Recent Progress in Functional Edible Food Packaging Based on Gelatin and Chitosan
Abstract
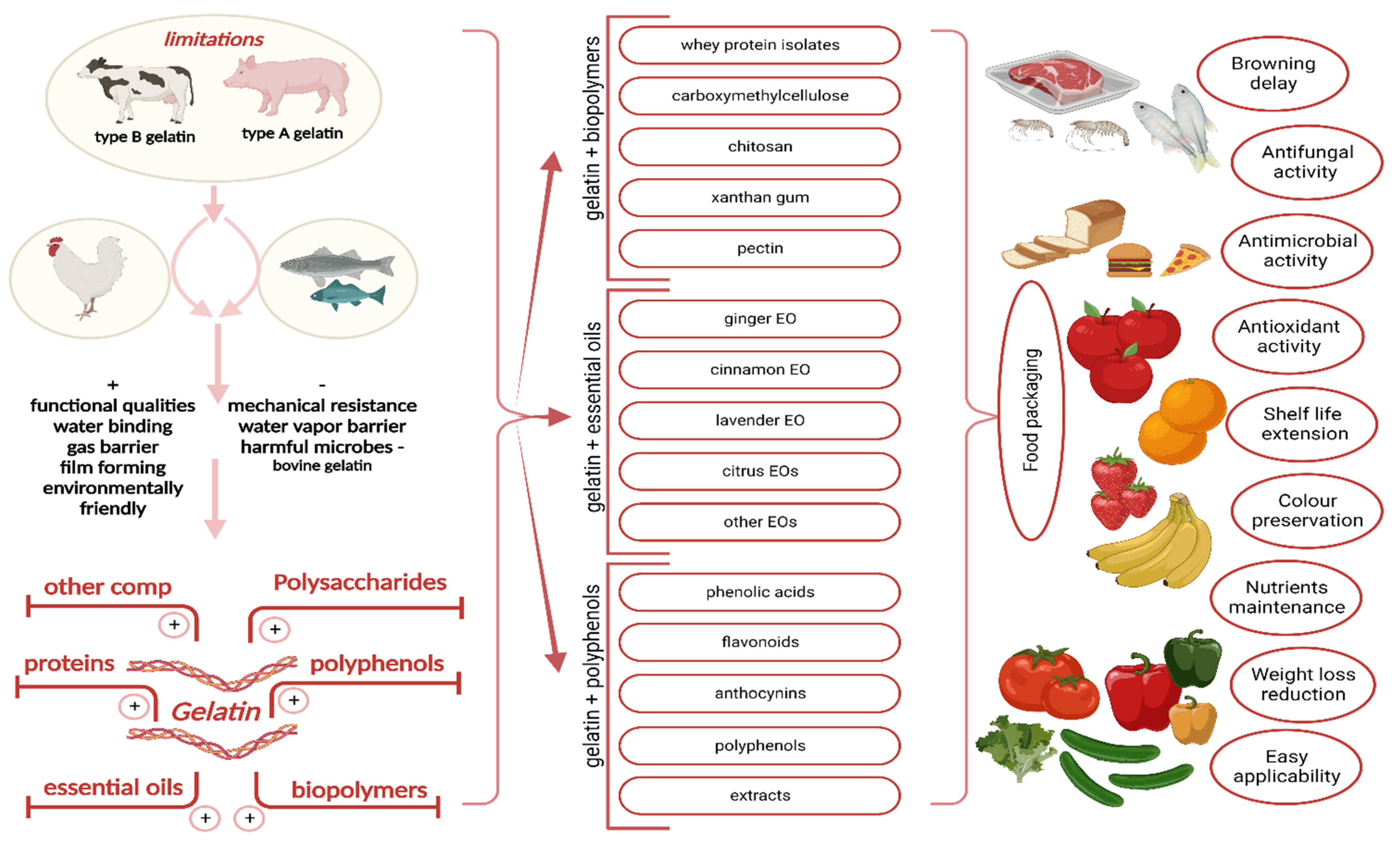
1. Introduction
2. Gelatin
2.1. Origination of Gelatin
2.1.1. Gelatin Obtained from Mammals
2.1.2. Gelatin Obtained from Poultry
2.1.3. Gelatin Obtained from Aquatic Species
2.2. Gelatin-Based Composites
2.2.1. Combined Gelatin and Other Biopolymers
| Formulation | Physical/Chemical/Mechanical/Biological Characteristics | References |
|---|---|---|
| Gelatin, whey protein isolate | synergistic interaction ↑ gelling properties, EM | [57] |
| Gelatin, soy protein isolate | ↑ mechanical properties when the weight ratio of soy protein isolate: gelatin is 1:3 | [64] |
| Gelatin, soy protein isolate | ↑ TS, EAB, EM, flexibility | [27] |
| Gelatin, chitosan | ↑ mechanical properties ↓ permeability good UV-light protection qualities | [25] |
| Gelatin, CMC | ↑ TS, puncture test of film, thermal stability, WVP, ↓ EAB, opacity, and UV-light penetration of the films | [60] |
| Gelatin, CMC, chitosan | ↓ WVP ↑ biodegradability | [62] |
| Gelatin, CMC, chitosan | ↑ flexibility, EAB, WVP, thickness ↓ TS and puncture force | [63] |
| Gelatin, chitosan, xanthan gum | ↑ thickness, WVP, UV-light protection, thermal stability, ↓ TS, EAB, VIS light transparency | [28] |
| Gelatin, starch | ↑ mechanical strength, water solubility (WS), WVP, thickness ↓ opacityimproved appearance of refrigerated Red Crimson grapes | [26] |
| Gelatin, potato starch | ↑ TS, EM, WVP, melting temperature, UV–VIS light protection ↓ WS, EAB | [61] |
| Gelatin, tapioca starch | ↑ TS, EAB, thickness, WVP, UV-light protection, thermal stability visible light transmission, film transparency | [29] |
| Gelatin, pectin | ↑ thickness, TS, antioxidant, and antibacterial activities ↓ WVP, EAB | [30] |
2.2.2. Combined Gelatin and Polyphenols/Extracts Rich in Polyphenols
| Formulation | Physical/Chemical/Mechanical Characteristics | Biological Properties | Applications | References |
|---|---|---|---|---|
| Gelatin, protocatechuic acid | ↑ thickness, EAB achieved fine look, ↓ light transmittance, TS, WVP | Antioxidant activity (DPPH), antimicrobial activity against E. coli and S. aureus, with high protocatechuic acid amounts. | Beef preservation | [31] |
| Gelatin, epigallocatechin gallate (EGCG) | ↑ bloom strength | Antioxidant activity (DPPH (50%–99%), FRAP (200–662 μg Vc/g)), antimicrobial activity against E. coli and S. aureus | Active packaging | [32] |
| Gelatin, Galla chinensis extract | ↑ gel strength and thermal stability, ↓ swelling of gelatin | Not determined | Packaging | [33] |
| Gelatin, eugenol/β-cyclodextrin emulsion | not determined | Reduced the H2S-producing bacteria, total viable Pseudomonas spp. and Psychrophilic counts, total volatile basic nitrogen, K value, free fatty acids | Chinese Seabass during superchilling storage | [34] |
| Gelatin, mango peel | ↓ WVP, solubility films more rigid and less flexible | Antioxidant activity (DPPH 70%–85%) | Active packaging | [35] |
| Gelatin, green tea extract grape seeds extract gingko leaf extract | ↓ TS, EAB, lowest WVP lowest TS, EAB, ↓ WVP ↓ TS, EAB, WVP | All the films presented antioxidant activity (DPPH) | Active food packaging | [70] |
| Gelatin, Fructus chebulae extract | ↑ gel strength, thermal stability | Not determined | Packaging | [76] |
| Gelatin, chlorogenic acid | not determined | Antioxidant activity (ABTS), antimicrobial activity against E. coli, P. aeruginosa, L. monocytogenes, and S. aureus | Fresh seafood preservation | [65] |
| Gelatin, epigallocatechin gallate | ↑ TS, EM, ↓EAB | Antioxidant activity (DPPH 67%) | Reduce the oxidation of cod-liver oil | [66] |
| Gelatin, green tea powder | ↓ TS, EM, EAB with high amounts of green tea powder | Antioxidant activity (DPPH 77%) | Reduce the oxidation of cod-liver oil | [66] |
| Gelatin, green tea extract | ↑ TS↓ EAB, WS, WVP | Antioxidant activity (DPPH 15%–55%) | Active packaging | [69] |
| Gelatin, rosmarinic acid | ↑ thickness, TS, EAB, light protection, ↓ WS, WVP | Antioxidant activity (DPPH 75%–90%) | Bacon preservation | [67] |
| Gelatin, rosmarinic acid | ↑ EAB, ↓ TS, EM, WVP | Antioxidant activity (ABTS), antimicrobial activity against E. coli and S. aureus | Active packaging | [74] |
| Gelatin, tannic acid | ↑ TS ↓ EAB, WVP, oxygen permeability | Antimicrobial activity against E. coli and S. aureus | Cherry tomatoes, grapes | [68] |
| Gelatin, mangrove extracts | ↑ thickness, EAB, TS ↓ WVP | Antioxidant activity (DPPH 15%–60%), antimicrobial activity against S. aureus, E. coli, Bacillus subtillis, Salmonella sp. | Active packaging | [19] |
| Gelatin, pomegranate peel powder | ↑ thickness, WVP, TS ↓ film solubility, EAB | Antioxidant activity (DPPH 59%–72%, ABTS 48%–80%), antimicrobial activity against S. aureus, L. monocytogenes, and E. coli | Active packaging | [71] |
| Gelatin, haskap berries extract | ↑TS, EAB ↓WVP, WS | Antioxidant activity (DPPH) | Shrimp spoilage | [75] |
| Gelatin, date by-products | ↓water holding capacity, WS color change | Antimicrobial activity against E. coli and S. aureus | Active packaging | [72] |
2.2.3. Combined Gelatin and Essential Oil
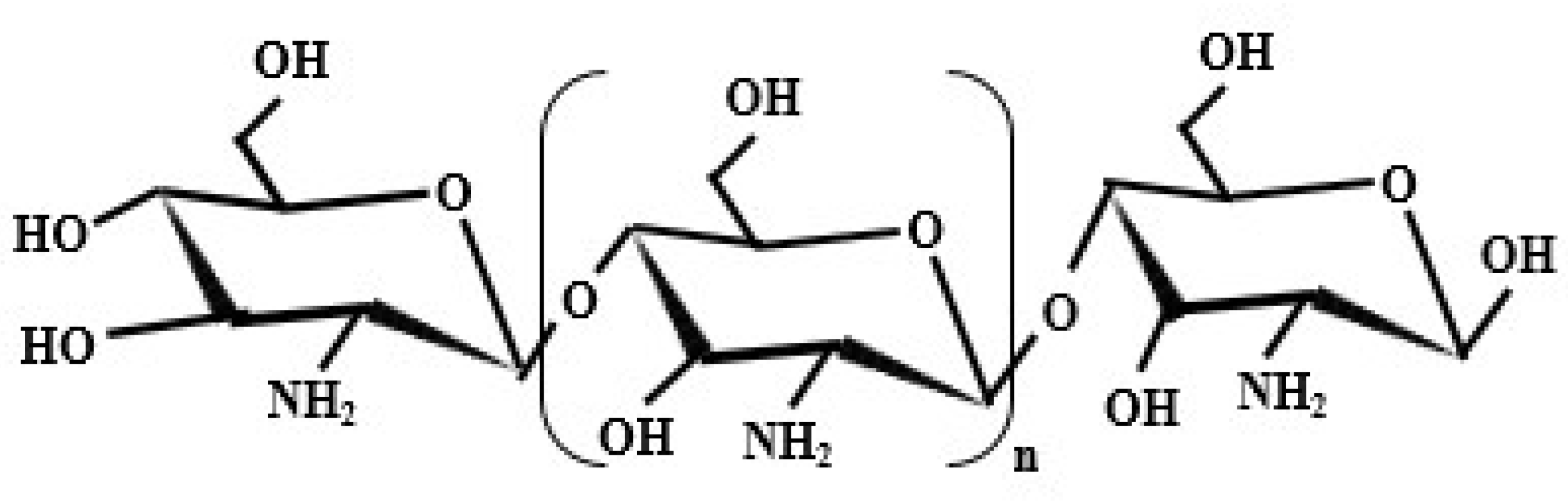
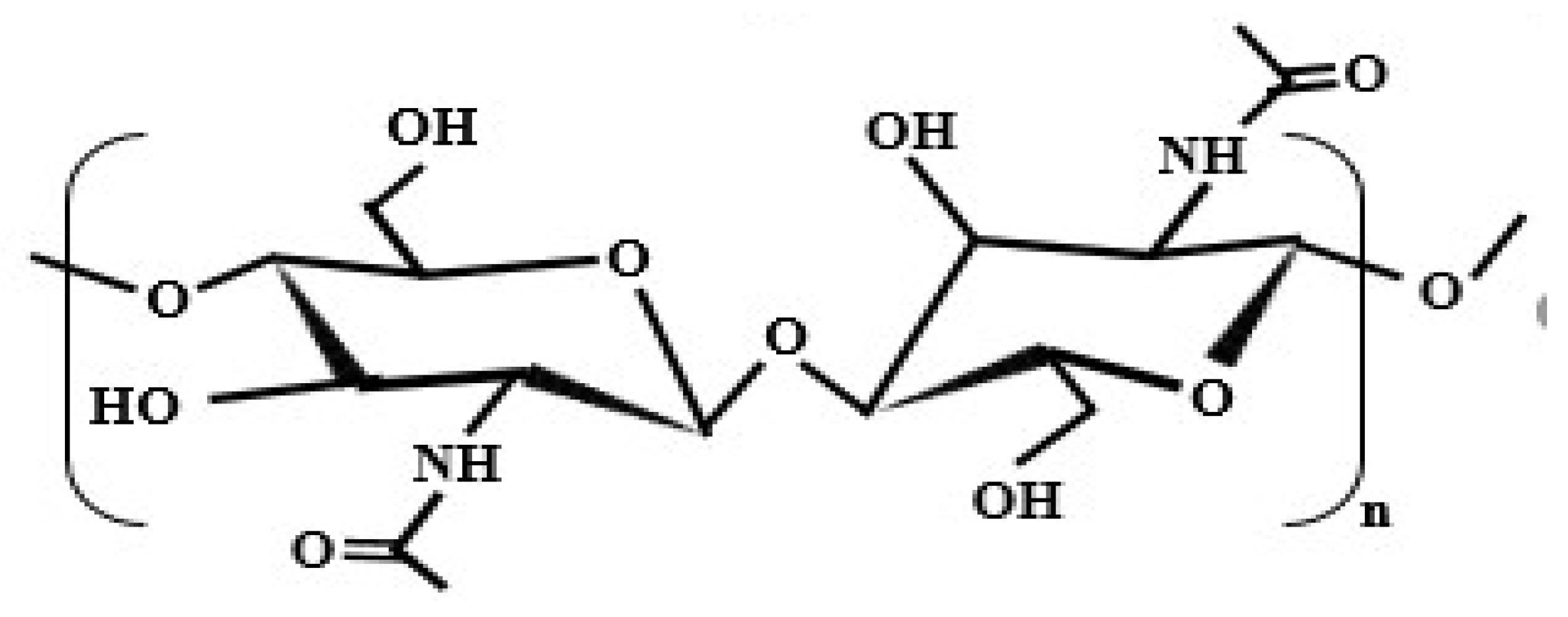
3. Chitosan
3.1. Origination of Chitosan
3.2. Chitosan-Based Composites
3.2.1. Combined Chitosan and Other Biopolymers
3.2.2. Combined Chitosan with Polyphenols/Extracts Rich in Polyphenols
| Formulation | Physical/Chemical/Mechanical Characteristics | Biological Properties | Applications | References |
|---|---|---|---|---|
