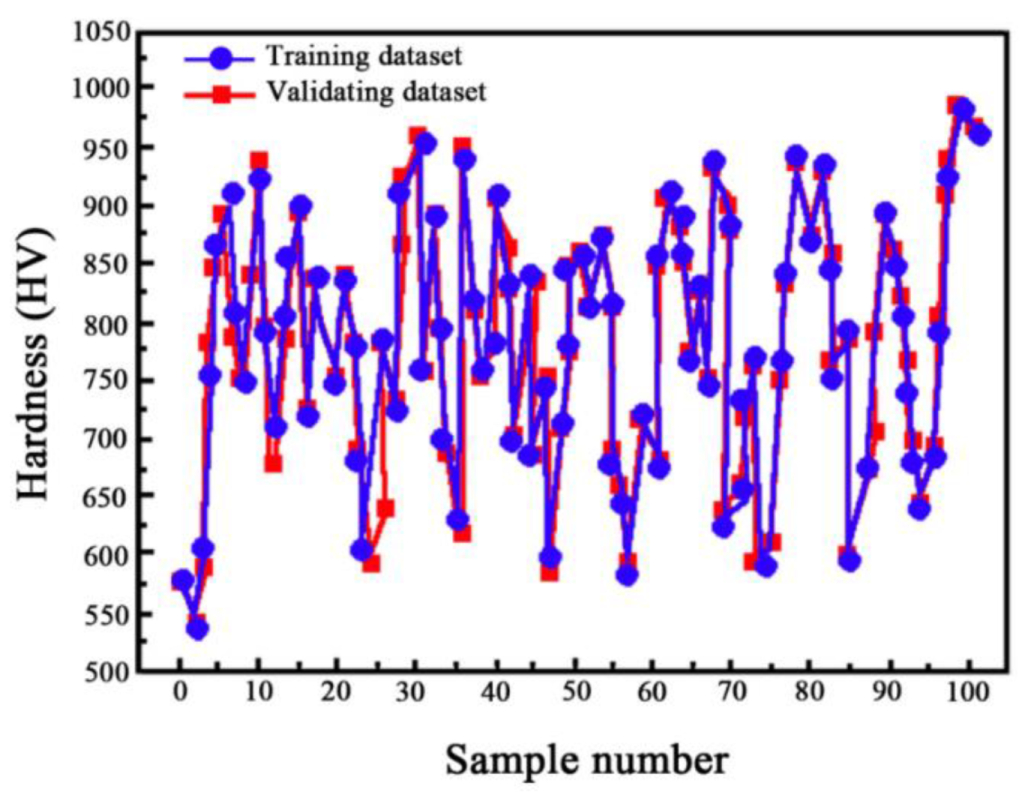
3.2. Validation and Training of the BP Model
3.3. BP Model Results
4. Conclusions
- According to the analysis results of microhardness of Ni-TiN nanoplatings, the optimum process parameters for preparing Ni-TiN nanoplatings by pulse electrodeposition were examined as follows: 8 g/L concentration of TiN particles, 5 A/dm2 current density, 80 Hz pulse frequency, and 0.7 duty cycle.
