Comparative Corrosion Characterization of Hybrid Zinc Coatings in Cl−-Containing Medium and Artificial Sea Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Stable CuO Suspension
2.2. Characterization of CuO Nanoparticles Dispersion
2.3. Electrodeposition of Hybrid Zinc Coatings on Steel
2.4. Surface Morphology
2.5. Corrosion Characterization and CVA Studies
2.6. XPS Measurements
2.7. Atomic Force Microscopy (AFM) Investigations
2.8. Contact Angle Measurements
2.9. Test Media and Reproducibility
3. Results and Discussion
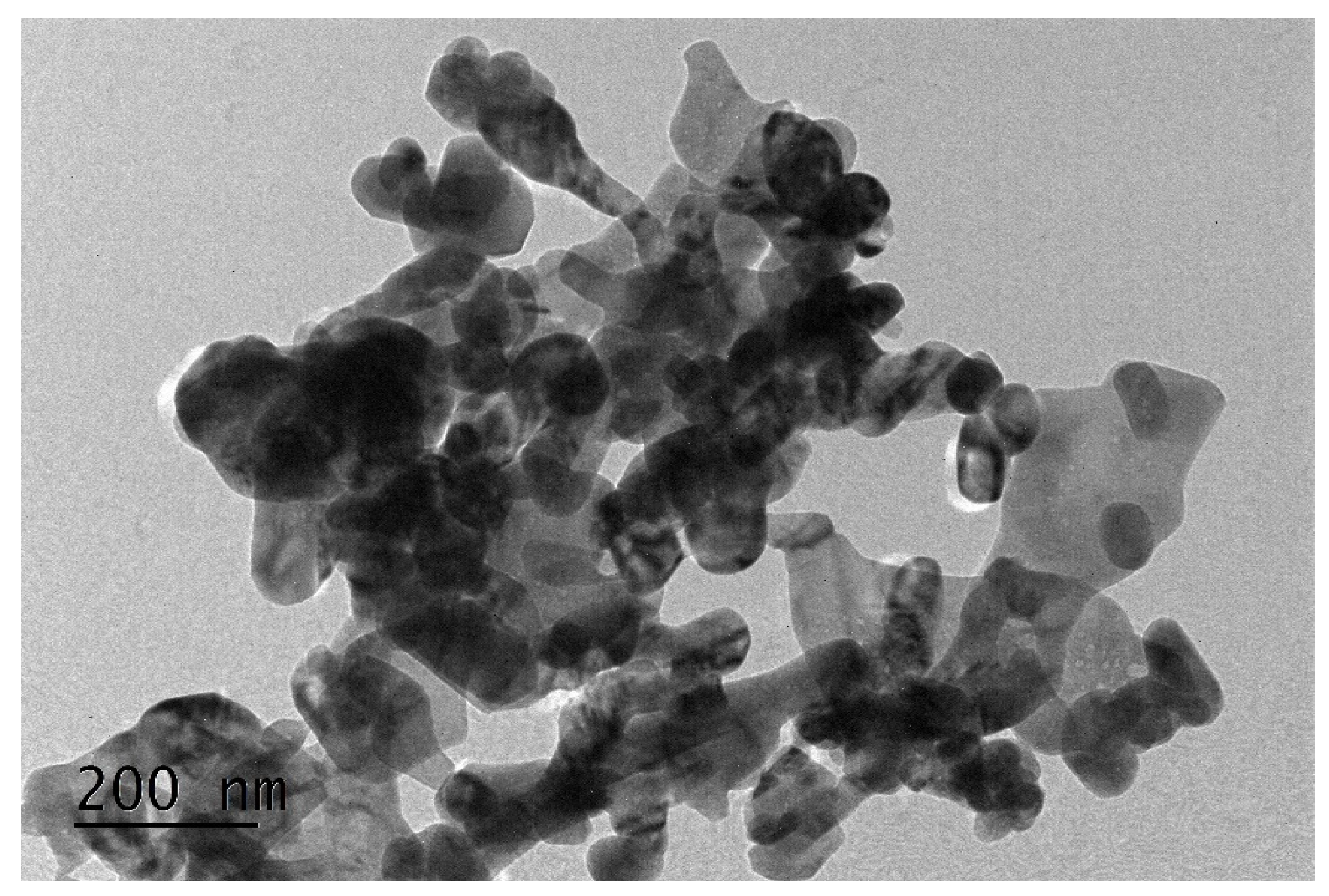
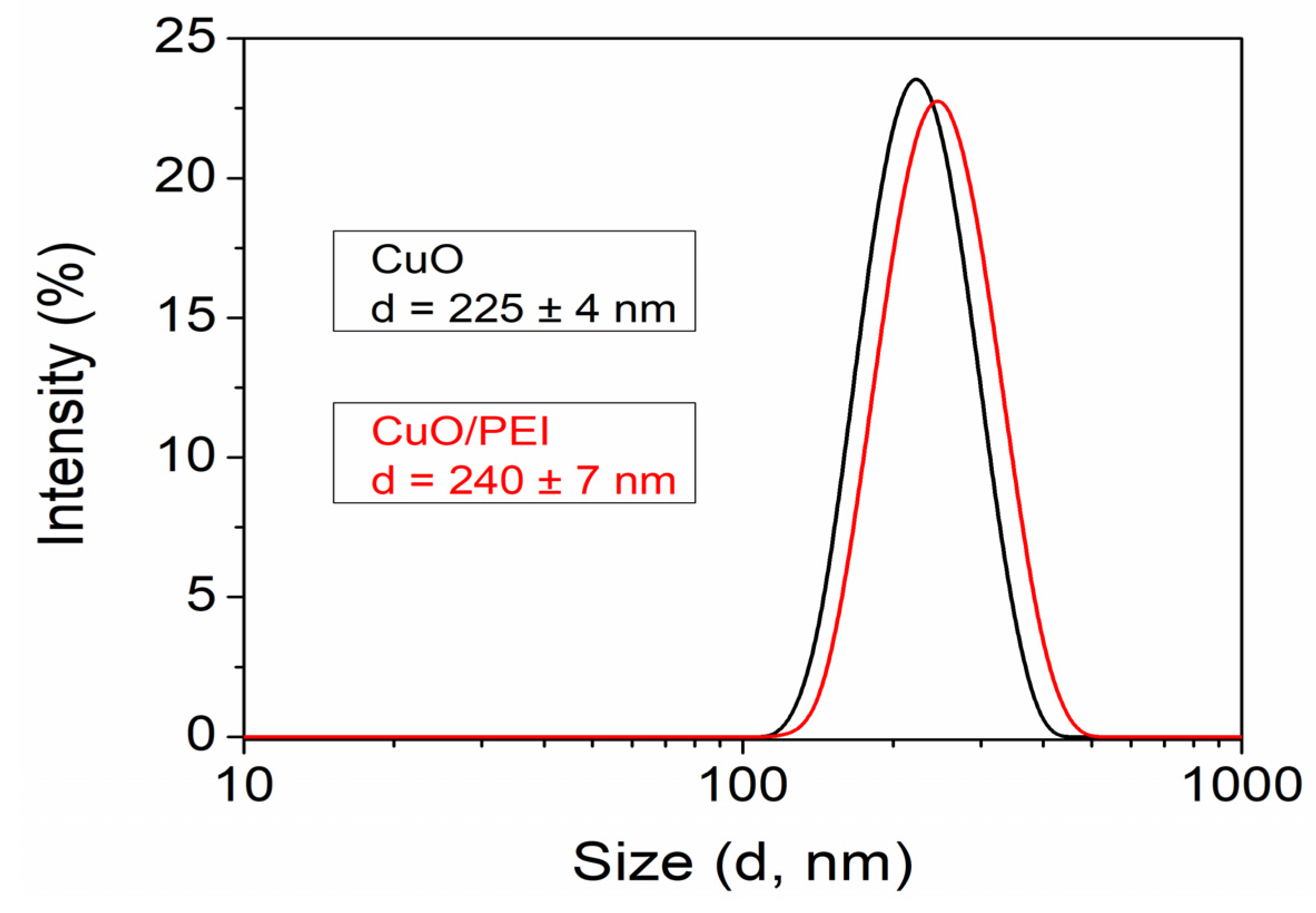