| Chitosan, propolis extract | ↑ TS, EAB, ↓ WVP, oxygen permeability color changes of the films | Antioxidant activity (DPPH), antimicrobial activity against S. aureus, Salmonella Enteritidis, E. coli, and P. aeruginosa | Active packaging | [103] |
| Chitosan, propolis extract | ↑ thickness, thermal stability, TS ↓ transparency, EAB, WS color change | Antioxidant activity (DPPH (49.8%–94.5%), ABTS (20.3%–83.6%)), antimicrobial activity against Staphylococcus hominis, Pantoea sp., Arthrobacter sp., Erwinia sp., B. cereus, E. coli, S. aureus, Metschnikowia rancensis, Cladosporium sp., Penicillium brevicompactum, Botrytis cinerea, and Alternaria sp. | Active packaging | [104] |
| Chitosan, gallic acid | ↑ TS (for chitosan:gallic acid ratio 1:0.1, 1:0.5) ↓ EAB and WVP (for chitosan:gallic acid ratio 1:0.1) | Antioxidant activity (DPPH, ABTS), antimicrobial activity against E. coli and L. monocytogenes | Active food packaging | [105] |
| Chitosan, epigallocatechin gallate nanocapsules (with zein) | ↑ TS, EAB, VIS-light protection | Antioxidant activity (DPPH) | Active packaging | [106] |
| Chitosan, ellagic acid | ↑ EAB, WVP ↓ TS, Young’s modulus, UV-light protection good thermal stability | Antioxidant activity (DPPH), antimicrobial activity against P. aeruginosa and S. aureus, prevent photo-oxidation of light-sensitive foods | Active packaging | [107] |
| Chitosan, protocatechuic acid | ↑ thickness, opacity, WS, UV-light barrier ↓ moisture content, WVP, EAB, color change TS increased up to 1% acid incorporation, afterwards decreased | Antioxidant activity (DPPH) | Active packaging | [108] |
| Chitosan, thinned young apple polyphenols | ↑ thickness, density, WS ↓ WVP, TS, EAB, water content | Antioxidant activity (DPPH 68%–92%), antimicrobial activity against E. coli, S aureus, L. monocytogenes, Colletotrichum fructicola, Botryosphaerial dothidea, and Alternaria tenuissima | Active packaging | [123] |
| Chitosan, apple peel polyphenols | ↑ thickness, density, WS, ↓ thermal stability, WVP, TS, EAB, moisture content, transparency color change | Antioxidant activity (DPPH 30%–67%, ABTS 70%–90%), antimicrobial activity against E. coli, B. cereus, S. aureus, and S. typhimurium | Active packaging | [101] |
| Chitosan, proanthocyanidins | ↓ thermal stability | Antioxidant activity (DPPH, ABTS), antimicrobial activity against M. luteus, B. subtilis, E. coli, S. aureus, Proteus vulgaris, and P. aeruginosa | Active packaging | [132] |
| Chitosan, proanthocyanidins | ↑ thickness, opacity, thermal stability, WS, WVP, TS, UV–VIS light barrier ↓ moisture content, EAB, oxygen permeability color change | Antioxidant activity (DPPH), antimicrobial activity against E. coli, Salmonella, S. aureus, and L. monocytogenes | Active packaging | [109] |
| Chitosan, syringic acid | ↑ thickness, density, WS, opacity, TS when the amount of syringic acid was under 0.5% and EAB when the amount of syringic acid was 0.25%, ↓ moisture content, thermal stability and WVP color change | Antimicrobial activity against S. aureus and E. coli | Preservation of quail egg Active packaging | [110] |
| Chitosan, phenolic acids (ferulic acid, caffeic acid, tannic acid, gallic acid) | ↑ TS, EAB, Young’s modulus, thermal stability, WVP color change | Antioxidant activity (DPPH 17%–89%) | Active packaging | [81] |
| Chitosan, curcumin | ↑ TS ↓ EAB, WVP, | Antimicrobial activity against S. aureus and Rhizoctonia solani | Active packaging | [111] |
| Chitosan, carvacrol | ↓ WVP, TS, EAB, thickness and transparency, change color to yellow | Antioxidant activity (FRAP), antimicrobial activity against E. coli and S. aureus | Active packaging | [114] |
| Chitosan, pomegranate peel extract | ↑ thickness, TS ↓ EAB and transparency change color | Antioxidant activity (FRAP), antimicrobial activity against S. aureus | Active packaging | [114] |
| Chitosan, pomegranate peel extract | ↑ EAB ↓ TS, WVP | Antioxidant activity (DPPH 21%–57%) | Active packaging | [119] |
| Chitosan, thyme extract | ↑ TS, EM, opacity decreased: EAB, color change | Antioxidant activity (DPPH) | Active packaging | [115] |
| Chitosan, turmeric extract | ↑ TS, Young’s modulus, WVP, UV–VIS barrier property | Antimicrobial activity against S. aureus and Salmonella | Active packaging | [116] |
| Chitosan, tea extract | ↑ thickness, WS ↓ water content, WVP, TS, EAB | Antioxidant activity (DPPH) | Active packaging | [124] |
| Chitosan, grapefruit seed extract | ↑ thickness, EAB, ↓TS | Antifungal activity | Bread preservation | [120] |
| Chitosan, maqui berry extract (Aristotelia chilensis) | not determined | Antioxidant activity (DPPH, FRAP), antimicrobial activity against Serratia marcescens, Alcaligenes faecalis, Aeromonas hydrophila, Pseudomonas fluorescens, Citrobacter freundii, Achromobacter denitrifican, S. putrefaciens | Active packaging | [131] |
| Chitosan, Lycium barbarum fruit extract | ↑ density ↓ TS, EAB, WVP, WS, moisture content | Antioxidant activity (DPPH) | Active packaging | [125] |
| Chitosan, honeysuckle flower extract (Lonicera japonica Thunb) | ↑ WS, density ↓ WVP, TS, EAB, moisture content | Antioxidant activity (DPPH), antimicrobial activity against E. coli | Active packaging | [126] |
| Chitosan, Berberis crataegina fruit extract | ↑ thickness, EAB ↓ transparency, TS, WS, Young’s modulus | Antioxidant activity (DPPH 86%), antimicrobial activity against E. coli, S. thypmurium, Proteus microbilis, Proteus vulgaris, P. aeruginosa, Enterobacter aerogenes, S. aureus, Streptococcus mutans, Bacillus thuringiensis | Active packaging | [121] |
| Chitosan, Nigella sativa seedcake extract | ↑ thickness, EAB, ↓ moisture content, WVP, TS color change | Antioxidant activity (DPPH, FRAP) | Active packaging | [122] |
| Chitosan, mango leaf extract | ↑ thickness, TS, EM, ↓ moisture content, WS, WVP, EAB | Antioxidant activity (DPPH, FRAP, ABTS) | Cashew nuts preservation | [117] |