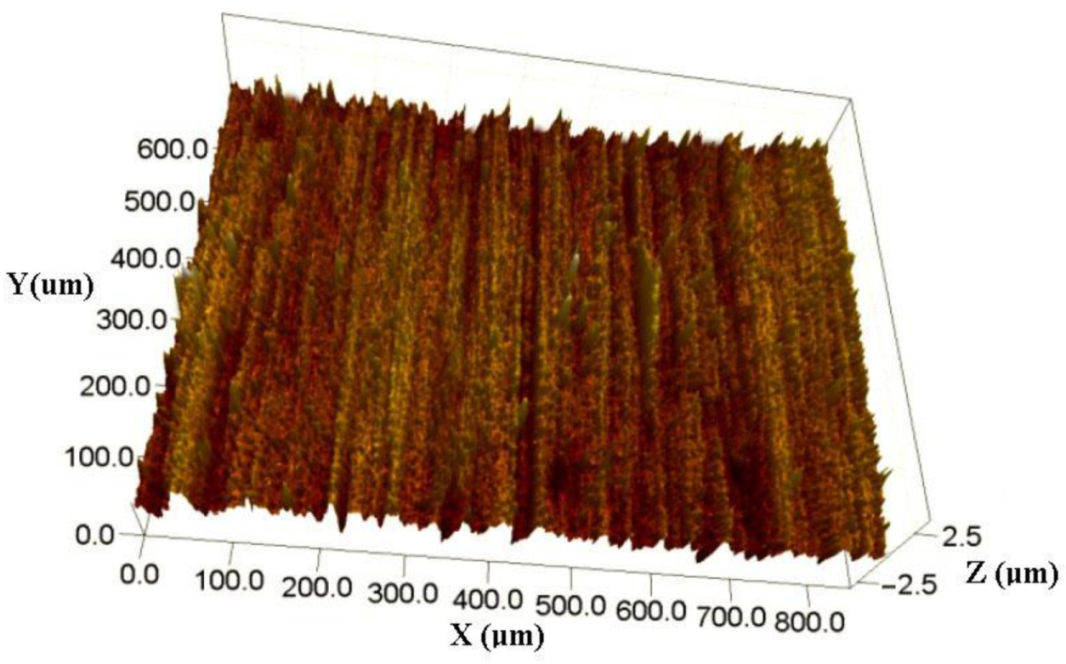
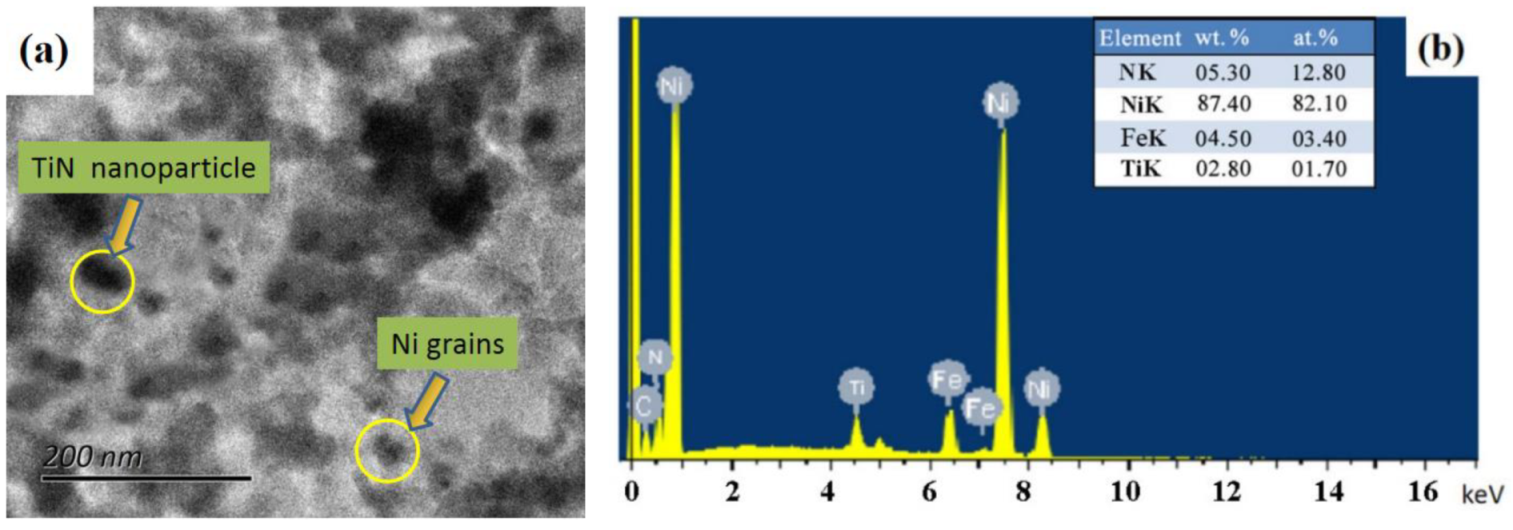
- The results by white light interferometry showed that the Ra value of the nanoplatings was about 0.122 µm. Furthermore, the mean sizes of Ni and TiN grains were determined to be 61.8 and 31.3 nm, respectively, using XRD and HRTEM.
