Interaction Mechanism of RGD Tripeptide on Different Surfaces of Mg and Mg Alloys: A First-Principles Study
Abstract
:Highlights
- The adsorption models of RGD on different surfaces of Mg and Mg alloys were set up.
- Alloying elements promoted the adsorption of RGD on different Mg surfaces.
- The ligand covalent bond between RGD and substrate was formed.
- The pronounced localization of electrons of Mg(110) and Mg(101) surfaces improved the adsorption.
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
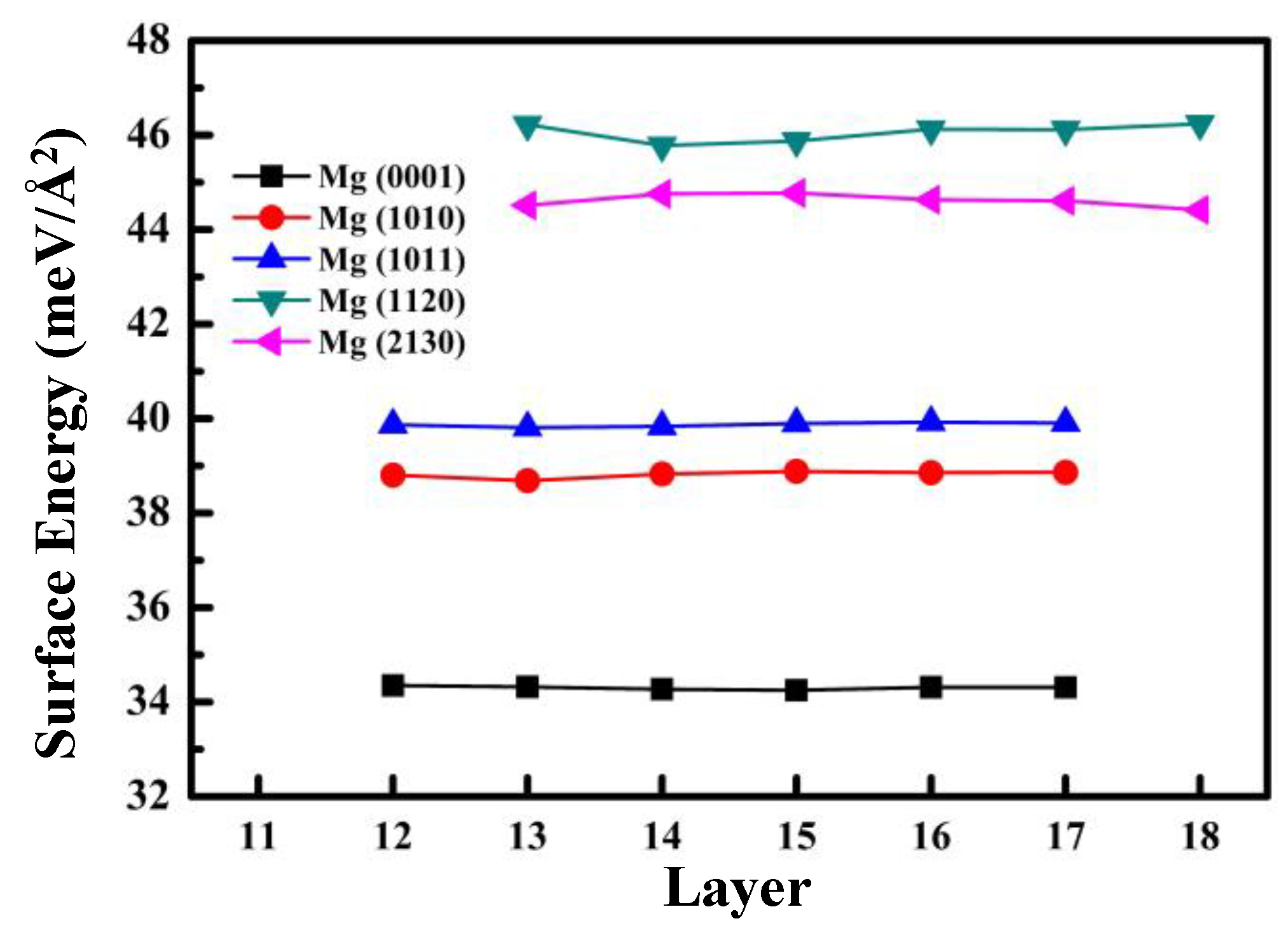
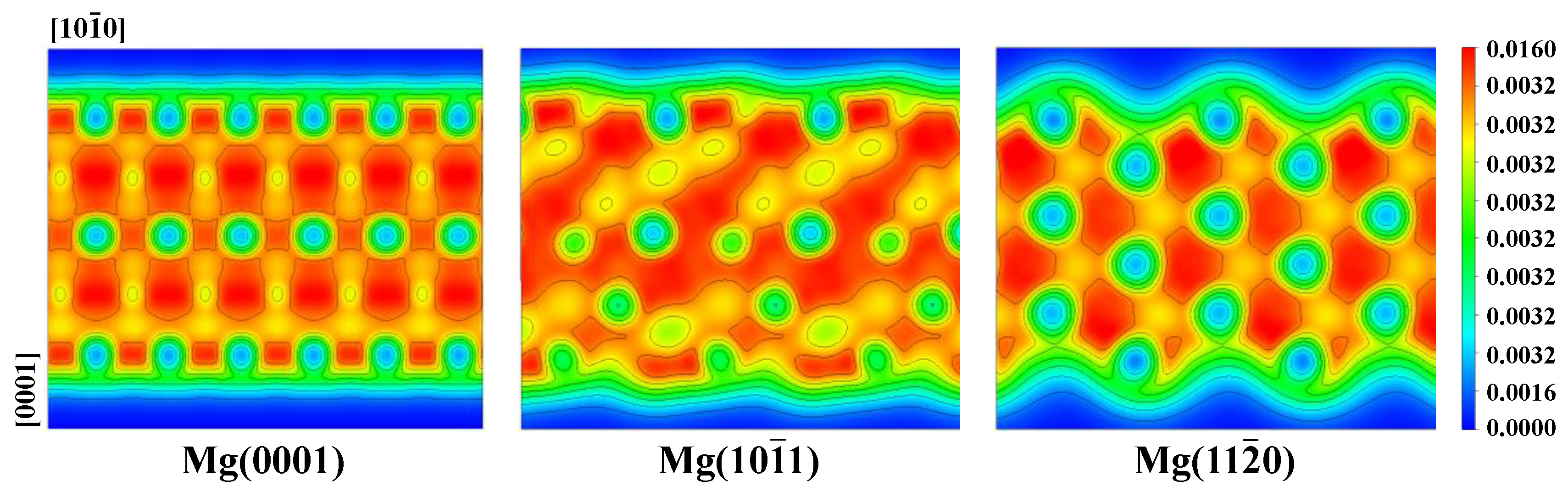
3.1. Properties of Different Surfaces of Pure Mg
3.2. Adsorption of RGD on Different Surfaces of Pure Mg and Mg Alloy
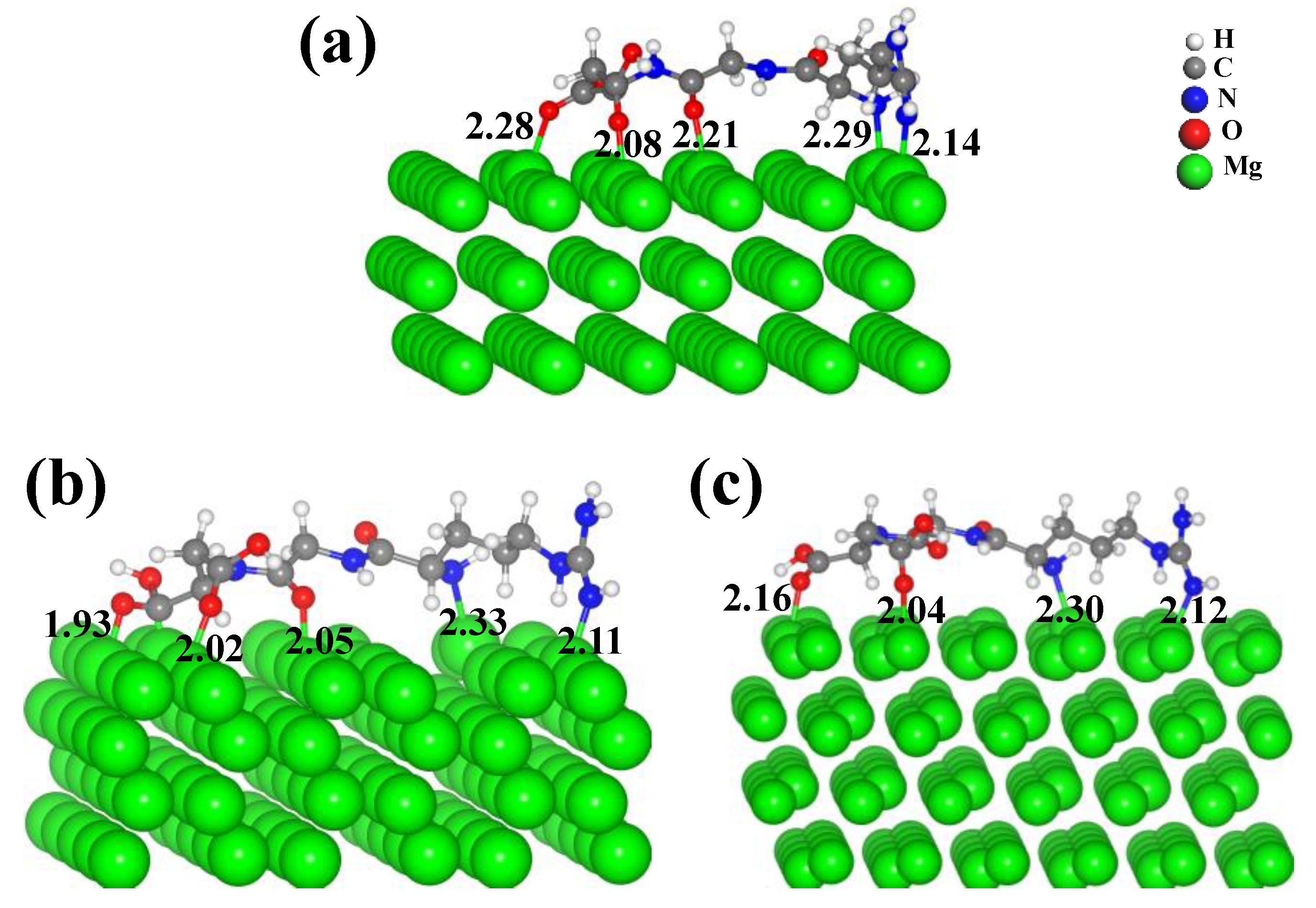
3.2.1. Adsorption of RGD on Different Surfaces of Pure Mg
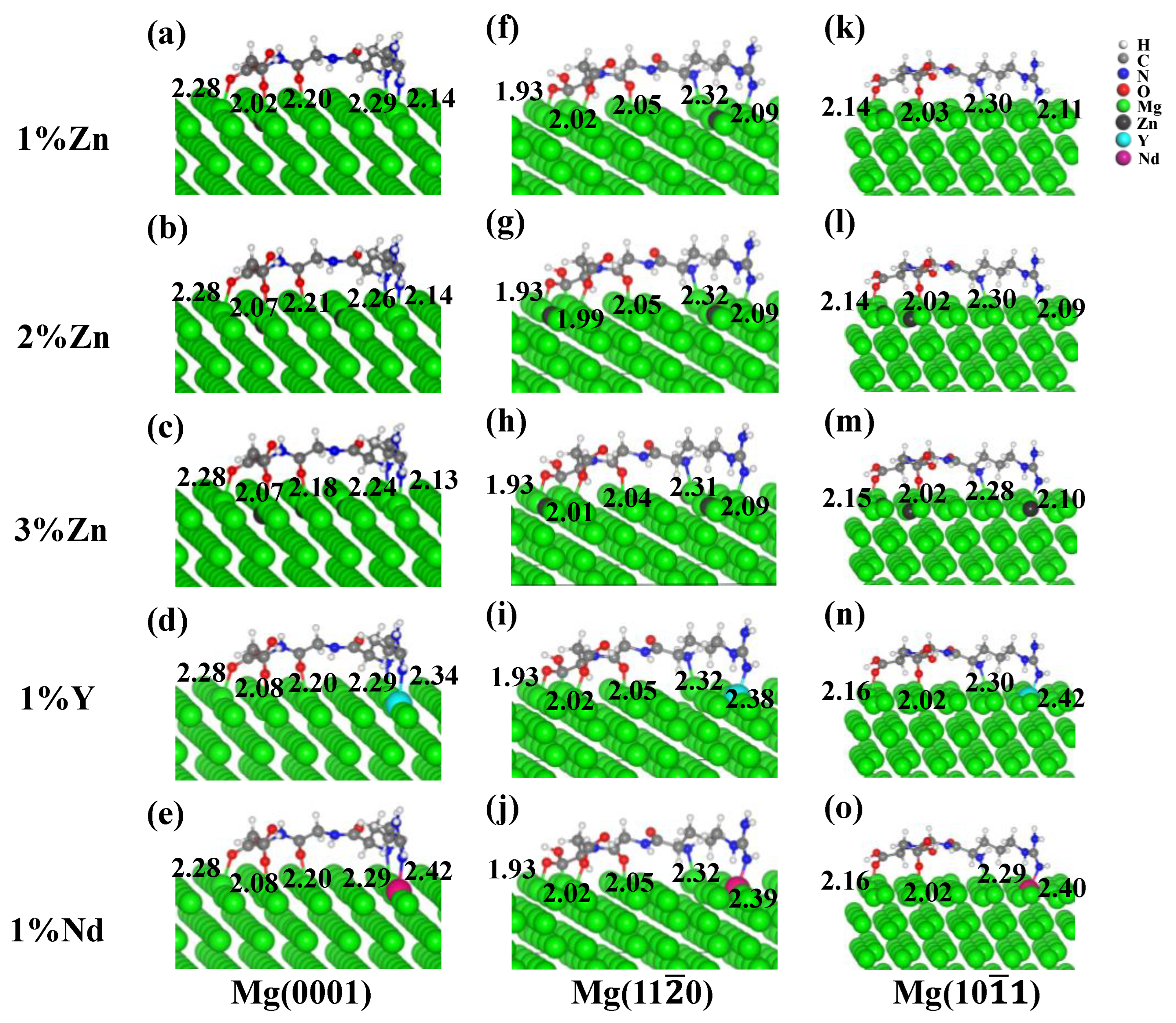
3.2.2. Effect of Zn, Y and Nd Alloying Elements on the Adsorption of RGD on Different Mg Alloys Surfaces
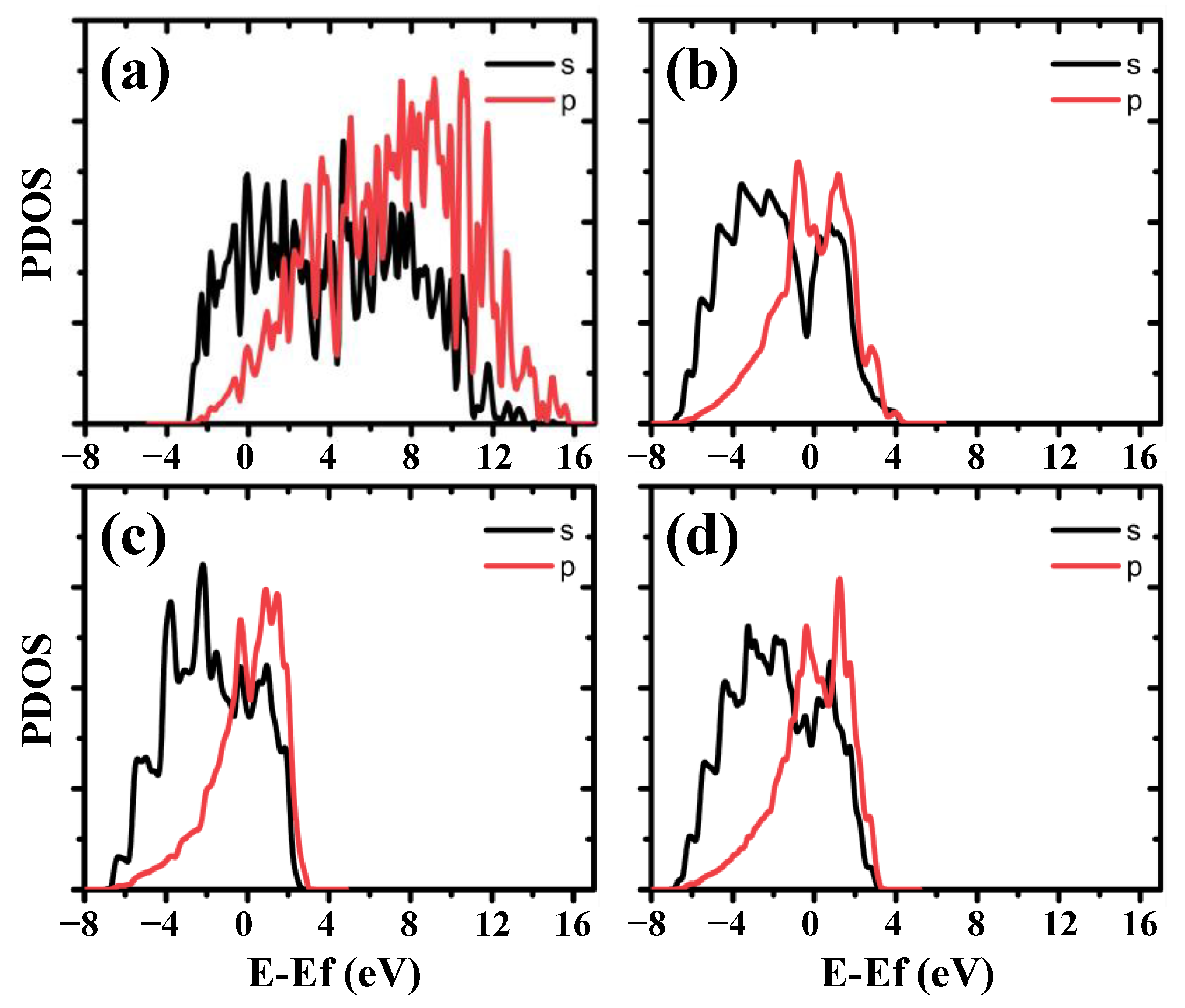
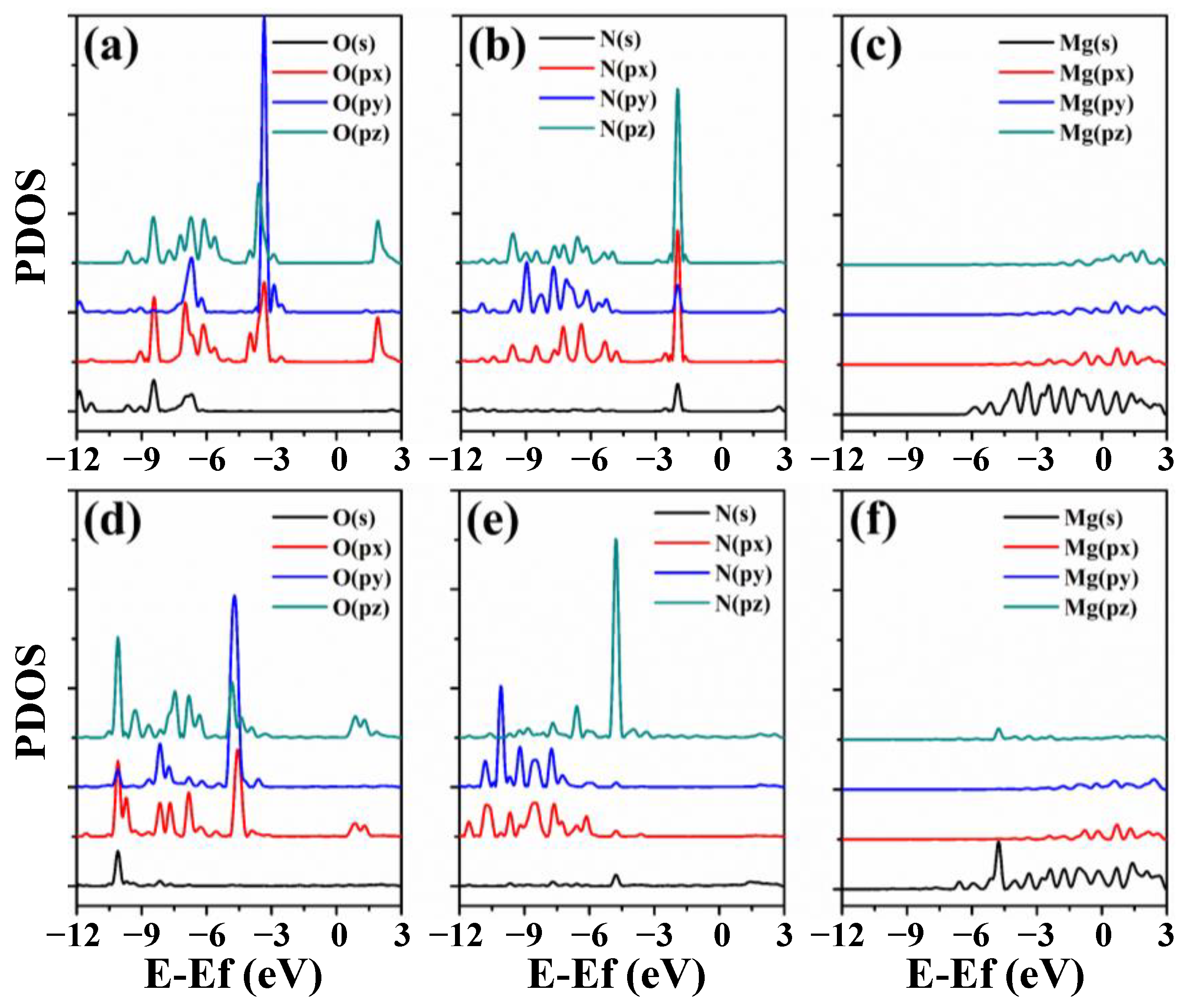
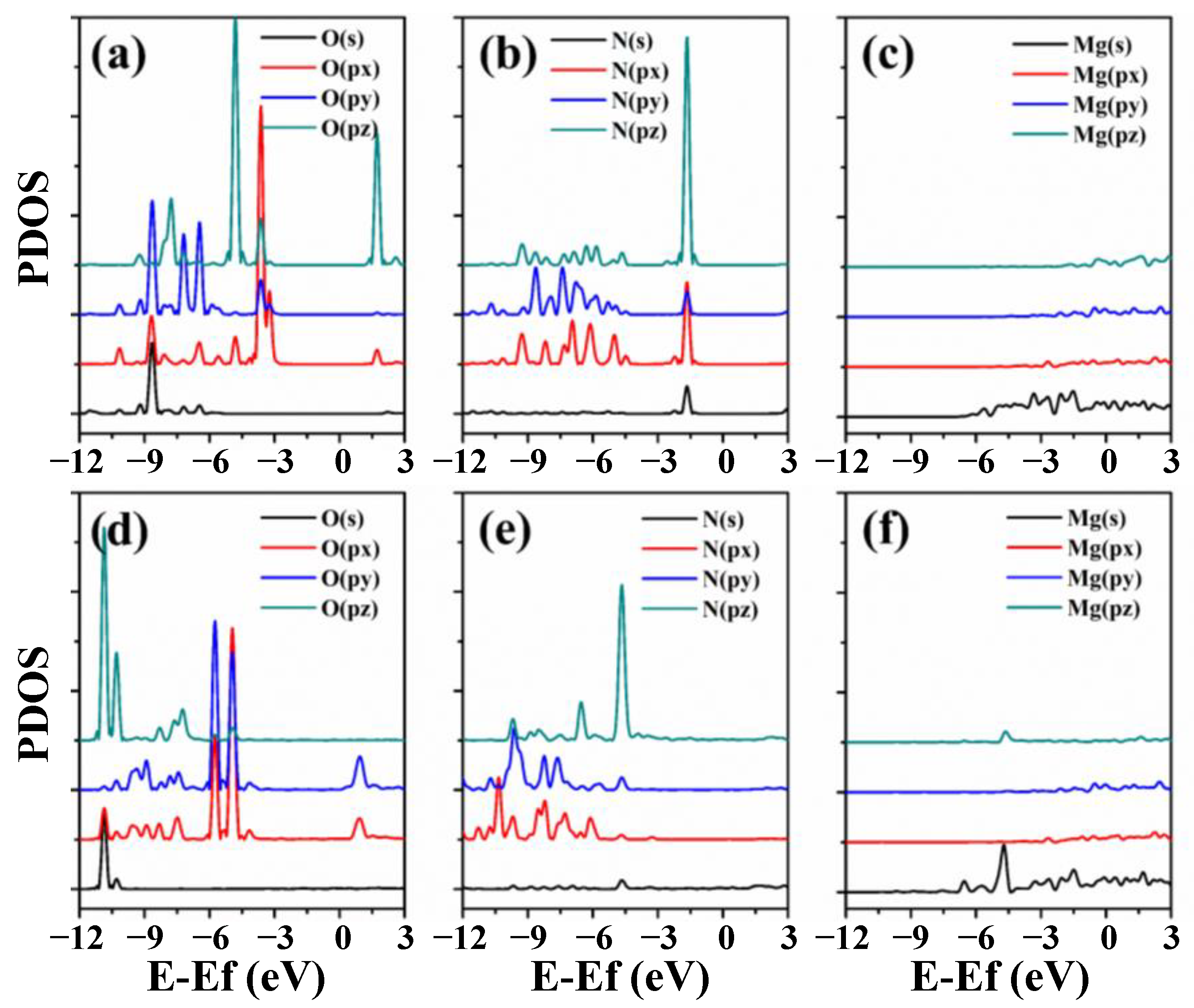
3.3. Electronic Properties of RGD on Mg and Mg-Based Alloy Surfaces
4. Conclusions
- (1)
- The order of Esurf for the above different surfaces was (110) > (210) > (101) > (100) > (0001). The higher surface energy made it much easier to interact with RGD, and the order of Eads was ΔE(110) > ΔE(101) > ΔE(0001).
- (2)