| Chitosan, Herba Lophatheri extract from dried leaves of Lophatherum gracile Brongn | ↑ opacity, density, ↓ WS, WVP, moisture content color change, higher oil resistance | Antioxidant activity (DPPH), antimicrobial activity against E. coli and S. aureus | Active packaging | [129] |
| Chitosan, Chinese chive (Allium tuberosum) root extract | ↑ thickness, thermal stability ↓ TS, EAB, WS, WVP, moisture content | Antioxidant activity (DPPH 20%–47%, ABTS 28%–57%), antimicrobial activity against B. cereus, S. aureus, E. coli, and S. typhimurium | Soybean oil packaging | [102] |
| Chitosan, Sonneratia caseolaris (L.) Engl. leaf extract | ↑ light barrier property, WS, WVP ↓ TS, EAB, moisture content change color | Antimicrobial activity against S. aureus and P. aeruginosa | Vietnamese banana preservation | [84] |
| Chitosan, olive leaves extract | ↑ WS, TS, and EAB, ↓ WVP | Antioxidant activity (ABTS), antimicrobial activity against L. monocytogenes and Campylobacter jejuni | Active packaging | [112] |
| Chitosan, blueberry extract by-products | ↑ thickness, WVP ↓ oxygen permeability, water content | Antioxidant activity (DPPH, ABTS, FRAP) | Active packaging | [128] |
| Chitosan, parsley extract by-products | ↑ thickness, WVP ↓ oxygen permeability, water content | Antioxidant activity (DPPH, ABTS, FRAP) | Active packaging | [128] |
| Chitosan, red grapes extract by-products | ↑ thickness, WVP ↓ oxygen permeability, water content | Antioxidant activity (DPPH, ABTS, FRAP), antimicrobial activity against E. coli | Active packaging | [128] |
| Chitosan, purple-fleshed sweet potato extract | ↑ thickness, WS, WVP when the extract exceeded 5 wt% and TS when the extract was 5 wt% ↓ EAB, WVP when the extract was 5 wt%, TS when the extract exceeded 5 wt%, moisture content and light transmittance | Antioxidant activity (DPPH), color variations of films to pH, pink-red (pH 3.0–6.0), purple-brown (pH 7.0–8.0), and greenish–green (pH 9.0–10.0) | Monitoring food spoilage | [118] |
| Chitosan, purple rice extract | ↑ thickness, EAB, TS, light barrier property, and WVP when the extract exceeded 1 wt% ↓ moisture content, change color | Antioxidant activity (DPPH), pH-sensitive in different buffer solutions | Monitor pork spoilage | [113] |
| Chitosan, black rice extract | ↑ thickness, EAB, light barrier property ↓ moisture content, TS when the extract exceeded 1 wt% change color | Antioxidant activity (DPPH) | Active packaging | [113] |
3.2.3. Combined Chitosan and Essential Oil
| Formulation | Physical/Chemical/Mechanical Characteristics | Biological Properties | Applications | References |
|---|---|---|---|---|
| Chitosan, citronella essential oil | ↑ EAB (low essential oil content), thermal stability ↓ WVP, TS, moisture content | Not determined | Packaging | [135] |
| Chitosan, cedarwood essential oil | ↑ EAB (low essential oil content), thermal stability ↓ WVP, TS, moisture content | Not determined | Packaging | [135] |
| Chitosan, basil essential oil | ↑ thickness, TS, EM ↓ WVP, EAB | Tested for antifungal activity, but the film did not inhibit the growth of A. niger, Botrytis cinerea, and R. stolonifer | Packaging | [138] |
| Chitosan, thyme essential oil | ↑ thickness, TS, EM ↓ WVP, EAB | Tested for antifungal activity, but the film did not inhibit the growth of A. niger, B. cinerea, and R. stolonifer | Packaging | [138] |
| Chitosan, fennel essential oil | ↑ density, thermal stability, and opacity ↓ WS, water swelling, thickness, and moisture content color change | Antioxidant activity (DPPH 68%) | Active packaging | [139] |
| Chitosan, peppermint essential oil | ↑ density, thermal stability, and opacity ↓ WS, water swelling, and thickness color change | Antioxidant activity (DPPH 66%) | Active packaging | [139] |
| Chitosan, Eucalyptus globulus essential oil | ↑ opacity ↓ moisture content, WS | Antioxidant activity (DPPH 23%–43%), antimicrobial activity against E. coli, S. aureus, P. aeruginosa, C. albicans, C. parapsilosis | Active packaging | [140] |
| Chitosan, apricot kernel essential oil | ↑ opacity, TS ↓ moisture content, WS, WVP EAB first increased, and then when the ratio of chitosan:essential oil exceeded 1: 0.5 decreased | Antioxidant activity (DPPH 26%–35%), antimicrobial activity against S. aureus and B. subtillis, antifungal activity against R. stolonifer | Inhibited the growth of fungi on bread, active food packaging | [136] |
| Chitosan, piper betle Linn oil | ↑ UV-light barrier, EAB (at 0.4 and 1% oil incorporation), ↓ thermal stability, TS, EM, and EAB (at 1.2% oil incorporated) | Antioxidant activity (DPPH), antimicrobial activity against S. aureus, E. coli, P. aeruginosa, and S. typhimurium | King orange preservation | [137] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef] [PubMed]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Kumar, V.A.; Hasan, M.; Mangaraj, S.; Pravitha, M.; Verma, D.K.; Srivastav, P.P. Trends in Edible Packaging Films and its Prospective Future in Food: A Review. Appl. Food Res. 2022, 2, 100118. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, N.; Islam, J.; Tahergorabi, R. Marine biopolymers: Applications in food packaging. Processes 2021, 9, 2245. [Google Scholar] [CrossRef]
- Kaur, J.; Rasane, P.; Singh, J.; Kaur, S. Edible Packaging: An Overview. In Edible Food Packaging; Springer Nature: Singapore, 2022; pp. 3–25. ISBN 9789811623837. [Google Scholar]