- The BP model could be used as an applicable method to effectively predict the microhardness of Ni-TiN nanoplatings, with a maximum error was about 1.04%. In comparison to experimental data, the BP model successfully predicted the microhardness of Ni-TiN nanoplatings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, F.; Li, C.; Ma, C.; Li, Q.; Xing, H. Effect of pulse current density on the microstructure and wear property of Ni-TiN nanocoatings deposited via pulse electrodeposition. Appl. Surf. Sci. 2021, 538, 148139. [Google Scholar] [CrossRef]
- Zimmerman, A.F.; Clark, D.G.; Aust, K.T.; Erb, U. Pulse electrodeposition of Ni–SiC nanocomposite. Mater. Lett. 2002, 52, 85–90. [Google Scholar] [CrossRef]
- Dehgahi, S.; Amini, R.; Alizadeh, M. Corrosion, passivation and wear behaviors of electrodeposited Ni-Al2O3-SiC nano-composite coatings. Surf. Coat. Technol. 2016, 304, 502–511. [Google Scholar] [CrossRef]
- Wu, M.; Jia, W.; Lv, P. Electrodepositing Ni-TiN nanocomposite layers with applying action of ultrasonic waves. Procedia Eng. 2017, 174, 717–723. [Google Scholar] [CrossRef]
- Ma, C.Y.; Liang, G.Q.; Zhu, Y.Y.; Mu, H.W.; Xia, F.F. Preparation and corrosion assessment of electrodeposited Ni-SiC composite thin films. Ceram. Int. 2014, 40, 3341–3346. [Google Scholar] [CrossRef]
- Borkar, T.; Harimkar, S. Microstructure and wear behaviour of pulse electrodeposited Ni-CNT composite coatings. Surf. Eng. 2011, 27, 524–530. [Google Scholar] [CrossRef]
- Sen, R.; Das, S.; Das, K. Effect of stirring rate on the microstructure and microhardness of Ni-CeO2 nanocomposite coating and investigation of the corrosion property. Surf. Coat. Technol. 2011, 205, 3847–3855. [Google Scholar] [CrossRef]
- Zhu, X.B.; Cai, C.; Zheng, G.Q.; Zhang, Z.; Li, J.F. Electrodeposition and corrosion behavior of nanostructured Ni-TiN composite films. Trans. Nonferrous Met. Soc. China 2011, 21, 2216–2224. [Google Scholar] [CrossRef]
- Xia, F.F.; Liu, C.; Ma, C.H.; Chu, D.Q.; Miao, L. Preparation and corrosion behavior of electrodeposited Ni-TiN composite coatings. Int. J. Refract. Met. Hard Mater. 2012, 35, 295–299. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, F.; Wang, J.; Xu, F. Influence of duty cycle and pulse frequency on structures and performances of electrodeposited Ni-W/TiN nanocomposites on oil-gas X52 steels. Coatings 2021, 11, 1182. [Google Scholar] [CrossRef]
- Hattab, N.; Motelica-heino, M. Application of an inverse neural network model for the identification of optimal amendment to reduce copper toxicity in phytoremediated contaminated soils. J. Geochem. Explor. 2014, 136, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Fang, J.C.; Zhao, Z.Y.; Zeng, H.P. Application of backward propagation network for forecasting hardness and porosity of coatings by plasma spraying. Surf. Coat. Tech. 2007, 201, 5085–5089. [Google Scholar] [CrossRef]
- Shafyei, A.; Mousavi Anijdan, S.H.; Bahrami, A. Prediction of porosity percent in Al–Si casting alloys using ANN. Mat. Sci. Eng. A. 2006, 431, 206–210. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.; Han, Z. Multi-parameter online optimization algorithm of BP neural network algorithm in Internet of Things service. Neural. Comput. Appl. 2021, 33, 505–515. [Google Scholar] [CrossRef]
