Preparation of a Poly (Ether-b-Amide) Mixed-Matrix Membrane and Its Application in Blast Furnace Gas
Abstract
:1. Introduction
2. Experiment Method
2.1. Material and Chemical Reagent
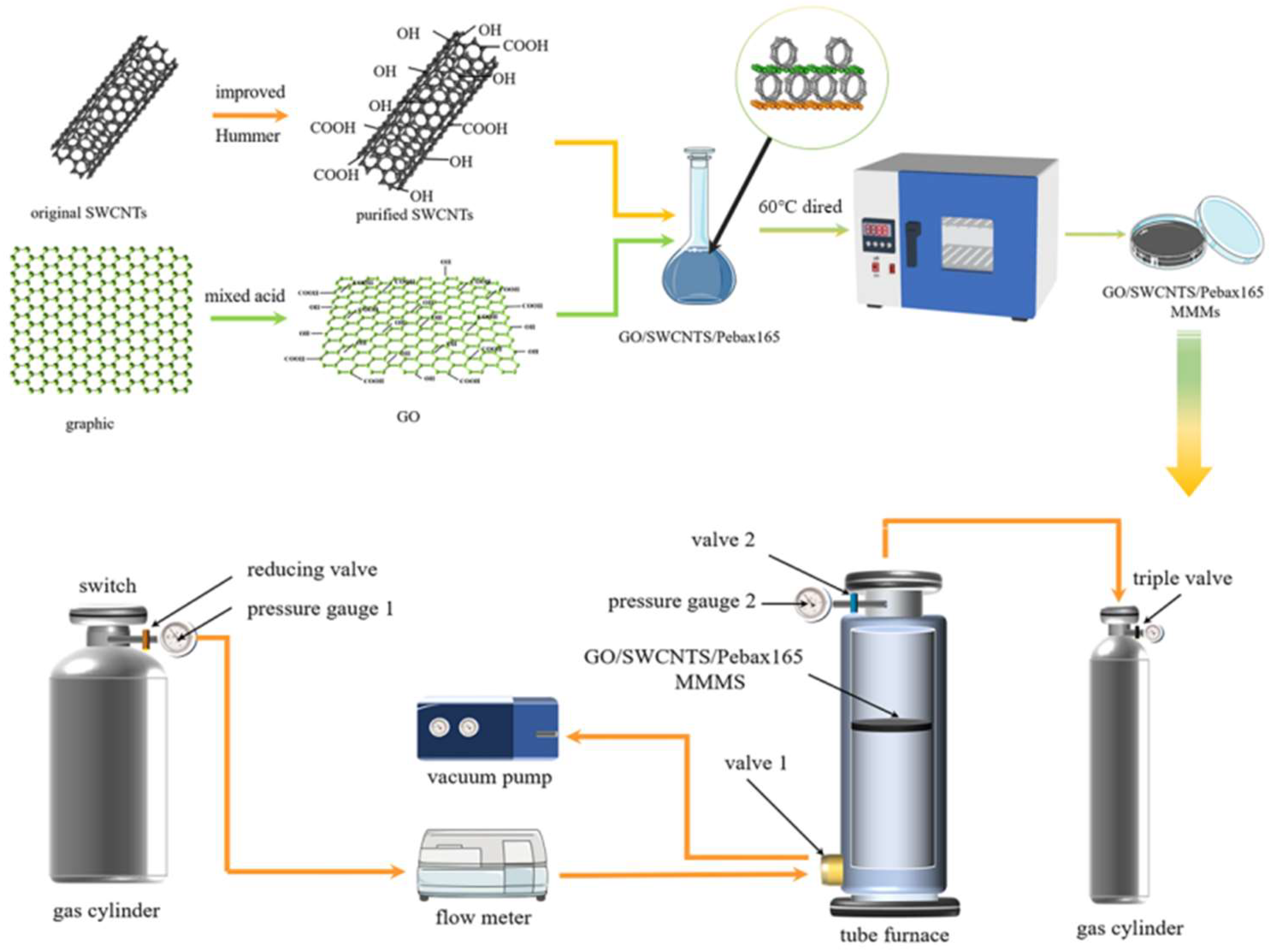
2.2. GO Preparation
2.3. Puried SWCNTs
2.4. Preparation of MMMs
2.5. Characterization Methods
2.6. Gas Permeation Experiment
3. Results and Discussion
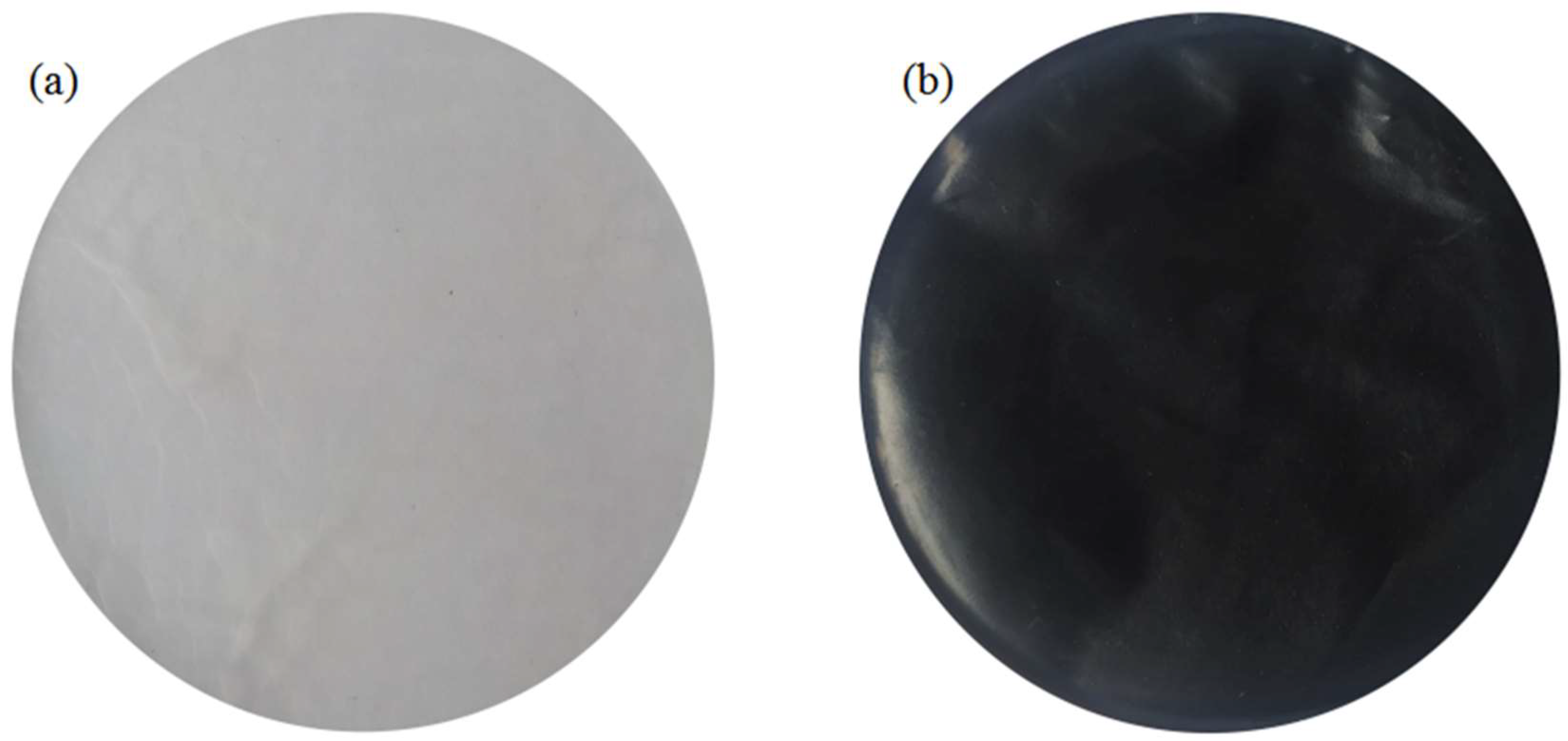
3.1. Membrane Shape
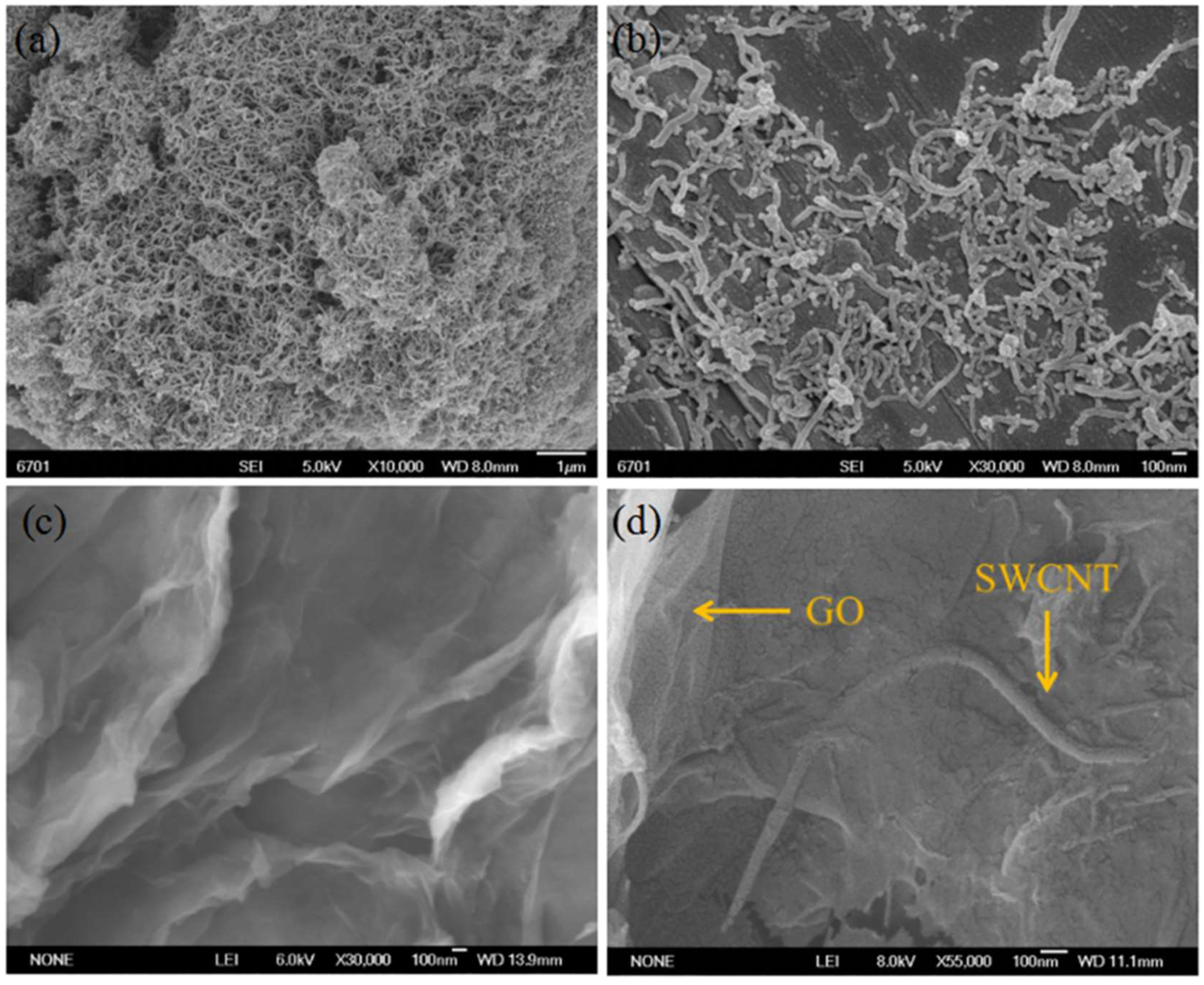
3.2. SEM
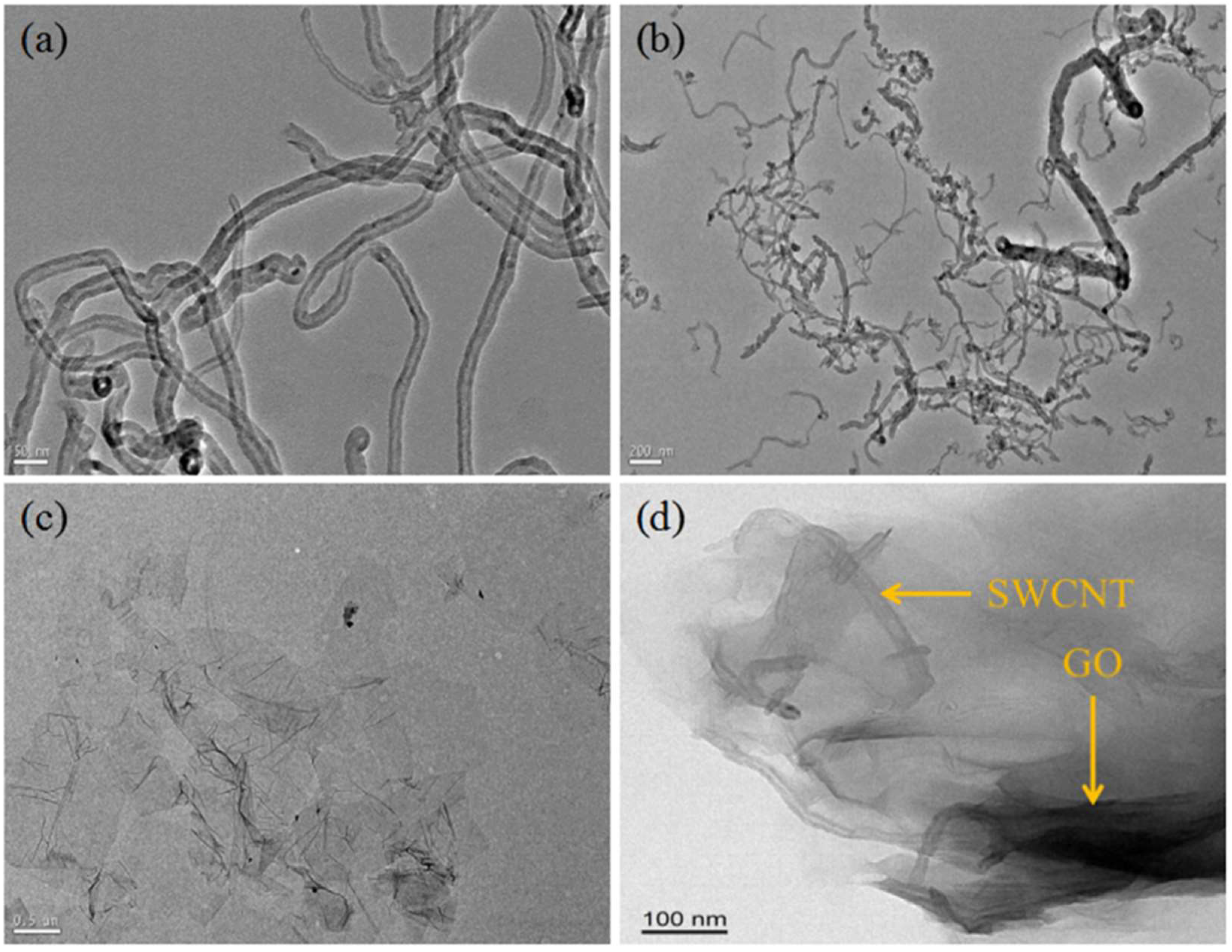
3.3. TEM
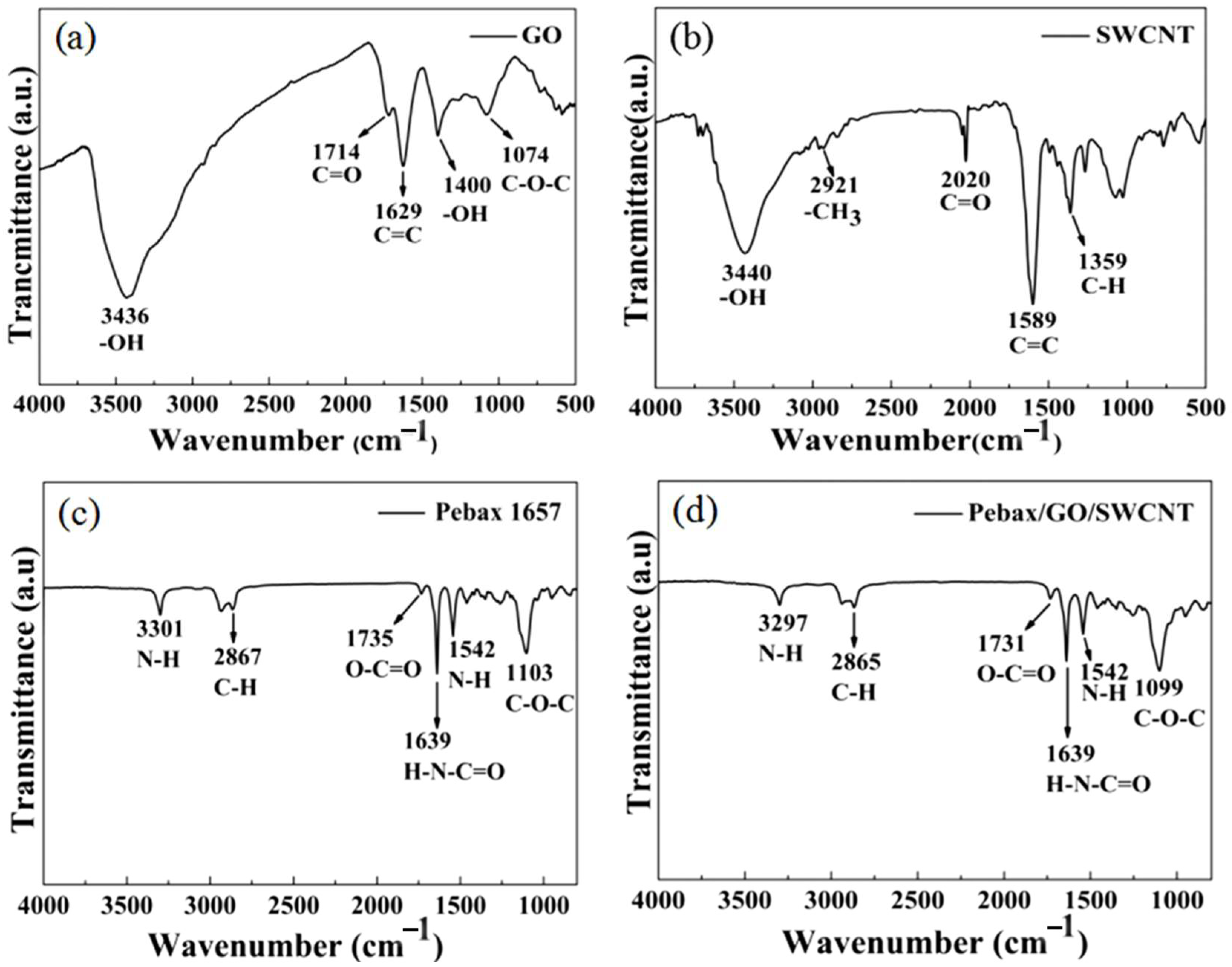
3.4. FTIR
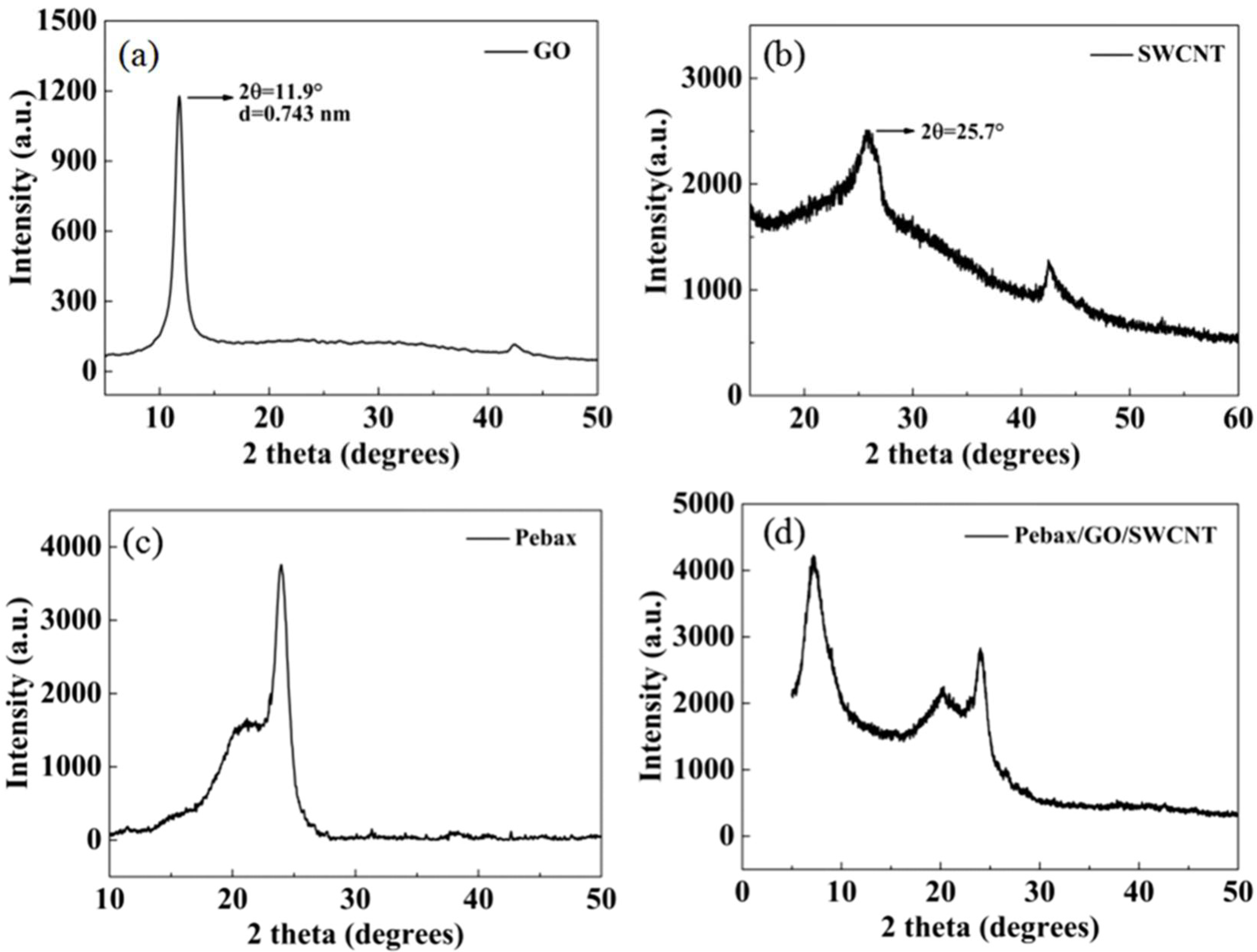
3.5. XRD
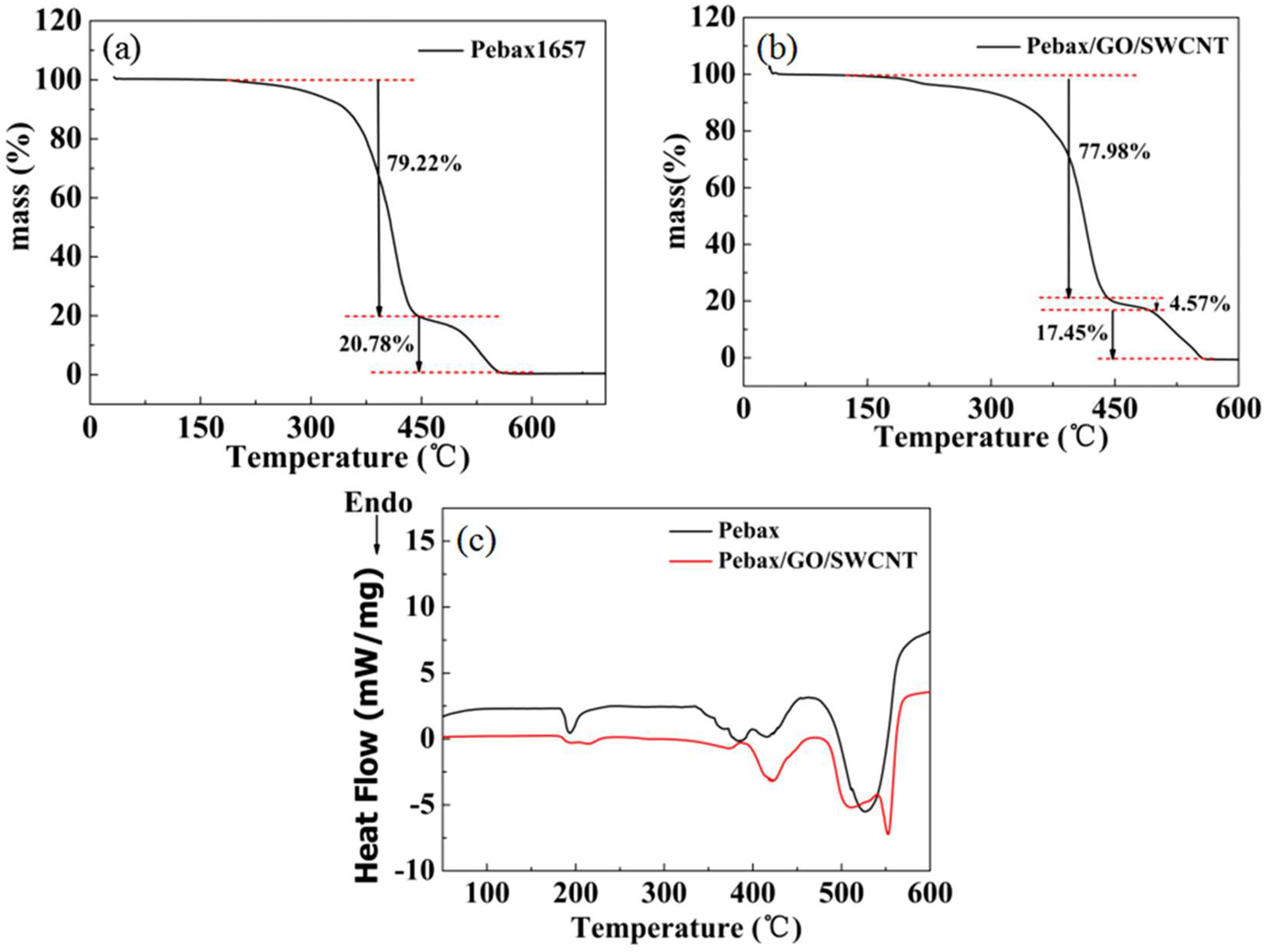
3.6. TGA and DSC
3.7. AFM
3.8. Gas-Separation Performance
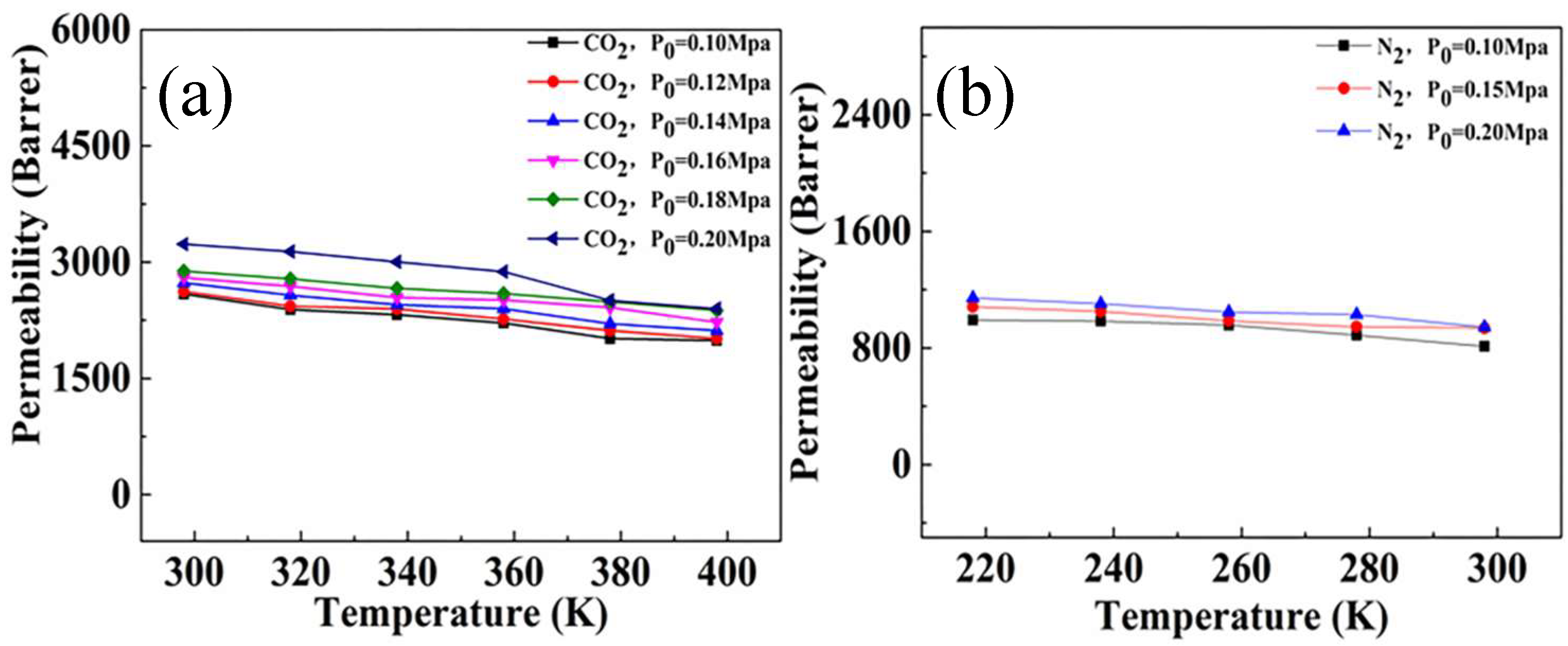
3.8.1. Single Gas Separation
3.8.2. Mixed-Gases Separation
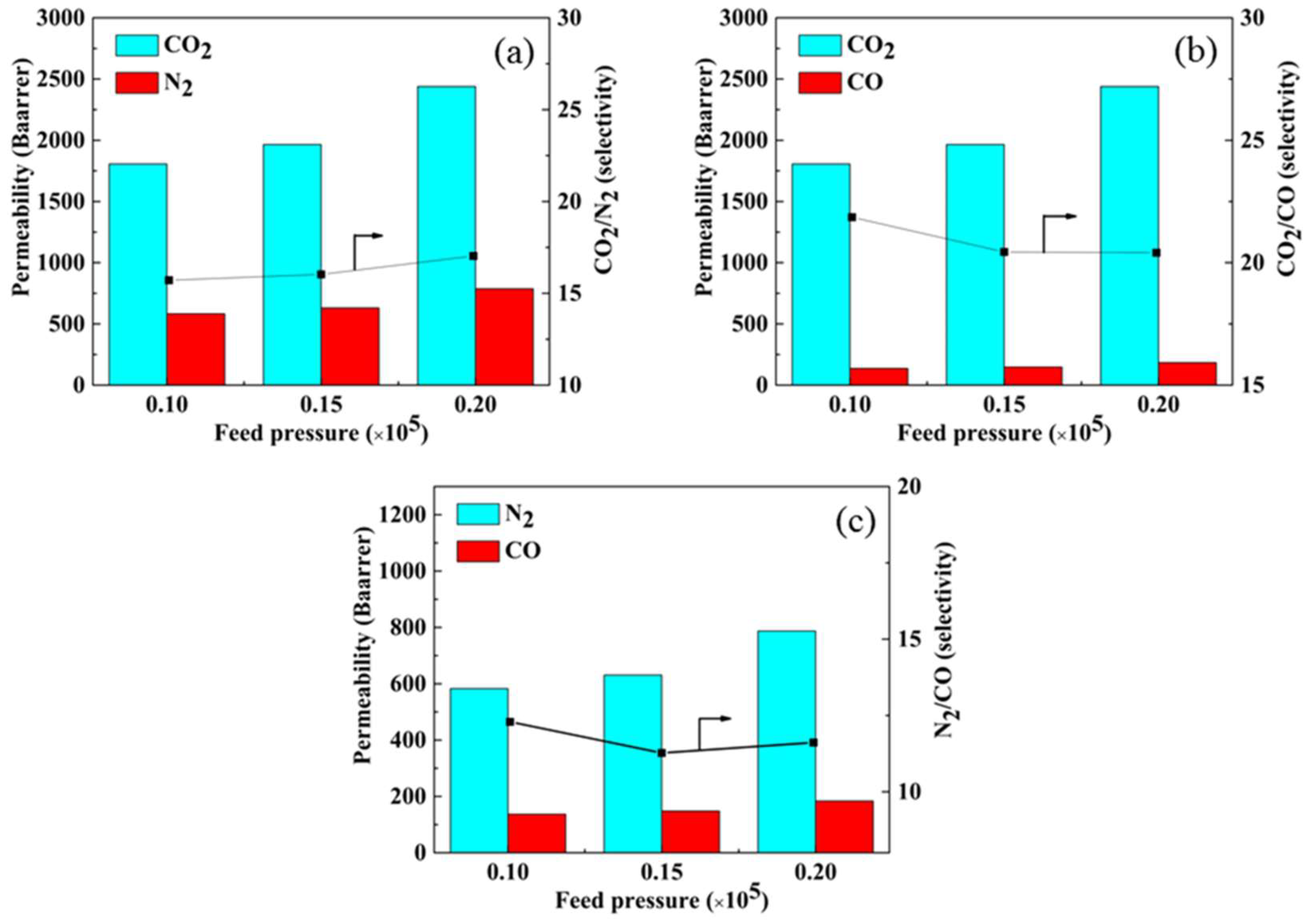
3.8.3. Selective Ability for Mixed Gases
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomes, C.D.N.; Jacquet, O.; Villiers, C.; Thuery, P.; Ephritikhine, M.; Cantat, T. A diagonal approach to chemical recycling of carbon dioxide: Organocatalytic transformation for the reductive functionalization of CO2. Angew. Chem. Int. Ed. 2012, 51, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, J.P.V.D.; Hendriks, C.A.; Blok, K. Feasibily of polymer membranes for carbon dioxide recovery from flue gases. Energy Convers. Manag. 1992, 33, 429–436. [Google Scholar] [CrossRef]