- For Mg alloys surfaces, the addition of Zn/Y/Nd alloying elements improved the association of RGD tripeptide with the different Mg alloys surfaces. The Eads also gradually increased with the increase of alloying element Zn content.
- (3)
- RGD tripeptide was bonded to the (0001), (110) and (101) surfaces of Mg through the ligand covalent bond. The pronounced localization of electrons of Mg(110) and Mg(101) surfaces promoted the adsorption of RGD tripeptide compared with that on the Mg(0001) surface. The calculated results provide insight for the interaction mechanism of RGD tripeptide on the Mg and Mg-based alloy surfaces, and also point out some directions for the design of functional biomolecular coatings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.-Q.; Yang, Y.-X.; Li, J.-A.; Zeng, R.-C.; Guan, S.-K. Advances in coatings on magnesium alloys for cardiovascular stents–A review. Bioact. Mater. 2021, 6, 4729–4757. [Google Scholar] [CrossRef]
- Wang, H.; Bai, M.; Yuan, H.; Hou, Y.; Liu, Y.; Fang, Z.; Sun, Y.; Wang, J.; Zhu, S.; Guan, S. Zn content mediated fibrinogen adsorption on biodegradable Mg-Zn alloys surfaces. J. Magnes. Alloy. 2021, 9, 2145–2154. [Google Scholar] [CrossRef]
- Li, M.; Jiang, M.; Gao, Y.; Zheng, Y.; Liu, Z.; Zhou, C.; Huang, T.; Gu, X.; Li, A.; Fang, J.; et al. Current status and outlook of biodegradable metals in neuroscience and their potential applications as cerebral vascular stent materials. Bioact. Mater. 2022, 11, 140–153. [Google Scholar] [CrossRef]