- Ma, X.; Guan, Y.; Mao, R.; Zheng, S.; Wei, Q. Modeling of lead removal by living Scenedesmus obliquus using backpropagation (BP) neural network algorithm. Environ. Technol. Innov. 2021, 22, 101410. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, H.; Zhang, X. Synergy evaluation model of container multimodal transport based on BP neural network. Neural Comput. Appl. 2021, 33, 1–9. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Qiang, W.; Zhao, Y.; Tang, C. A network traffic forecasting method based on SA optimized ARIMA-BP neural network. Comput. Netw. 2021, 193, 108102. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Li, Q.; Chen, S.; Ma, C. Microstructure and performance of magnetic feld assisted, pulse-electrodeposited Ni-TiN thin coatings with various TiN grain sizes. Ceram. Int. 2021, 47, 18532–18539. [Google Scholar] [CrossRef]
- Jiang, S.; Gao, S.; Kong, J.; Jin, X. Study on the synthesis of β-SiC nanoparticles from diamond-wire silicon cutting waste. RSC Adv. 2019, 9, 23785–23790. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.T.; Wang, Y.; Sun, D.B.; Yu, H.Y. Influence of pulse parameters on the microstructure and microhardness of nickel electrodeposits. Surf. Coat. Tech. 2008, 202, 1895–1903. [Google Scholar]
- Vaezi, M.R.; Sadrnezhaad, S.K.; Nikzad, L. Electrodeposition of Ni-SiC nano–composite coatings and evaluation of wear and corrosion resistance and electroplating characteristics. Colloids Surf. Asp. 2008, 315, 176–182. [Google Scholar] [CrossRef]
- Lajevardi, S.A.; Shahrabi, T. Effects of pulse electrodeposition parameters on the properties of Ni–TiO2 nanocomposite coatings. Appl. Surf. Sci. 2010, 256, 6775–6781. [Google Scholar] [CrossRef]


| Plating Parameters | Content |
|---|---|
| TiN particle concentration | 8 g/L |
| Pulse current density | 5 A/dm2 |
| Pulse current frequency | 80 Hz |
| Duty cycle | 0.6 |
| Electroplating time | 90 min |
| Plating Parameters | Values |
|---|---|
| TiN particle concentration | 4, 5, 6, 7, 8, 9, 10 g/L |
| Pulse current density | 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0 A/dm−2 |
| Pulse current frequency | 50, 60, 70, 80, 90, 100 Hz |
| Duty cycle | 0.4, 0.5, 0.6, 0.7, 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Han, X.; Kang, L.; Wang, B.; Xiang, H. Forecast the Microhardness of Ni-TiN Nanoplatings via an Artificial Neural Network Model. Coatings 2022, 12, 145. https://doi.org/10.3390/coatings12020145
Liu Y, Han X, Kang L, Wang B, Xiang H. Forecast the Microhardness of Ni-TiN Nanoplatings via an Artificial Neural Network Model. Coatings. 2022; 12(2):145. https://doi.org/10.3390/coatings12020145
Chicago/Turabian StyleLiu, Yan, Xingguo Han, Li Kang, Binwu Wang, and Hongxia Xiang. 2022. "Forecast the Microhardness of Ni-TiN Nanoplatings via an Artificial Neural Network Model" Coatings 12, no. 2: 145. https://doi.org/10.3390/coatings12020145
APA StyleLiu, Y., Han, X., Kang, L., Wang, B., & Xiang, H. (2022). Forecast the Microhardness of Ni-TiN Nanoplatings via an Artificial Neural Network Model. Coatings, 12(2), 145. https://doi.org/10.3390/coatings12020145





