1. Introduction
There has been a tremendous increase in wastewater production in the last few decades because of the growing pace of industrialization. It is one of the world’s most severe issues today, owing to various industries [
1]. The release of harmful chemicals has caused severe health and environmental issues. Industrial wastewater contains a wide range of toxins, including dyes and pollutants of both organic and inorganic origins. Heavy metals, hydrocarbons, microbes, endocrine disruptors, and nutrients (nitrogen and phosphorus) are the primary contaminants in wastewater. These contaminants and organic matter make it suitable for the growth of pathogenic organisms, such as bacteria, viruses, and fungi. These organisms are responsible for various waterborne diseases [
2]. Moreover, most of the dyes in this wastewater are carcinogenic and toxic [
3]. Water pollution also negatively impacts aquatic ecosystems, as the amount of sunlight penetrating the water is considerably reduced. Consequently, gas solubility and photosynthetic activities are decreased [
4].
Water toxicity is due to various sources, including households and agricultural wastes, but the most significant source is water from industries [
5]. Among major industries, the textile industry is responsible for the maximum discharge of dyes into the environment. Moreover, cosmetic production, the printing industry, and plastic manufacturing all significantly use dye as a vital ingredient in their manufacturing [
6]. The textile industry is one of the oldest industries, and with the increasing demand for textile products, the discharge of harmful effluents from these industries has been increasing daily. This tremendous increase in wastewater pollutants may cause health and environmental problems. Among these pollutants, dyes have gained significant importance and are known for their adverse effects worldwide. The environmental issues related to these textile industries are primarily associated with toxic chemicals used during processing and untreated effluents discharged into water bodies.
Dyes are very stable, as they can last in the air for a long time if they are not adequately treated [
7]. When discharged directly into water bodies, such as canals and rivers, these effluents pollute the aquatic environment and adversely affect flora and fauna [
8]. When exposed now to these dyes, organisms can show chronic or acute effects, depending upon dye concentration and exposure time. The most complicated side effect of polluted water is the depletion of dissolved oxygen, which is highly important for the aquatic ecosystem. This also limits the self-purification potential of water. In addition to these issues caused during the dyeing process, industries also produce many effluents. These pollutants are a complex mixture of toxins, ranging from original colors (lost during dyeing) to heavy metals and pesticides. As environmental pollutants are not properly treated, they can seriously contaminate the environment and water sources [
9].
Adsorption has been recognized as one of the most effective options for the decontamination of water from heavy metals, dyes, and organic compounds. It is preferred over other techniques because of its insensitivity to contaminants and water reuse terms [
10]. In recent years, much research has been carried out to observe the removal efficiency of different nanoadsorbents. Different types of nanoadsorbents have been applied [
11]. Nanostructured adsorbents have significantly higher efficiencies and faster adsorption rates than conventional materials because of their large surface area. For potential applications in the detoxification of drinking water, industrial effluents, surface and ground water, low-cost, environmentally friendly, and efficient nanomaterials with distinct functionalities have been suggested. The most efficient and ideal adsorbent for wastewater treatment should have high selectivity, mainly for contaminants found in low concentrations in water. Moreover, these adsorbents should be environmentally benign and demonstrate a high adsorption capacity. The pollutants that are adsorbed on its surface should be easily removed and recyclable [
12].
The treatment of wastewater using indigenous rocks and different waste materials, such as marble, limestone, shell, granite, mineral zeolite, refuse concrete, refuse cement, and charcoal, has been studied [
13]. According to a study, marble powder is an attractive option for dye removal from wastewater. Marble powder shows higher adsorption efficiency due to micro- and macrospores, resulting in longer contact time between dye and adsorbent [
14]. Marble dust, a low-cost sorbent, is commonly available worldwide [
15]. The chemical name of marble is calcium carbonate. Adsorption is the fundamental mechanism for the processing of dyeing water. Several disperse dyes, such as Foron black (FBL), Foron red (FR), Foron turquoise (FT), Foron violet (FV), and Foron brown (FB), have been used in the different studies, but there is limited literature on the decolorization of Foron dyes. Foron class of dyes usually require 1–2 h for complete color removal [
16].
Marble is preferred over other adsorbents in wastewater treatment due to its suitability and cost-effectiveness. Several studies have shown that marble is a promising adsorbent for dye removal. The attractive properties of marble include its cost-effectiveness and environmental friendliness. Compared to activated carbon, the dye removal rate with marble is faster, which is one of the critical requirements for the practical application of this treatment approach [
15]. Marble is a metamorphic rock created from limestone that has been subjected to extreme heat and pressure deep inside the Earth’s crust. Limestone is a sedimentary rock composed of sea corals and animal skeletons from the sea. Calcite is a mineral that is required for the formation of limestone. These calcite crystals melt and recrystallize into larger, coarser crystals that interlock and form the carbonate rock known as marble. Contamination-free limestone yields shining, clean marble [
17].