- Ubeda, S.; Aznar, M.; Rosenmai, A.K.; Vinggaard, A.M.; Nerín, C. Migration studies and toxicity evaluation of cyclic polyesters oligomers from food packaging adhesives. Food Chem. 2020, 311, 125918. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, H.; Auras, R.; Burgess, G.; Dolan, K.; Soto-Valdez, H. Migration of antioxidants from polylactic acid films, a parameter estimation approach: Part I—A model including convective mass transfer coefficient. Food Res. Int. 2018, 105, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Nechita, P.; Roman, M. Review on Polysaccharides Used in Coatings for Food. Coatings 2020, 10, 566. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gavara, R.; Catalá, R.; Hernández-Muñoz, P. The Potential of Proteins for Producing Food Packaging Materials: A Review. Packag. Technol. Sci. 2016, 29, 203–224. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef] [PubMed]
- Díaz-montes, E.; Castro-muñoz, R. Trends in chitosan as a primary biopolymer for functional films and coatings manufacture for food and natural products. Polymers 2021, 13, 767. [Google Scholar] [CrossRef] [PubMed]
- Calinoiu, L.F.; Ştefanescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan coating applications in probiotic microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan- and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.d.L.L.R.; da Rocha Pires, N.; da Cunha, P.L.R.; de Freitas Rosa, M.; de Souza, B.W.S.; de Andrade Feitosa, J.P.; de Souza, M.D.S.M. Effect of tannic acid as crosslinking agent on fish skin gelatin-silver nanocomposite film. Food Packag. Shelf Life 2019, 19, 7–15. [Google Scholar] [CrossRef]
- Nurdiani, R.; Ma’rifah, R.D.A.; Busyro, I.K.; Jaziri, A.A.; Prihanto, A.A.; Firdaus, M.; Talib, R.A.; Huda, N. Physical and functional properties of fish gelatin-based film incorporated with mangrove extracts. PeerJ 2022, 10, e13062. [Google Scholar] [CrossRef] [PubMed]
- Hanani, Z.A.N. Gelatin. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2015; pp. 191–195. ISBN 9780123849533. [Google Scholar]
- Kuan, Y.H.; Nafchi, A.M.; Huda, N.; Ariffin, F.; Karim, A.A. Comparison of physicochemical and functional properties of duck feet and bovine gelatins. J. Sci. Food Agric. 2017, 97, 1663–1671. [Google Scholar] [CrossRef]
- Karayannakidis, P.D.; Zotos, A. Fish Processing By-Products as a Potential Source of Gelatin: A Review. J. Aquat. Food Prod. Technol. 2016, 25, 65–92. [Google Scholar] [CrossRef]
- Ahmad, T.; Ismail, A.; Ahmad, S.A.; Khalil, K.A.; Kumar, Y.; Adeyemi, K.D.; Sazili, A.Q. Recent advances on the role of process variables affecting gelatin yield and characteristics with special reference to enzymatic extraction: A review. Food Hydrocoll. 2017, 63, 85–96. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Physical and Mechanical Characteristics of Gelatin-Based Films as a Potential Food Packaging Material: A Review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Fakhreddin Hosseini, S.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin-chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Nur Hazirah, M.A.S.P.; Isa, M.I.N.; Sarbon, N.M. Effect of xanthan gum on the physical and mechanical properties of gelatin-carboxymethyl cellulose film blends. Food Packag. Shelf Life 2016, 9, 55–63. [Google Scholar] [CrossRef]
- Loo, C.P.Y.; Sarbon, N.M. Chicken skin gelatin films with tapioca starch. Food Biosci. 2020, 35, 100589. [Google Scholar] [CrossRef]
- Jridi, M.; Abdelhedi, O.; Salem, A.; Kechaou, H.; Nasri, M.; Menchari, Y. Physicochemical, antioxidant and antibacterial properties of fish gelatin-based edible films enriched with orange peel pectin: Wrapping application. Food Hydrocoll. 2020, 103, 105688. [Google Scholar] [CrossRef]
- Zhong, C.; Hou, P.F.; Li, Y.X.; Yang, W.Y.; Shu, M.; Wu, G.P. Characterization, antioxidant and antibacterial activities of gelatin film incorporated with protocatechuic acid and its application on beef preservation. LWT 2021, 151, 112154. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, J.; Yu, H.; Zhang, J.; Yuan, Y.; Shen, X.; Li, C. The effects of EGCG on the mechanical, bioactivities, cross-linking and release properties of gelatin film. Food Chem. 2019, 271, 204–210. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Yang, W.; Xue, C.; Wang, Y.; Dong, J.; Xue, Y. Modification of gelatine with galla chinensis extract, a natural crosslinker. Int. J. Food Prop. 2016, 19, 731–744. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, P.; Fang, S.; Liu, W.; Mei, J.; Xie, J. Preservative effects of gelatin active coating enriched with eugenol emulsion on Chinese seabass (Lateolabrax maculatus) during superchilling (−0.9 °C) storage. Coatings 2019, 9, 489. [Google Scholar] [CrossRef]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.A.N. Utilization of mango peel extracts on the biodegradable films for active packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Li, X.; Tu, Z.C.; Sha, X.M.; Ye, Y.H.; Li, Z.Y. Flavor, antimicrobial activity and physical properties of gelatin film incorporated with of ginger essential oil. J. Food Sci. Technol. 2022, 59, 815–824. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lee, K.Y.; Beak, S.E.; Kim, H.; Song, K. Bin Antimicrobial activity of gelatin films based on duck feet containing cinnamon leaf oil and their applications in packaging of cherry tomatoes. Food Sci. Biotechnol. 2017, 26, 1429–1435. [Google Scholar] [CrossRef]