- Cheon, K.-H.; Park, C.; Kang, M.-H.; Park, S.; Kim, J.; Jeong, S.-H.; Kim, H.-E.; Jung, H.-D.; Jang, T.-S. A combination strategy of functionalized polymer coating with Ta ion implantation for multifunctional and biodegradable vascular stents. J. Magnes. Alloy. 2021, 9, 2194–2206. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Luo, X.; Xiong, P.; Gao, S.; Yan, J.; Li, Y.; Cheng, Y.; Xi, T. A tannic acid-modified fluoride pre-treated Mg–Zn–Y–Nd alloy with antioxidant and platelet-repellent functionalities for vascular stent application. J. Mater. Chem. B 2019, 7, 7314–7325. [Google Scholar] [CrossRef]
- Zong, J.; He, Q.; Liu, Y.; Qiu, M.; Wu, J.; Hu, B. Advances in the development of biodegradable coronary stents: A translational perspective. Mater. Today Bio 2022, 16, 100368. [Google Scholar] [CrossRef]
- Wang, P.; Xiong, P.; Liu, J.; Gao, S.; Xi, T.; Cheng, Y. A silk-based coating containing GREDVY peptide and heparin on Mg–Zn–Y–Nd alloy: Improved corrosion resistance, hemocompatibility and endothelialization. J. Mater. Chem. B 2018, 6, 966–978. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Chu, C.-C.; Xi, T. A novel biodegradable and biologically functional arginine-based poly(ester urea urethane) coating for Mg–Zn–Y–Nd alloy: Enhancement in corrosion resistance and biocompatibility. J. Mater. Chem. B 2017, 5, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Cui, J.; Chen, S.; Zhou, X.; Li, J.; Zhang, K. Extracellular Matrix Coatings on Cardiovascular Materials—A Review. Coatings 2022, 12, 1039. [Google Scholar] [CrossRef]