3.1. Characterization of CuO Nanoparticles Dispersion
3.2. Surface Morphology
3.3. Cyclic Voltammetry (CVA) Studies
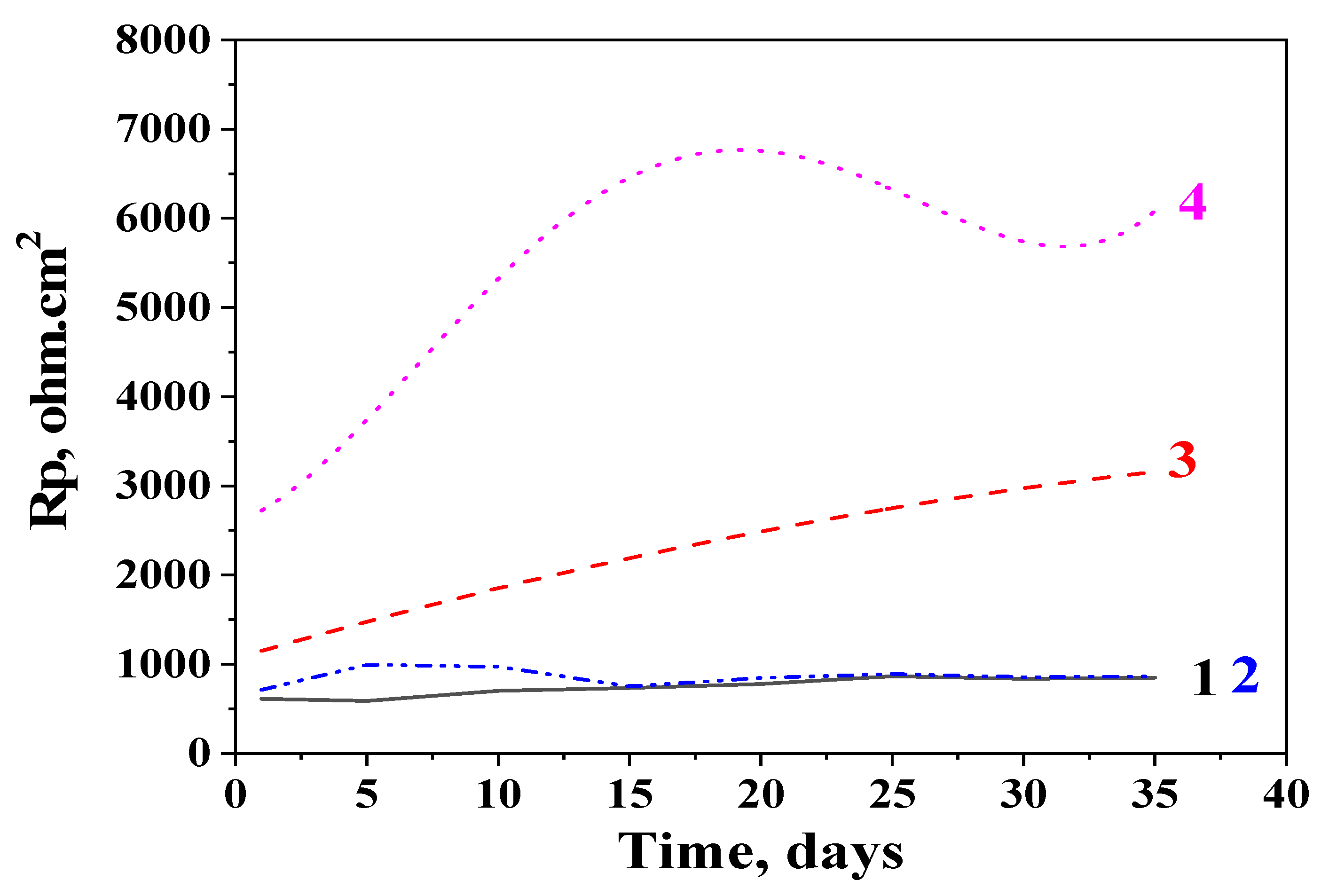
3.4. Potentiodynamic Polarization (PDP) Curves