- Martucci, J.F.; Gende, L.B.; Neira, L.M.; Ruseckaite, R.A. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crops Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lee, J.H.; Yang, H.J.; Song, K. Bin Production and characterisation of skate skin gelatin films incorporated with thyme essential oil and their application in chicken tenderloin packaging. Int. J. Food Sci. Technol. 2016, 51, 1465–1472. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Physico-chemical properties, morphology and antioxidant activity of film from fish skin gelatin incorporated with root essential oils. J. Food Eng. 2013, 117, 350–360. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Kavoosi, G.; Rahmatollahi, A.; Mohammad Mahdi Dadfar, S.; Mohammadi Purfard, A. Effects of essential oil on the water binding capacity, physico-mechanical properties, antioxidant and antibacterial activity of gelatin films. LWT—Food Sci. Technol. 2014, 57, 556–561. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Pires, C.; Ramos, C.; Batista, I.; Saraiva, J.A.; Nunes, M.L. Characterization of fish protein films incorporated with essential oils of clove, garlic and origanum: Physical, antioxidant and antibacterial properties. LWT—Food Sci. Technol. 2014, 59, 533–539. [Google Scholar] [CrossRef]
- Mihaly Cozmuta, A.; Turila, A.; Apjok, R.; Ciocian, A.; Mihaly Cozmuta, L.; Peter, A.; Nicula, C.; Galić, N.; Benković, T. Preparation and characterization of improved gelatin films incorporating hemp and sage oils. Food Hydrocoll. 2015, 49, 144–155. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Prodpran, T.; Tanaka, M. Characterization of edible films from skin gelatin of brownstripe red snapper and bigeye snapper. Food Hydrocoll. 2006, 20, 492–501. [Google Scholar] [CrossRef]
- Mhd Sarbon, N.; Badii, F.; Howell, N.K. Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocoll. 2013, 30, 143–151. [Google Scholar] [CrossRef]
- Abedinia, A.; Ariffin, F.; Huda, N.; Mohammadi Nafchi, A. Preparation and characterization of a novel biocomposite based on duck feet gelatin as alternative to bovine gelatin. Int. J. Biol. Macromol. 2018, 109, 855–862. [Google Scholar] [CrossRef]
- Rahman, M.N.; Jamalulail, S.A.S.K.A. Extraction, Physicochemical Characterizations and Sensory. Borneo Sci. 2012, 30, 1–13. [Google Scholar]
- Nik Muhammad, N.A.; Huda, N.; Karim, A.A.; Mohammadi Nafchi, A. Effects of acid type extraction on characterization and sensory profile of duck feet gelatin: Towards finding bovine gelatin alternative. J. Food Meas. Charact. 2018, 12, 480–486. [Google Scholar] [CrossRef]
- Ninan, G.; Joseph, J.; Aliyamveettil, Z.A. A comparative study on the physical, chemical and functional properties of carp skin and mammalian gelatins. J. Food Sci. Technol. 2014, 51, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- da Trindade Alfaro, A.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Howell, N.K. Elucidation of protein protein interactions in gels and foams. In Gums and Stabilizers for the Food Industry 7; Oxford University Press: Wales, UK, 1994; pp. 77–89. [Google Scholar]
- Sarbon, N.M.; Badii, F.; Howell, N.K. The effect of chicken skin gelatin and whey protein interactions on rheological and thermal properties. Food Hydrocoll. 2015, 45, 83–92. [Google Scholar] [CrossRef]
- Ngarize, S.; Adams, A.; Howell, N. A comparative study of heat and high pressure induced gels of whey and egg albumen proteins and their binary mixtures. Food Hydrocoll. 2005, 19, 984–996. [Google Scholar] [CrossRef]
- Kehoe, J.J.; Foegeding, E.A. The characteristics of heat-induced aggregates formed by mixtures of β-lactoglobulin and β-casein. Food Hydrocoll. 2014, 39, 264–271. [Google Scholar] [CrossRef]
- Nazmi, N.N.; Isa, M.I.N.; Sarbon, N.M. Preparation and characterization of chicken skin gelatin/CMC composite film as compared to bovine gelatin film. Food Biosci. 2017, 19, 149–155. [Google Scholar] [CrossRef]
- Alias, S.A.; Mhd Sarbon, N. Rheological, physical, and mechanical properties of chicken skin gelatin films incorporated with potato starch. NPJ Sci. Food 2019, 3, 26. [Google Scholar] [CrossRef]
- Jahit, I.S.; Nazmi, N.N.M.; Isa, M.I.N.; Sarbon, N.M. Preparation and physical properties of gelatin/CMC/chitosan composite films as affected by drying temperature. Int. Food Res. J. 2016, 23, 1068–1074. [Google Scholar]
- Bakry, N.F.; Isa, M.I.N.; Sarbon, N.M. Effect of sorbitol at different concentrations on the functional properties of gelatin/carboxymethyl cellulose (CMC)/chitosan composite films. Int. Food Res. J. 2017, 24, 1753–1762. [Google Scholar]
- Fatyasari Nata, I.; Irawan, C.; Ramadhan, L.; Rizky Ramadhani, M. Influence of soy protein isolate on gelatin-based edible film properties. MATEC Web Conf. 2018, 156, 01014. [Google Scholar] [CrossRef][Green Version]
- Fu, S.; Wu, C.; Wu, T.; Yu, H.; Yang, S.; Hu, Y. Preparation and characterisation of Chlorogenic acid-gelatin: A type of biologically active film for coating preservation. Food Chem. 2017, 221, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Tammineni, N.; Ünlü, G.; Rasco, B.; Powers, J.; Sablani, S.; Nindo, C. Trout-Skin Gelatin-Based Edible Films Containing Phenolic Antioxidants: Effect on Physical Properties and Oxidative Stability of Cod-Liver Oil Model Food. J. Food Sci. 2012, 77, E342–E347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, L.; Yu, Y.; Zhou, H.; Guo, T.; Dai, H.; Zhang, Y. Physico-mechanical and antioxidant properties of gelatin film from rabbit skin incorporated with rosemary acid. Food Packag. Shelf Life 2019, 19, 121–130. [Google Scholar] [CrossRef]
- Halim, A.L.A.; Kamari, A.; Phillip, E. Chitosan, gelatin and methylcellulose films incorporated with tannic acid for food packaging. Int. J. Biol. Macromol. 2018, 120, 1119–1126. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Ge, S.; Miao, J.; Li, J.; Zhang, Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocoll. 2013, 32, 42–51. [Google Scholar] [CrossRef]