- Freeman, B.D.; Pinnau, I. Polymer Membranes for Gas and Vapor Separation. Chem. Mater. Sci. 1999, 733, 135–150. [Google Scholar]
- Chung, T.S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Samarasinghe, S.A.S.C.; Chuah, C.Y.; Yang, Y.Q.; Bae, T.H. Tailoring CO2/CH4 separation properties of mixed-matrix membranes via combined use of two-and three-dimensional metal-organic frameworks. J. Membr. Sci. 2018, 557, 30–37. [Google Scholar] [CrossRef]
- Bondar, V.; Freeman, B.; Pinnau, I. Gas sorption and charaterization of poly (ether-b-amide) segmented block copolymers. J. Polym. Sci. Part B Polym. Phys. 2015, 37, 2463–2475. [Google Scholar] [CrossRef]
- Tocci, E.; Gugliuzza, A.; De Lorenzo, L.; Macchione, M.; Luca, G.D.; Drioli, E. Transoprt properties of a co-poly(amide-12-b-ethylene oxide) membrane: A comparative study between expermental and molecular modelling result. J. Membr. Sci. 2008, 323, 316–327. [Google Scholar] [CrossRef]
- Reijerkerk, S.R.; Knoef, M.H.; Nijmeijer, K.; Wessling, M. Poly(ethylene glycol) and poly(dimethyl siloxane): Combining their advantages into efficient CO2 gas separation membranes. J. Membr. Sci. 2010, 352, 126–135. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Gas solubility, diffusivity and permeability in poly (ethlene oxide). J. Membr. Sci. 2015, 239, 105–117. [Google Scholar] [CrossRef]