- Li, Y.; McRobb, L.S.; Khachigian, L.M. Inhibition of intimal thickening after vascular injury with a cocktail of vascular endothelial growth factor and cyclic Arg-Gly-Asp peptide. Int. J. Cardiol. 2016, 220, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Tugulu, S.; Silacci, P.; Stergiopulos, N.; Klok, H.-A. RGD—Functionalized polymer brushes as substrates for the integrin specific adhesion of human umbilical vein endothelial cells. Biomaterials 2007, 28, 2536–2546. [Google Scholar] [CrossRef] [PubMed]
- Kou, F.; Liu, C.; Wang, L.; Yasin, A.; Li, J.; Guan, S. Fabrication of Citric Acid/RGD Multilayers on Mg-Zn-Y-Nd Alloy via Layer-by-Layer Self-Assembly for Promoting Surface Biocompatibility. Adv. Mater. Interfaces 2021, 8, 2002241. [Google Scholar] [CrossRef]
- Schieber, R.; Mas-Moruno, C.; Lasserre, F.; Roa, J.J.; Ginebra, M.-P.; Mücklich, F.; Pegueroles, M. Effectiveness of Direct Laser Interference Patterning and Peptide Immobilization on Endothelial Cell Migration for Cardio-Vascular Applications: An In Vitro Study. Nanomaterials 2022, 12, 1217. [Google Scholar] [CrossRef]
- Wang, C.; Hao, H.; Wang, J.; Xue, Y.; Huang, J.; Ren, K.; Ji, J. High-throughput hyaluronic acid hydrogel arrays for cell selective adhesion screening. J. Mater. Chem. B 2021, 9, 4024–4030. [Google Scholar] [CrossRef]
- Deguchi, S.; Hakamada, M.; Mabuchi, M. Adsorption of RGD Tripeptide on Au (111) Surface. Mater. Trans. 2019, 60, 1711–1715. [Google Scholar] [CrossRef] [Green Version]
