A Study on the Improvement of the Photothermal Characteristics of the Adsorbent for Sorption-Based Atmospheric Water Harvesting Driven by Solar
Abstract
1. Introduction
2. Experimental Section
2.1. Selection of Composite Adsorbent Materials and Mixing Ratio
2.2. Composite Adsorbent Preparation Method
2.3. Characterization
2.4. Water Sorption
2.5. Water Release
3. Results and Discussion
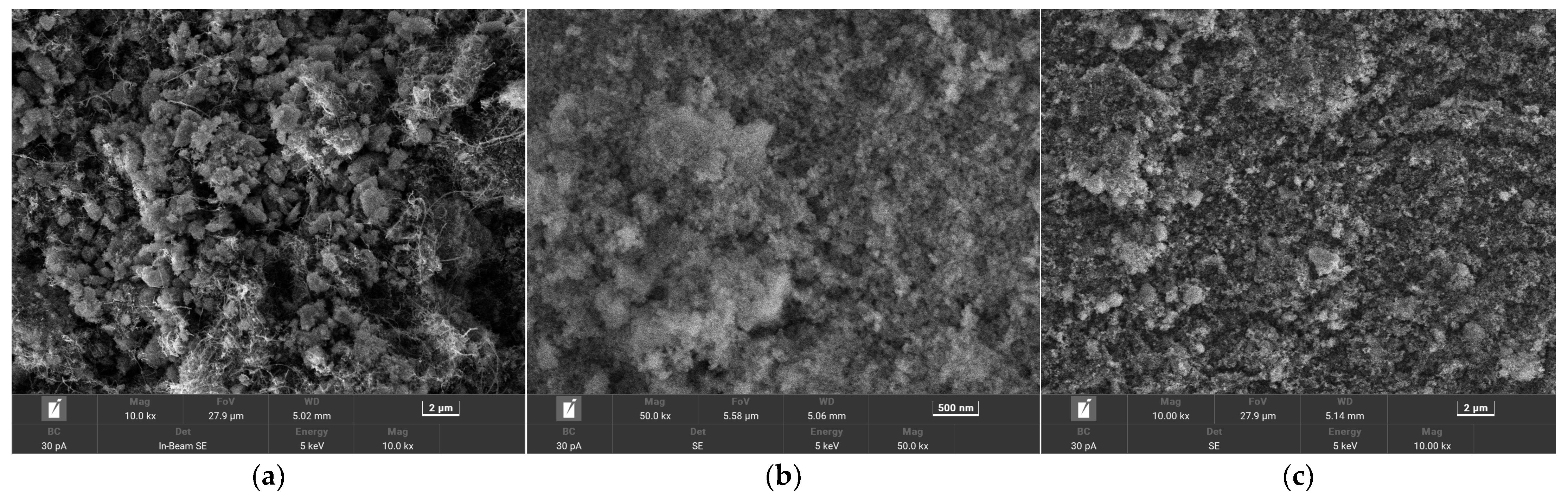
3.1. Structure and Morphology Characterization
3.2. Effects of Different Carbon Materials on the Adsorption Properties of Composite Adsorbents
3.3. Effects of Humidity on the Adsorption Properties of Composite Adsorbents
3.4. Effects of Temperature on the Adsorption Properties of Composite Adsorbents
3.5. Light Absorption Properties of Composite Adsorbent Desorption
3.6. Photothermal Conversion Properties of Composite Adsorbent Desorption
4. Conclusions
- (1)
- The new composite adsorbent MOF-801/CNT prepared in this paper has better adsorption and desorption properties than the existing MOF-801/G adsorbent. In terms of adsorption properties, the saturated adsorption capacity of MOF-801/CNT was 30% higher than that of MOF-801/G at the same temperature and humidity, and the humidity absorption rate was increased. In terms of desorption characteristics, under the same light intensity, MOF-801/CNT has the best light absorption performance and photothermal conversion performance, the desorption speed is 50% higher than MOF-801/G, and the water release is improved.
- (2)
- The structure and morphology of MOF-801/CNT adsorbent were clarified. The BET specific surface area was 354.77 m2/g, and the average pore size of the adsorption was up to 4.26 nm, which has excellent qualities as an adsorbent.