- Li, J.H.; Miao, J.; Wu, J.L.; Chen, S.F.; Zhang, Q.Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Bessaleh, S.; Jebahi, S.; Abbassi, R.; Ben Belgecem, S.; Faraz, A. Bioactive Gelatin-based Date By-Product for Packaging Applications: Physico-Chemical and Biological Characterization. J. Mater. Appl. 2022, 11, 10–16. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-based composite films and their application in food packaging: A review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, M.; Li, X.; Xu, Y.; Ma, X.; Shi, R.; Li, D.; Mu, C. Development of active rosmarinic acid-gelatin biodegradable films with antioxidant and long-term antibacterial activities. Food Hydrocoll. 2018, 83, 308–316. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Kan, J.; Liu, J. Preparation and characterization of active and intelligent films based on fish gelatin and haskap berries (Lonicera caerulea L.) extract. Food Packag. Shelf Life 2019, 22, 100417. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z. Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. Int. J. Food Prop. 2018, 20, S2822–S2832. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Y.; Cao, X.; Yao, F.; Shi, L.; Liu, Y. A Film of Chitosan Blended with Ginseng Residue Polysaccharides as an Antioxidant Packaging for Prolonging the Shelf Life of Fresh-Cut Melon. Coatings 2022, 12, 468. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Zasada, L.; Grabska-Zielińska, S. The Physicochemical, Antioxidant, and Color Properties of Thin Films Based on Chitosan Modified by Different Phenolic Acids. Coatings 2022, 12, 126. [Google Scholar] [CrossRef]
- Salgado-Cruz, M.d.l.P.; Salgado-Cruz, J.; García-Hernández, A.B.; Calderón-Domínguez, G.; Gómez-Viquez, H.; Oliver-Espinoza, R.; Fernández-Martínez, M.C.; Yáñez-Fernández, J. Chitosan as a coating for biocontrol in postharvest products: A bibliometric review. Membranes 2021, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.d.l.L.; Caballero, A.H. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Thi Dao, U.T.; Thi Bui, Q.P.; Bach, G.L.; Ha Thuc, C.N.; Ha Thuc, H. Enhanced antimicrobial activities and physiochemical properties of edible film based on chitosan incorporated with Sonneratia caseolaris (L.) Engl. leaf extract. Prog. Org. Coatings 2020, 140, 105487. [Google Scholar] [CrossRef]
- Tokatlı, K.; Demirdöven, A. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Sci. Hortic. (Amst.) 2020, 259, 108656. [Google Scholar] [CrossRef]
- Hu, W.; Sarengaowa; Feng, K. Effect of Edible Coating on the Quality and Antioxidant Enzymatic Activity of Postharvest Sweet Cherry (Prunus avium L.) during Storage. Coatings 2022, 12, 581. [Google Scholar] [CrossRef]
- Bigi, F.; Haghighi, H.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Characterization of chitosan-hydroxypropyl methylcellulose blend films enriched with nettle or sage leaf extract for active food packaging applications. Food Hydrocoll. 2021, 120, 106979. [Google Scholar] [CrossRef]
- Kumari, S.; Kishor, R. Chitin and chitosan: Origin, properties, and applications. In Handbook of Chitin and Chitosan; Volume 1: Preparation and Properties; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. ISBN 9780128179703. [Google Scholar]
- Lisitsyn, A.; Semenova, A.; Nasonova, V.; Polishchuk, E.; Revutskaya, N.; Kozyrev, I.; Kotenkova, E. Approaches in animal proteins and natural polysaccharides application for food packaging: Edible film production and quality estimation. Polymers 2021, 13, 1592. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Sagheer, F.A.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-García, M.; Reyes-Basurto, A.; García-Almendárez, B.E.; Hernández-Hernández, E.; Calderón-Domínguez, G.; Rossi-Márquez, G.; Regalado-González, C. Modified starch-chitosan edible films: Physicochemical and mechanical characterization. Coatings 2017, 7, 224. [Google Scholar] [CrossRef]
- Kaya, M.; Akyuz, L.; Sargin, I.; Mujtaba, M.; Salaberria, A.M.; Labidi, J.; Cakmak, Y.S.; Koc, B.; Baran, T.; Ceter, T. Incorporation of sporopollenin enhances acid–base durability, hydrophobicity, and mechanical, antifungal and antioxidant properties of chitosan films. J. Ind. Eng. Chem. 2017, 47, 236–245. [Google Scholar] [CrossRef]
- Younis, H.G.R.; Zhao, G. Physicochemical properties of the edible films from the blends of high methoxyl apple pectin and chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066. [Google Scholar] [CrossRef]
- Costa, S.M.; Ferreira, D.P.; Teixeira, P.; Ballesteros, L.F.; Teixeira, J.A.; Fangueiro, R. Active natural-based films for food packaging applications: The combined effect of chitosan and nanocellulose. Int. J. Biol. Macromol. 2021, 177, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Azaza, Y.B.; Hamdi, M.; Charmette, C.; Jridi, M.; Li, S.; Nasri, M.; Nasri, R. Development and characterization of active packaging films based on chitosan and sardinella protein isolate: Effects on the quality and the shelf life of shrimps. Food Packag. Shelf Life 2022, 31, 100796. [Google Scholar] [CrossRef]
- Lan, W.; He, L.; Liu, Y. Preparation and properties of sodium carboxymethyl cellulose/sodium alginate/chitosan composite film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef]
- Mao, H.; Wei, C.; Gong, Y.; Wang, S.; Ding, W. Mechanical and water-resistant properties of eco-friendly chitosan membrane reinforced with cellulose nanocrystals. Polymers 2019, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Lagnika, C.; Luo, H.; Dai, Z.; Nie, M.; Hashim, M.M.; Liu, C.; Song, J.; Li, D. Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int. J. Biol. Macromol. 2020, 150, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Vitchayakitti, W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of chitosan-based biodegradable active films using bio-waste enriched with polyphenol propolis extract envisaging food packaging applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef]