- Blume, I.; Pinnau, I. Composite Membrane, Method of Preparation and Use. U.S. Patent 4963165, 16 October 1990. [Google Scholar]
- Azizi, N.; Mohammadi, T.; Behbahani, R.M. Comparison of permeability performance of PEBAX-1074/TiO2, PEBAX-1074/SiO2 and PEBAX-1074/Al2O3 nanocomposite membranes for CO2/CH4 separation. Chem. Eng. Res. Des. 2017, 117, 177–189. [Google Scholar] [CrossRef]
- Chen, K.; Xu, K.; Xiang, L.; Dong, X.; Han, Y.; Wang, C.Q.; Sun, L.B.; Pan, Y.C. Enhance CO2/CH4 separation performance of mixed-matrix membranes through dispersion of sorption-selective MOF nanocrystals. J. Membr. Sci. 2018, 536, 360–370. [Google Scholar] [CrossRef]
- Dorosti, F.; Alizadehdakhel, A. Fabrication and investigation of PEBAX/FE-BTC, a high permeable and CO2 selective mixed matrix membrane. Chem. Eng. Res. Des. 2018, 136, 119–128. [Google Scholar] [CrossRef]
- Isanejad, M.; Mohammadi, T. Effect of a mine modification on morphology and performance of poly (ether-block-amide)/fumed silica nanocomposite membranes for CO2/CH4 separation. Mater. Chem. Phys. 2018, 205, 303–314. [Google Scholar] [CrossRef]