- Höffling, B.; Ortmann, F.; Hannewald, K.; Bechstedt, F. Single cysteine adsorption on Au(110): A first-principles study. Phys. Rev. B 2010, 81. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, J.; Zhu, S.; Yang, X.; Jia, Y.; Sun, Q.; Guan, S. A DFT study of the adsorption of short peptides on Mg and Mg-based alloy surfaces. Phys. Chem. Chem. Phys. 2018, 20, 3602–3607. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, J.; Yang, X.; Sun, Q.; Jia, Y.; Liu, H.; Xi, T.; Guan, S. Adsorption of arginine, glycine and aspartic acid on Mg and Mg-based alloy surfaces: A first-principles study. Appl. Surf. Sci. 2017, 409, 149–155. [Google Scholar] [CrossRef]
- Nikfar, Z.; Shariatinia, Z. The RGD tripeptide anticancer drug carrier: DFT computations and molecular dynamics simulations. J. Mol. Liq. 2019, 281, 565–583. [Google Scholar] [CrossRef]
- Zhang, H.-P.; Lu, X.; Fang, L.-M.; Weng, J.; Huang, N.; Leng, Y. Molecular dynamics simulation of RGD peptide adsorption on titanium oxide surfaces. J. Mater. Sci. Mater. Electron. 2008, 19, 3437–3441. [Google Scholar] [CrossRef]
- Zhang, H.-P.; Lu, X.; Leng, Y.; Watari, F.; Weng, J.; Feng, B.; Qu, S. Effects of aqueous environment and surface defects on Arg-Gly-Asp peptide adsorption on titanium oxide surfaces investigated by molecular dynamics simulation. J. Biomed. Mater. Res. Part A 2011, 96A, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Tang, J.; Zhao, Y.; Wang, H.; Yao, X.; Liu, J.; Zhu, M. Express penetration of hydrogen on Mg(10(1)over-bar3) along the close-packed-planes. Sci. Rep. 