- Wu, C.; Tian, J.; Li, S.; Wu, T.; Hu, Y.; Chen, S.; Sugawara, T.; Ye, X. Structural properties of films and rheology of film-forming solutions of chitosan gallate for food packaging. Carbohydr. Polym. 2016, 146, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yan, H.; Zhang, J.; Dai, W.; Gao, X.; Zhou, Y.; Wan, X.; Puligundla, P. Preparation and characterization of antioxidant edible chitosan films incorporated with epigallocatechin gallate nanocapsules. Carbohydr. Polym. 2017, 171, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Pinto, R.J.B.; Coelho, J.; Domingues, M.R.M.; Daina, S.; Sadocco, P.; Santos, S.A.O.; Freire, C.S.R. Bioactive chitosan/ellagic acid films with UV-light protection for active food packaging. Food Hydrocoll. 2017, 73, 120–128. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocoll. 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Bai, R.; Liu, Y.; Liu, J.; Liu, J. Preparation and characterization of antioxidant and antimicrobial packaging films based on chitosan and proanthocyanidins. Int. J. Biol. Macromol. 2019, 134, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Dang, H.; Liu, L.; Hu, X.; Li, X.; Ma, Z.; Wang, X.; Ren, T. Effect of syringic acid incorporation on the physical, mechanical, structural and antibacterial properties of chitosan film for quail eggs preservation. Int. J. Biol. Macromol. 2019, 141, 876–884. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Jiang, X.; Wu, J.; Le, X. Molecular interactions, characterization and antimicrobial activity of curcumin-chitosan blend films. Food Hydrocoll. 2016, 52, 564–572. [Google Scholar] [CrossRef]
- Musella, E.; El Ouazzani, I.C.; Mendes, A.R.; Rovera, C.; Farris, S.; Mena, C.; Teixeira, P.; Poças, F. Preparation and characterization of bioactive chitosan-based films incorporated with olive leaves extract for food packaging applications. Coatings 2021, 11, 1339. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J.; Qin, Y.; Bai, R.; Zhang, X.; Liu, J. Antioxidant and pH-sensitive films developed by incorporating purple and black rice extracts into chitosan matrix. Int. J. Biol. Macromol. 2019, 137, 307–316. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Torlak, E.; Akın-Evingür, G.; Özen, İ.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Z.H.; Qin, Y.Y.; Zhao, T.R.; Cheng, C.S. Characterization of antioxidant chitosan film incorporated with pomegranate peel extract. Adv. Mater. Res. 2013, 706, 24–27. [Google Scholar] [CrossRef]
- Tan, Y.M.; Lim, S.H.; Tay, B.Y.; Lee, M.W.; Thian, E.S. Functional chitosan-based grapefruit seed extract composite films for applications in food packaging technology. Mater. Res. Bull. 2015, 69, 142–146. [Google Scholar] [CrossRef]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erkul, S.K. Production and characterization of chitosan based edible films from Berberis crataegina’s fruit extract and seed oil. Innov. Food Sci. Emerg. Technol. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Kadam, D.; Shah, N.; Palamthodi, S.; Lele, S.S. An investigation on the effect of polyphenolic extracts of Nigella sativa seedcake on physicochemical properties of chitosan-based films. Carbohydr. Polym. 2018, 192, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wu, Y.; Li, Y. Development of tea extracts and chitosan composite films for active packaging materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, F.; Feng, Z.; Fan, X.; Pan, Z.; Zhou, J. Antioxidant activity and physicochemical properties of chitosan films incorporated with Lycium barbarum fruit extract for active food packaging. Int. J. Food Sci. Technol. 2015, 50, 458–464. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Tong, J.; Zhou, J. Physicochemical Properties of Chitosan Films Incorporated with Honeysuckle Flower Extract for Active Food Packaging. J. Food Process Eng. 2017, 40, e12305. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Dordevic, S.; Dordevic, D.; Sedlacek, P.; Kalina, M.; Tesikova, K.; Antonic, B.; Tremlova, B.; Treml, J.; Nejezchlebova, M.; Vapenka, L.; et al. Incorporation of natural blueberry, red grapes and parsley extract by-products into the production of chitosan edible films. Polymers 2021, 13, 3388. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, H.; Wang, J.; Jiang, G.; Du, F.; Liu, X. Effects of Herba Lophatheri extract on the physicochemical properties and biological activities of the chitosan film. Int. J. Biol. Macromol. 2019, 133, 51–57. [Google Scholar] [CrossRef]
- Zhu, F. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar] [CrossRef] [PubMed]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernandez-Lopez, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristotelia chilensis). LWT 2015, 64, 1057–1062. [Google Scholar] [CrossRef]
- Jing, Y.; Huang, J.; Yu, X. Preparation, characterization, and functional evaluation of proanthocyanidin-chitosan conjugate. Carbohydr. Polym. 2018, 194, 139–145. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Ngiwngam, K.; Tongdeesoontorn, W. Polysaccharide-Based Active Coatings Incorporated with Bioactive Compounds for Reducing Postharvest Losses of Fresh Fruits. Coatings 2022, 12, 8. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Morsi, R.E.; Fathy, M. Chitosan-Oregano Essential Oil Blends Use as Antimicrobial Packaging Material; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128007235. [Google Scholar]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Sauraj; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.T.T.; Van Tran, T.; Van Tan, L.; Danh, L.T.; Than, V.T. Development of antibacterial, antioxidant, and uv-barrier chitosan film incorporated with piper betle linn oil as active biodegradable packaging material. Coatings 2021, 11, 351. [Google Scholar] [CrossRef]