- Estahbanati, E.G.; Omidkhah, M.; Amooghin, A.E. Preparation and characterization of novel Ionic liquide/Pebax membranes for efficient CO2/light gases separation. J. Ind. Eng. Chem. 2017, 51, 77–89. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, C.H.; Jung, J.P.; Kim, J.H.; Kim, J.H. Dual-phases all-polymeric membrane with graft copolymer filler for CO2 capture. Chem. Eng. J. 2017, 334, 939–947. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Weng, T.H.; Tseng, H.H.; Wey, M.Y. Preparation and characterization of multiwalled carbon nanotube/PBNPI nanocomposite membrane for H2/CH4 separation. Int. J. Hydrog. Energy 2009, 34, 8707–8715. [Google Scholar] [CrossRef]
- Chen, H.; Sholl, D.S. Prediction of selectivity and flux for CH4/H2 separations using single walled carbon nanotubes as membranes. J. Membr. Sci. 2006, 269, 152–160. [Google Scholar] [CrossRef]
- Sholl, D.S.; Johnson, J.K. Making high-flux membranes with carbon nanotubes. Science 2006, 312, 1003–1004. [Google Scholar] [CrossRef]
- Kim, S.; Jinschek, J.R.; Chen, H.; Sholl, D.S.; Marand, E. Scalable fabrication of carbon nanotube/polymer nanocomposite membranes for high flflux gas transport. Nano Lett. 2007, 7, 2806–2811. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawsk, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2009, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Bolling, L.; Priolo, M.A.; Grunlan, J.C. Super gas barrier and selectivity of graphene oxide-polymer multilayer thin fifilms. Adv. Mater. 