2015, 5, 10776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.J.; Ye, J.H.; Fang, Y.X.; Lin, Z.; Zhao, Y.J. Transition metal substitution on Mg(103) and Mg(0001) surfaces for improved hydrogenation and dehydrogenation: A systematic first-principles study. Appl. Surf. Sci. 2019, 479, 626–633. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, X.; Wu, M.; Sun, Q.; Jia, Y. First-principles study of hydrogen dissociation and diffusion on transition metal-doped Mg(0001) surfaces. Appl. Surf. Sci. 2014, 305, 40–45. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, C.G.S.; Majumder, C. First-principles study of the H2 interaction with transition metal (Ti, V, Ni) doped Mg(0001) surface: Implications for H-storage materials. J. Chem. Phys. 2008, 129, 174703. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhao, Y.; Wang, H.; Wang, J.; Zhu, S.; Jia, Y.; Cho, J.-H.; Guan, S. Influence of surface charge density on ligand-metal bonding: A DFT study of NH3 and HCOOH on Mg (0 0 0 1) surface. Appl. Surf. Sci. 2019, 470, 893–898. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Klimes, J.; Bowler, D.; Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 2011, 83, 195131. [Google Scholar] [CrossRef] [Green Version]
- Lüder, J.; Sanyal, B.; Eriksson, O.; Puglia, C.; Brena, B. Comparison of van der Waals corrected and sparse-matter density functionals for the metal-free phthalocyanine/gold interface. Phys. Rev. B 2014, 89, 045416. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Punkkinen, M.; Schönecker, S.; Nabi, Z.; Kádas, K.; Zólyomi, V.; Koo, Y.; Hu, Q.-M.; Ahuja, R.; Johansson, B.; et al. The surface energy and stress of metals. Surf. Sci. 2018, 674, 51–68. [Google Scholar] [CrossRef] [Green Version]
- Lautar, A.K.; Kopač, D.; Rejec, T.; Bančič, T.; Dominko, R. Morphology evolution of magnesium facets: DFT and KMC simulations. Phys. Chem. Chem. Phys. 2018, 21, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Wachowicz, E.; Kiejna, A. Bulk and surface properties of hexagonal-close-packed Be and Mg. J. Physics: Condens. Matter 2001, 13, 10767–10776. [Google Scholar] [CrossRef]
- Ji, D.-P.; Zhu, Q.; Wang, S.-Q. Detailed first-principles studies on surface energy and work function of hexagonal metals. Surf. Sci. 2016, 651, 137–146. [Google Scholar] [CrossRef]
- Sargent, W. Table of Periodic Properties of the Elements; Sargent-Welch Scientific: Skokie, IL, USA, 1980. [Google Scholar]
- Evtimova, J.; Drioli, E.; De Luca, G. A density functional theory study of hydrogen occupation in VNiTi alloys used for dense metal membranes. J. Alloys Compd. 2016, 665, 225–230. [Google Scholar] [CrossRef]
- Allred, A. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 1961, 17, 215–221. [Google Scholar] [CrossRef]

| Surface | Surface Energy(meV/Å2) | Work Function (eV) | ||
|---|---|---|---|---|
| This Work | Other Works | This Work | Other Works | |
| 0001 | 34.30 | 34.37 [33], 34.61 [34] | 3.69 | 3.70 [34], 3.80 [35] |
| 100 | 38.81 | 39.90 [34] | 3.61 | 3.60 [34], 3.64 [36] |