- Perdones, Á.; Chiralt, A.; Vargas, M. Properties of film-forming dispersions and films based on chitosan containing basil or thyme essential oil. Food Hydrocoll. 2016, 57, 271–279. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Chi, F.; Tan, Z.; Liu, L. Development and characterization of novel active chitosan films containing fennel and peppermint essential oils. Coatings 2020, 10, 936. [Google Scholar] [CrossRef]
- Hafsa, J.; ali Smach, M.; Khedher, M.R.B.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT 2016, 68, 356–364. [Google Scholar] [CrossRef]

| Formulation | Physical/Chemical/Mechanical Characteristics | Biological Properties | Applications | References |
|---|---|---|---|---|
| Gelatin, ginger essential oil | ↑ thickness, WVP, EAB, ↓ TS | Antimicrobial activity against E. coli and S. aureus | Antimicrobial active packaging | [36] |
| Gelatin, cinnamon leaf oil | ↓ TS, slightly decreased WVP | Antimicrobial activity against Salmonella typhimurium, E. coli, L. monocytogenes, and S. aureus | Cherry tomatoes | [37] |
| Gelatin, oregano essential oil | Insignificant modification | Antioxidant activity (DPPH 12%–60%, FRAP), antimicrobial activity against E. coli and S. aureus | Food active packaging | [38] |
| Gelatin, lavender essential oil | ↓ WVP, TS | Antioxidant activity (DPPH 1%–9%, FRAP), antimicrobial activity against E. coli and S. aureus | Food active packaging | [38] |
| Gelatin, thyme essential oil | ↑ EAB, ↓ TS, WVP | Antimicrobial activity against L. monocytogenes and E. coli | Chicken tenderloin packaging | [39] |
| Gelatin, citrus essential oils (bergamot, kaffir lime, lemon, lime) | ↑TS, ↓EAB, WVP (glycerol 20%)↑ EAB, ↓ TS (glycerol 30%) | Antioxidant activity (DPPH, ABTS, FRAP) | Active packaging | [40] |
| Gelatin, root essential oils (ginger, turmeric, plai) | ↑ EAB, ↓ TS and WVP | Antioxidant activity (DPPH, ABTS)plai > turmeric > ginger essential oils | Active packaging | [41] |
| Gelatin, Zataria multiflora (thyme-like plant) essential oil | ↑ WVP, EAB, light barrier properties, ↓ TS | Antioxidant activity (ABTS), antimicrobial activity against P. aeruginosa, E. coli, S. aureus, B. subtilis | Antioxidant, antimicrobial active packaging | [43] |
| Gelatin, essential oils (bergamot, lemongrass) | ↓ TS, EAB, WVP (lemongrass), solubility, transparency ↑ heat stability | Antimicrobial activity Lemongrass: E. coli, L. monocytogenes, S. aureus, S. typhimurium Bergamot: L. monocytogenes, S. aureus | Active packaging | [42] |
| Gelatin, essential oils (clove, garlic, origanum) | ↓ thickness, WS, EABslightly decreased WVP | Antioxidant activity (DPPH 38%–72%), antimicrobial activity against Brochothrix thermosphacta, Listeria innocua, L. monocytogenes, Shewanella putrefaciens | Biodegradable food packaging systems | [44] |
| Gelatin, sage essential oil | ↓ WVP, ↑ thickness | Antimicrobial activity against E. coli, S. aureus, L. innocua, Saccharomyces cerevisiae, Penicillium expansum | Fruits, vegetables, and meat packaging | [45] |
| Formulation | Physical/Chemical/Mechanical Characteristics | Biological Properties | Applications | References |
|---|---|---|---|---|
| Chitosan, corn starch | ↑ WS, TS, EAB, ↓ WVP by comparison with corn starch film color change | Not determined | Active packaging | [93] |
| Chitosan, starch | ↑ thickness and WS, ↓ WVP | Antimicrobial activity against L. innocua | Active packaging | [94] |
| Chitosan, sporopollenin | ↓ thickness, light transmittance, ↑ TS, EAB, Young’s modulus successfully incorporate sporopollenin into chitosan, enhanced hydrophobicity of films | Antifungal activity against Aspergillus niger, antioxidant activity | Active packaging | [95] |
| Chitosan, pectin | ↑ thickness, WVP, WS, TS, EAB, Young’s modulus ↓ density and opacity | Not determined | Packaging | [96] |
| Chitosan, nanocellulose | ↑ thermal stability, oxygen barrier properties, thickness, WVP, TS, Young’s modulus, ↓ film’s transparency | Antimicrobial activity against S. aureus, E. coli, and Candida albicans | Chicken meat | [97] |
| Chitosan, Sardinella protein isolate | ↑ thickness, moisture content, opacity, UV–VIS light barrier, WS, ↓ WVP, TS, and EAB, color change | Antioxidant activity (DPPH), antimicrobial activity against S. aureus, Micrococcus luteus, L. monocytogenes, Bacillus cereus, Salmonella enterica, P. aeruginosa, E. coli, Klebsiella pneumoniae | Shrimp packaging | [98] |
| Chitosan, CMC, sodium alginate | The optimal contents of the chitosan, CMC, and sodium alginate for the preparation of this composite film were 1.5%, 0.5%, and 1.5%. ↑ TS, EAB, WVP | Antimicrobial activity against E. coli and S. aureus | Packaging | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ștefănescu, B.E.; Socaciu, C.; Vodnar, D.C. Recent Progress in Functional Edible Food Packaging Based on Gelatin and Chitosan. Coatings 2022, 12, 1815. https://doi.org/10.3390/coatings12121815
Ștefănescu BE, Socaciu C, Vodnar DC. Recent Progress in Functional Edible Food Packaging Based on Gelatin and Chitosan. Coatings. 2022; 12(12):1815. https://doi.org/10.3390/coatings12121815
Chicago/Turabian StyleȘtefănescu, Bianca Eugenia, Carmen Socaciu, and Dan Cristian Vodnar. 2022. "Recent Progress in Functional Edible Food Packaging Based on Gelatin and Chitosan" Coatings 12, no. 12: 1815. https://doi.org/10.3390/coatings12121815
APA StyleȘtefănescu, B. E., Socaciu, C., & Vodnar, D. C. (2022). Recent Progress in Functional Edible Food Packaging Based on Gelatin and Chitosan. Coatings, 12(12), 1815. https://doi.org/10.3390/coatings12121815