2013, 25, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Miura, Y.; Macosko, C.W. Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity. Chem. Mater. 2010, 22, 3441–3450. [Google Scholar] [CrossRef]
- Zulhairun, A.K.; Ismail, A.F. The role of layered silicate loadings and their dispersion states on the gas separation performance of matrix membrane. J. Membr. Sci. 2014, 468, 20–30. [Google Scholar] [CrossRef]
- Chua, M.L.; Xiao, Y.C.; Chung, T.S. Modlfying the molecular structure and gas separation performance of themally labile polyimide-based membranes for enhanced natural gas purifification. Chem. Eng. Sci. 2013, 104, 1056–1064. [Google Scholar] [CrossRef]
- Jiang, L.L.; Li, C.T.; Yu, H.T.; Zou, Z.S.; Hou, X.G.; Shen, F.M.; Li, C.T.; Yao, X.Y. Amino and thiol modified magnetic multi-walled carbon nanotubes for the simultaneous removal of lead, zinc, and phenol from aqeous solutions. Appl. Surf. Sci. 2016, 369, 398–416. [Google Scholar] [CrossRef]
- Chua, M.L.; Xiao, Y.C.; Chung, T.S. Effects of thermally labile saccharide units on the gas separation performance of highly permeable polyimide membranes. J. Membr. Sci. 2012, 415, 375–382. [Google Scholar] [CrossRef]