- (3)
- The effects of temperature and relative humidity on the adsorption properties of MOF-801/CNT were clarified, that is, the low temperature and high humidity environment are beneficial to improve the adsorption properties of the adsorbent.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tortajada, C.; Biswas, A.K. Water management in post-2020 world. Int. J. Water Resour. Dev. 2020, 36, 874–878. [Google Scholar] [CrossRef]
- Chen, L.; Li, K.; Chen, S.; Wang, X.; Tang, L. Industrial activity, energy structure, and environmental pollution in China. Energy Econ. 2021, 104, 105633. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, J.; Ge, J. Dynamic game in agriculture and industry cross-sectoral water pollution governance in developing countries. Agric. Water Manag. 2021, 243, 106417. [Google Scholar] [CrossRef]
- Chen, C.; Kuang, Y.; Hu, L. Challenges and Opportunities for Solar Evaporation. Joule 2019, 3, 683–718. [Google Scholar] [CrossRef]
- Lord, J.; Thomas, A.; Treat, N.; Forkin, M.; Bain, R.; Dulac, P.; Behroozi, C.H.; Mamutov, T.; Fongheiser, J.; Kobilansky, N.; et al. Global potential for harvesting drinking water from air using solar energy. Nature 2021, 598, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wang, R.; Zhang, Y.; Wang, J. Progress and Expectation of Atmospheric Water Harvesting. Joule 2018, 2, 1452–1475. [Google Scholar] [CrossRef]
- Jänchen, J.; Ackermann, D.; Stach, H.; Brösicke, W. Studies of the water adsorption on Zeolites and modified mesoporous materials for seasonal storage of solar heat. Sol. Energy 2004, 76, 339–344. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.-B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal–Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.; Roshandel, R.; Vakiloroaya, V. An innovative solar assisted desiccant-based evaporative cooling system for co-production of water and cooling in hot and humid climates. Energy Convers. Manag. 2019, 185, 396–409. [Google Scholar] [CrossRef]
- Sleiti, A.K.; Al-Khawaja, H.; Al-Khawaja, H.; Al-Ali, M. Harvesting water from air using adsorption material—Prototype and experimental results. Sep. Purif. Technol. 2021, 257, 117921. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Yan, T.; Wu, S.; Wu, M.; Chao, J.; Huo, X.; Wang, P.; Wang, R. Ultrahigh solar-driven atmospheric water production enabled by scalable rapid-cycling water harvester with vertically aligned nanocomposite sorbent. Energy Environ. Sci. 2021, 14, 5979–5994. [Google Scholar] [CrossRef]