| 101 | 39.87 | 40.90 [34] | 3.69 | 3.80 [34], 3.70 [34] |
| 110 | 45.94 | 45.70 [34] | 3.66 | 4.00 [34] |
| 210 | 44.61 | 46.62 [36] | 3.49 | 3.49 [36] |
| Mg Surfaces | Pure | 1%Y | 1%Nd | 1%Zn | 2%Zn | 3%Zn |
|---|---|---|---|---|---|---|
| Mg(0001) | −3.24 | −3.89 | −4.03 | −3.53 | −3.64 | −3.73 |
| Mg(110) | −4.44 | −5.09 | −4.91 | −4.47 | −4.49 | −4.55 |
| Mg(101) | −3.32 | −3.56 | −3.47 | −3.51 | −3.52 | −3.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Ding, H.; Li, P.; Qiao, H.; Liang, E.; Jia, Y.; Guan, S. Interaction Mechanism of RGD Tripeptide on Different Surfaces of Mg and Mg Alloys: A First-Principles Study. Coatings 2022, 12, 1814. https://doi.org/10.3390/coatings12121814
Fang Z, Ding H, Li P, Qiao H, Liang E, Jia Y, Guan S. Interaction Mechanism of RGD Tripeptide on Different Surfaces of Mg and Mg Alloys: A First-Principles Study. Coatings. 2022; 12(12):1814. https://doi.org/10.3390/coatings12121814
Chicago/Turabian StyleFang, Zhe, Huili Ding, Ping Li, Huijie Qiao, Erjun Liang, Yu Jia, and Shaokang Guan. 2022. "Interaction Mechanism of RGD Tripeptide on Different Surfaces of Mg and Mg Alloys: A First-Principles Study" Coatings 12, no. 12: 1814. https://doi.org/10.3390/coatings12121814
APA StyleFang, Z., Ding, H., Li, P., Qiao, H., Liang, E., Jia, Y., & Guan, S. (2022). Interaction Mechanism of RGD Tripeptide on Different Surfaces of Mg and Mg Alloys: A First-Principles Study. Coatings, 12(12), 1814. https://doi.org/10.3390/coatings12121814