- Zhu, J.C.; Meng, X.X.; Zhao, J.P.; Jin, Y.Y.; Zhang, N.T.; Sunarso, S.G.; Liu, J.K.; Liu, S. Facile hydrogen/nitrogen separation through graphene oxide membranes supported on YSZ ceramic hollw fibers. J. Membr. Sci. 2017, 535, 143–150. [Google Scholar] [CrossRef]
- Chua, M.L.; Shao, L.; Low, B.T.; Xiao, Y.; Chung, T.S. Polytheramine-polyhedral oligomeroc silsequioxane organin-inorganic hybrid membranes foe CO2/H2 and CO2/N2 separation. J. Membr. Sci. 2011, 385, 40–48. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, L.; Liu, N.; Liu, M.; Wang, Y.; Liu, Z. Synthesis of nitrogen-doped graphene using embedded carbon and nitrogen sources. Adv. Mater. 2011, 23, 1020–1024. [Google Scholar] [CrossRef]
- Dong, G.; Hou, J.; Wang, J.; Zhang, Y.T.; Liu, J.D. Enhanced CO2/N2 separation by porous reduced graphene oxide/Pebax mixed matrix membranes. J. Membr. Sci. 2016, 520, 860–868. [Google Scholar] [CrossRef]
- Jomekian, A.; Bazooyar, B.; Behbahani, R.M.; Mohammadi, T.; Kargari, A. Ionic liquid-modified Pebax 1657 membrane filled by ZIF-8 particles for separation of CO2 from CH4, N2 and H2. J. Membr. Sci. 2017, 524, 652–662. [Google Scholar] [CrossRef]
- Estahbanati, E.G.; Omidkhah, M.; Amooghin, A.E. Interfacial Design of Ternary Mixed Matrix Membranes Containing Pebax 1657/Silver-Nanopowder/[BMIM][BF4] for Improved CO2 Separation Performance. ACS Appl. Mater. Interfaces 2017, 9, 10094–10105. [Google Scholar] [CrossRef]