- Lei, C.; Guo, Y.; Guan, W.; Lu, H.; Shi, W.; Yu, G. Polyzwitterionic Hydrogels for Efficient Atmospheric Water Harvesting. Angew. Chem. Int. Ed. 2022, 61, e202200271. [Google Scholar] [CrossRef] [PubMed]
- Kalmutzki, M.J.; Diercks, C.S.; Yaghi, O.M. Metal–Organic Frameworks for Water Harvesting from Air. Adv. Mater. 2018, 30, 1704304. [Google Scholar] [CrossRef]
- Fathieh, F.; Kalmutzki, M.J.; Kapustin, E.A.; Waller, P.J.; Yang, J.; Yaghi, O.M. Practical water production from desert air. Sci. Adv. 2018, 4, eaat3198. [Google Scholar] [CrossRef] [PubMed]
- Hanikel, N.; Prévot, M.S.; Fathieh, F.; Kapustin, E.A.; Lyu, H.; Wang, H.; Diercks, N.J.; Glover, T.G.; Yaghi, O.M. Rapid Cycling and Exceptional Yield in a Metal-Organic Framework Water Harvester. ACS Cent. Sci. 2019, 5, 1699–1706. [Google Scholar] [CrossRef]
- Hu, Y.; Fang, Z.; Wan, X.; Ma, X.; Wang, S.; Fan, S.; Dong, M.; Ye, Z.; Peng, X. Carbon nanotubes decorated hollow metal–organic frameworks for efficient solar-driven atmospheric water harvesting. Chem. Eng. J. 2022, 430, 133086. [Google Scholar] [CrossRef]


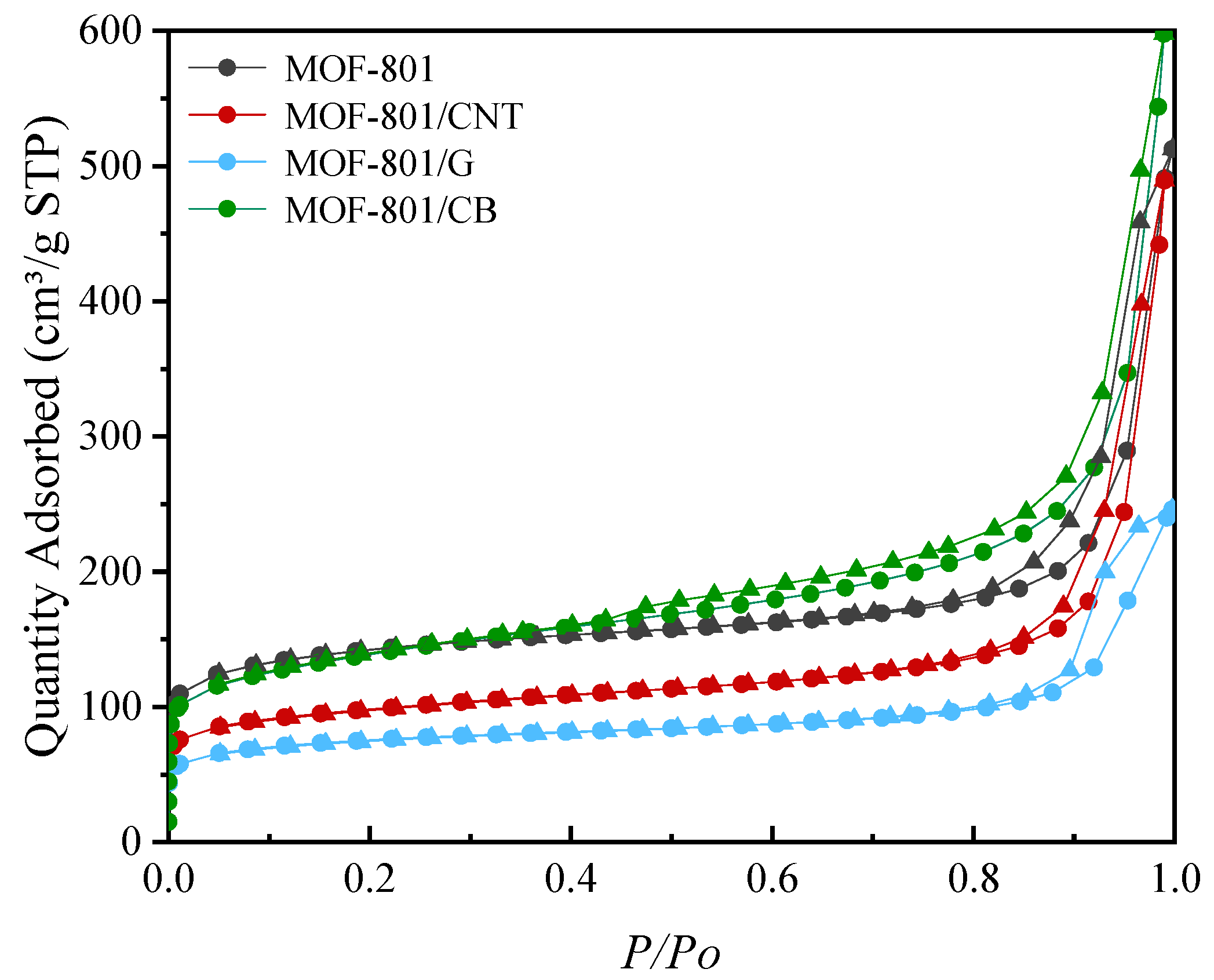





| Adsorbent | BET Surface Area/m2/g | t-Plot Micropore Volume/cm3/g | Average Adsorption Pore Size/nm |
|---|---|---|---|
| MOF-801 | 511.49 | 0.136 | 3.38 |
| MOF-801/CNT | 354.77 | 0.0842 | 4.26 |
| MOF-801/G | 270.68 | 0.0698 | 3.92 |
| MOF-801/CB | 486.45 | 0.0896 | 4.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Sui, Z.; Du, X.; Zhang, Y.; Ma, T. A Study on the Improvement of the Photothermal Characteristics of the Adsorbent for Sorption-Based Atmospheric Water Harvesting Driven by Solar. Coatings 2023, 13, 154. https://doi.org/10.3390/coatings13010154
Wu J, Sui Z, Du X, Zhang Y, Ma T. A Study on the Improvement of the Photothermal Characteristics of the Adsorbent for Sorption-Based Atmospheric Water Harvesting Driven by Solar. Coatings. 2023; 13(1):154. https://doi.org/10.3390/coatings13010154
Chicago/Turabian StyleWu, Jiangbo, Ziyi Sui, Xiaoze Du, Yaocong Zhang, and Tao Ma. 2023. "A Study on the Improvement of the Photothermal Characteristics of the Adsorbent for Sorption-Based Atmospheric Water Harvesting Driven by Solar" Coatings 13, no. 1: 154. https://doi.org/10.3390/coatings13010154
APA StyleWu, J., Sui, Z., Du, X., Zhang, Y., & Ma, T. (2023). A Study on the Improvement of the Photothermal Characteristics of the Adsorbent for Sorption-Based Atmospheric Water Harvesting Driven by Solar. Coatings, 13(1), 154. https://doi.org/10.3390/coatings13010154