The present study has focused on removing used Foron blue, Foron black, and Foron red dyes from water using marble waste material. The effects of numerous parameters, for example, initial dye concentration, pH, time, temperature, and adsorbent dose, were optimized on dye uptake capacity. Furthermore, the obtained data were discussed in detail using isothermal and kinetic models.
4. Conclusions
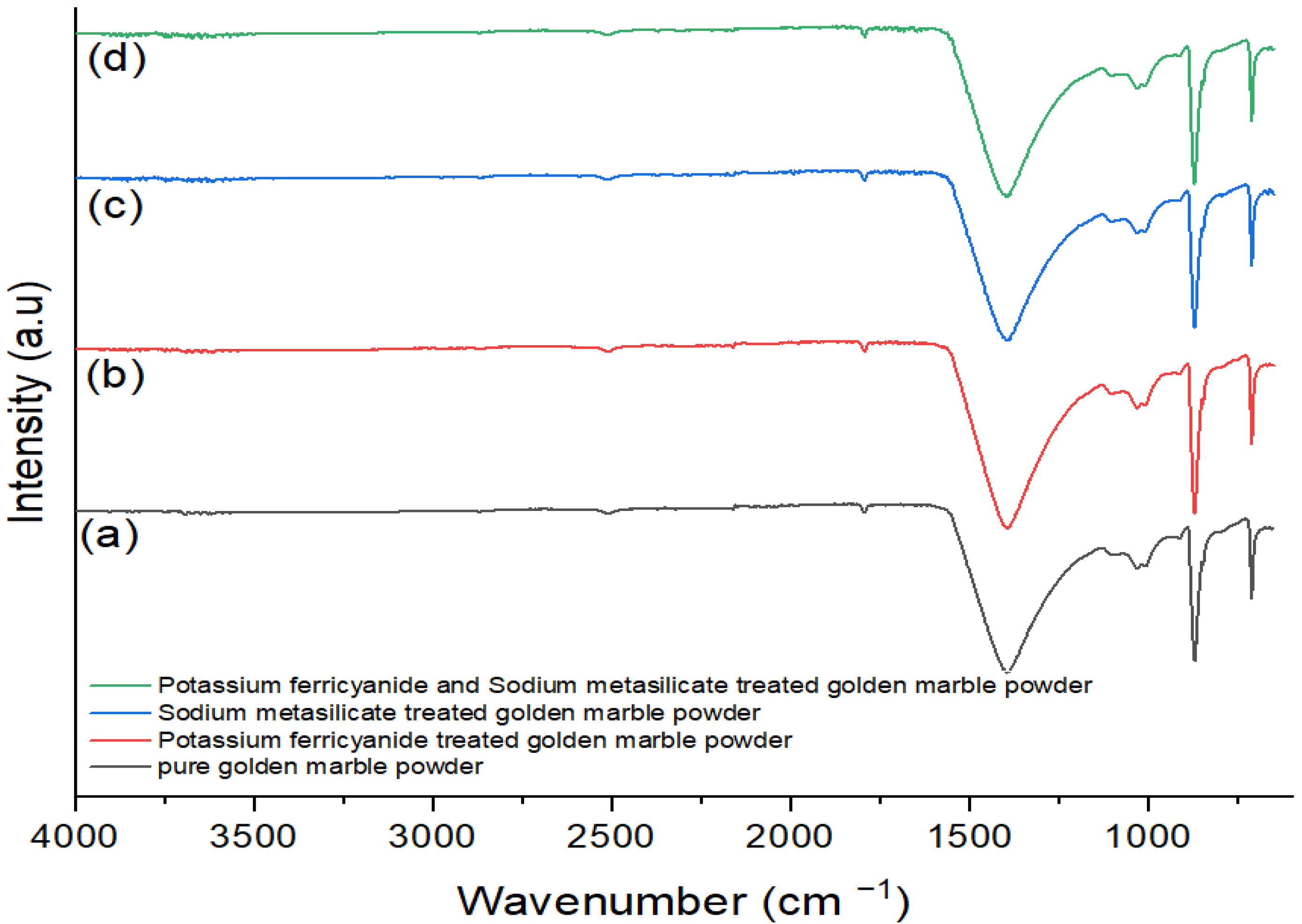
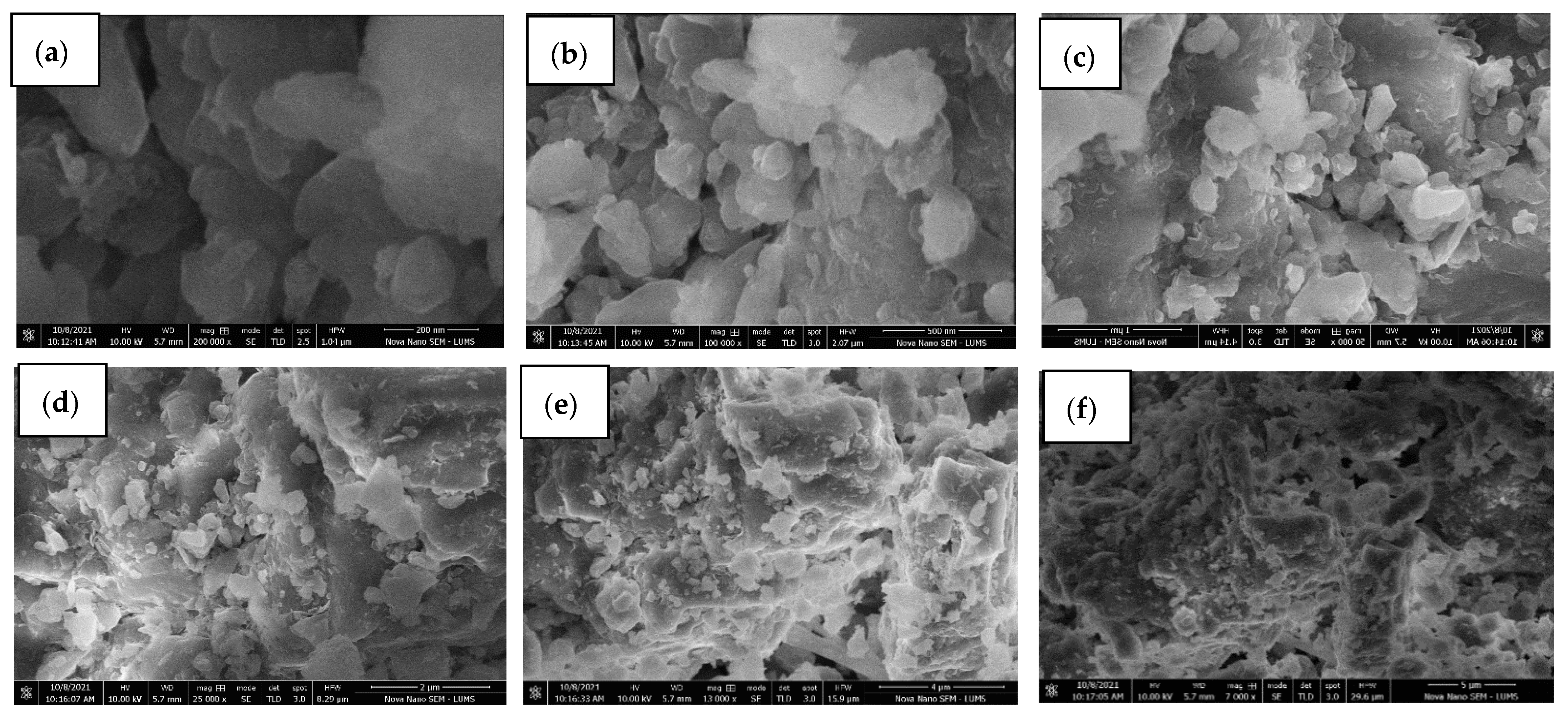
Treated golden marble waste composites have shown significant advantages over pure or untreated golden marble because of their high adsorption ability. Furthermore, nontoxic, and environmentally compatible marble composites appeared to be an excellent alternative for removing various pollutants from industrial wastewater. The adsorption method offers unique advantages owing to the use of natural adsorbents, such as marble waste composites for dye removal, due to their easy availability, low cost, eco-friendly nature, ease of operation, simplicity of design, flexibility, and great affinity for dyes. Golden marble waste nanocomposites are prepared for dye removal. Adsorption, a cheap and effective method, showed excellent results for all dyes discussed in the study without producing any waste. Removal efficiency has been significantly increased using the adsorption technique. Different factors greatly influence the adsorption capacity, such as absorbance, pH, time, temperature, concentration, and dose of the adsorbent. Spectrophotometric analysis suggested that treated golden marble composite material had better removal efficiency than pure golden marble waste composites. Optimization of a parameter, such as concentration, contact time, and temperature, was also discussed, and their results were calculated using isothermal and kinetics models. It was observed that golden marble waste composites gave better R2 values for second-order kinetics in the kinetic model. The results obtained from comparing the Langmuir, Freundlich, Temkin, Dubinin, and Harkin Jura isotherms showed that the R2 values of the Langmuir isotherm for Foron red, the Temkin isotherm for Foron blue, and the Freundlich isotherm for Foron black dye fit the adsorption for both treated and untreated composites. Characterization techniques, such as scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR), are also discussed for the structural determination of golden marble waste composites.