- Rostamian, R.; Behnejad, H. A comparative adsorption study of sulfamethoxale onto graphene and graphene oxide nanosheets through equilibrium, kinetic and thermodynamic modeling. Process Saf. Environ. Prot. 2016, 102, 20–29. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Chen, Z.; Waje, M.; Yan, Y.S. Carbon nanotube film by filtration as cathode catalyst support for proton-exchange membrane fuel cell. Langmuir 2005, 21, 9386–9389. [Google Scholar] [CrossRef]
- Li, F.Y.; Li, Y.; Chung, T.S.; Kawi, S. Facilitated transport by hybrid POSS–Matrimid–ZN2+ nanocomposite membranes for the separation of natural gas—ScienceDirect. J. Membr. Sci. 2010, 356, 14–21. [Google Scholar] [CrossRef]
- Zornoza, B.; Téllez, C.; Coronas, J. Mixed matrix membranes comprising glassy polymers and dispersed mesoporous silica spheres for gas separation. Fuel Energy Abstr. 2011, 368, 100–109. [Google Scholar] [CrossRef]
- Dan, Z.; Jiazhong, R. High CO2 separation performance of Pebax/CNTs/GTA mix matrix membranes. J. Membr. Sci. 2016, 61, 114–711. [Google Scholar]
- Bondar, V.I.; Freeman, B.D.; Pinnau, I. Gas transport properties of poly(ether-b-amide) segmented block copolymers. J. Polym. Sci. Part B Polym. Phys. 2015, 38, 2051–2062. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. High pressure gas separation performance of mixed-matrix polymer membranes containing mesoporous Fe(BTC). J. Membr. Sci. 2014, 459, 33–44. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Materials selection guidelines for membranes that remove CO2 from gas mixtures. J. Mol. Struct. 2005, 739, 57–74. [Google Scholar] [CrossRef]


| Membranes | Roughness | |
|---|---|---|
| Ra (nm) | Rq (nm) | |
| Pebax1657 | 24.33 | 30.83 |
| Pebax1657/SWCNT/GO | 7.77 | 10.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Chen, Y.; Hou, X. Preparation of a Poly (Ether-b-Amide) Mixed-Matrix Membrane and Its Application in Blast Furnace Gas. Coatings 2022, 12, 1851. https://doi.org/10.3390/coatings12121851
Jiang L, Chen Y, Hou X. Preparation of a Poly (Ether-b-Amide) Mixed-Matrix Membrane and Its Application in Blast Furnace Gas. Coatings. 2022; 12(12):1851. https://doi.org/10.3390/coatings12121851
Chicago/Turabian StyleJiang, Lili, Yixing Chen, and Xingang Hou. 2022. "Preparation of a Poly (Ether-b-Amide) Mixed-Matrix Membrane and Its Application in Blast Furnace Gas" Coatings 12, no. 12: 1851. https://doi.org/10.3390/coatings12121851
APA StyleJiang, L., Chen, Y., & Hou, X. (2022). Preparation of a Poly (Ether-b-Amide) Mixed-Matrix Membrane and Its Application in Blast Furnace Gas. Coatings, 12(12), 1851. https://doi.org/10.3390/coatings12121851