and Polarization Resistance (Rp) Measurements
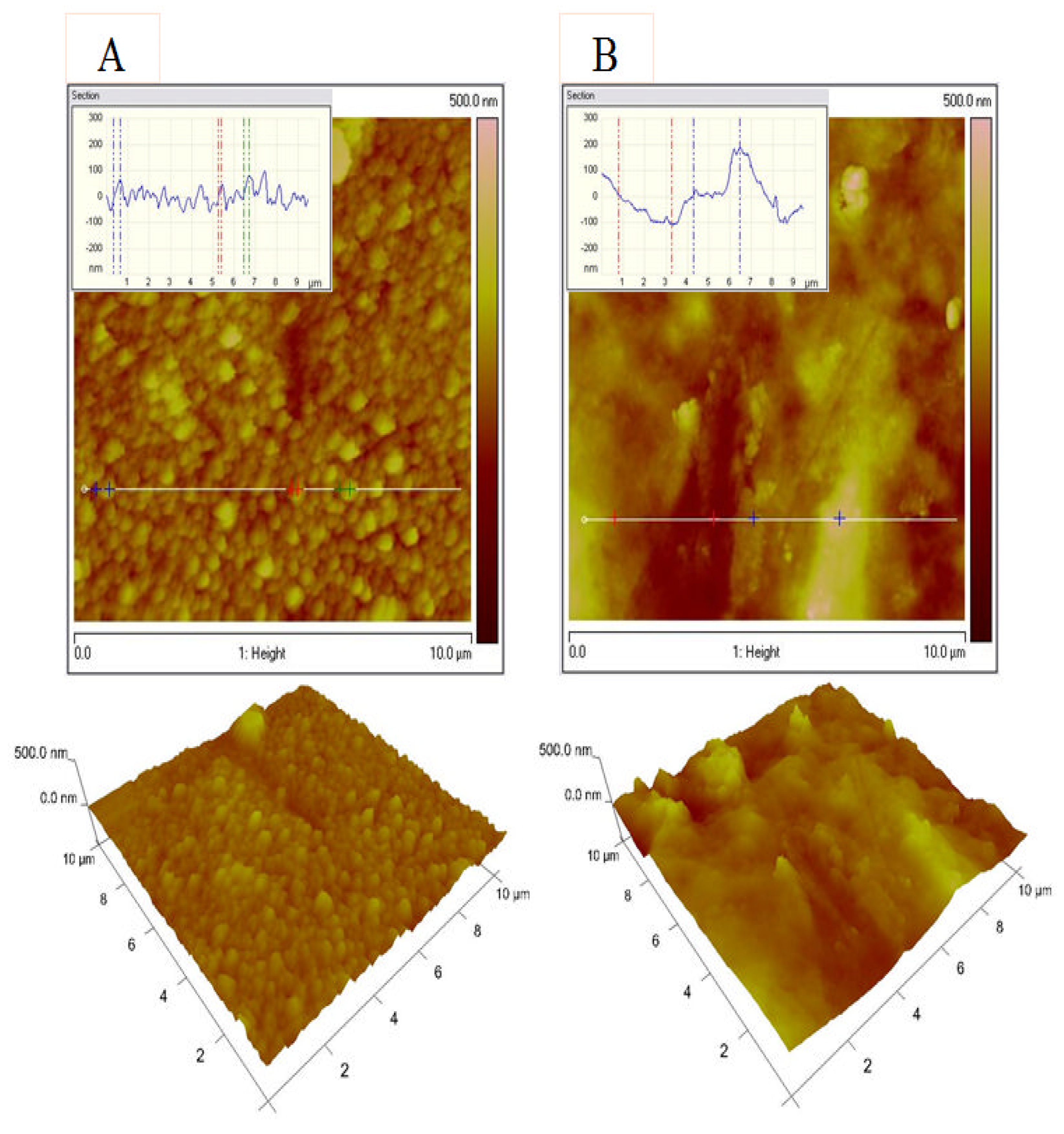
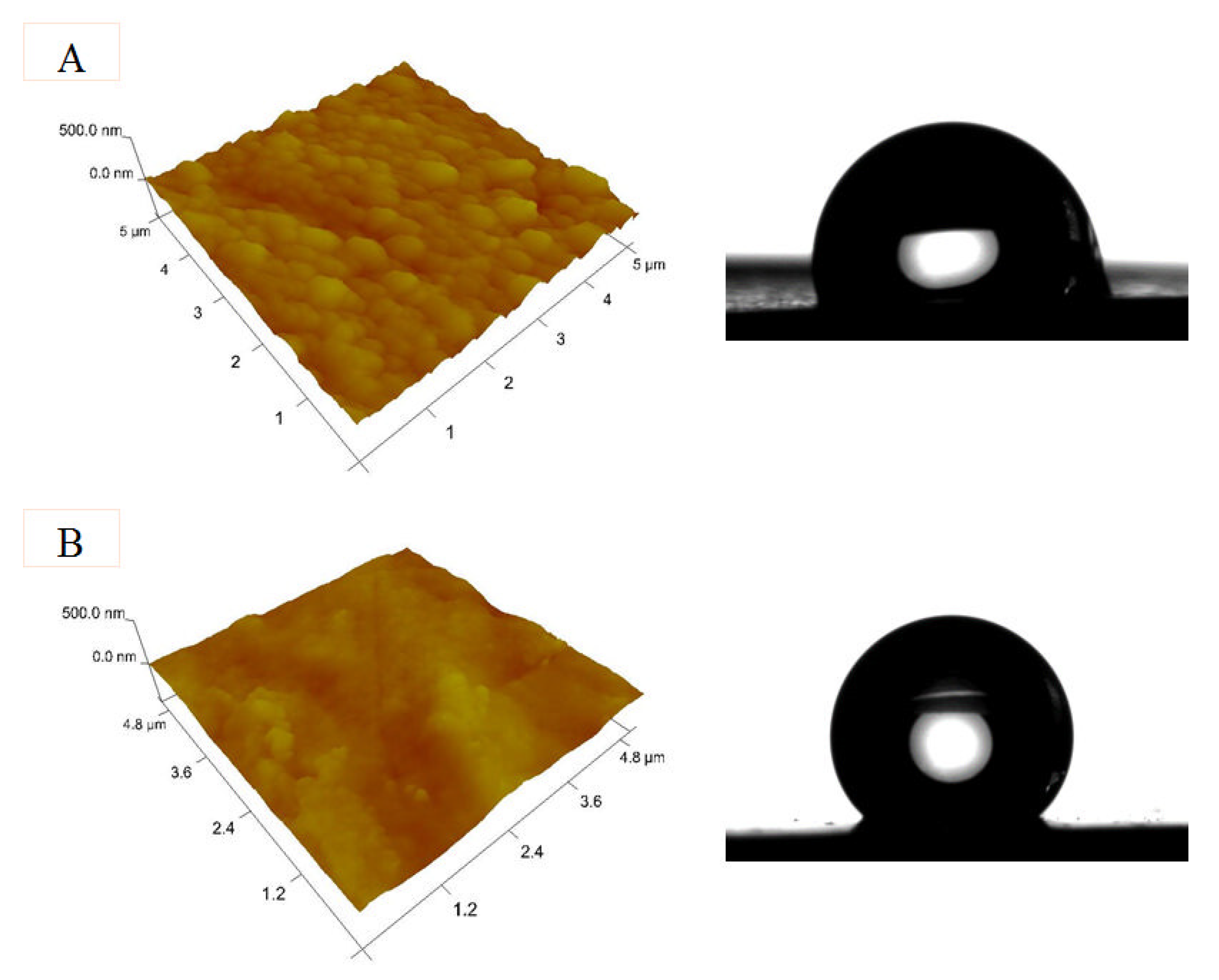
3.5. Atomic Force Microscopy (AFM) and Contact Angle Investigations
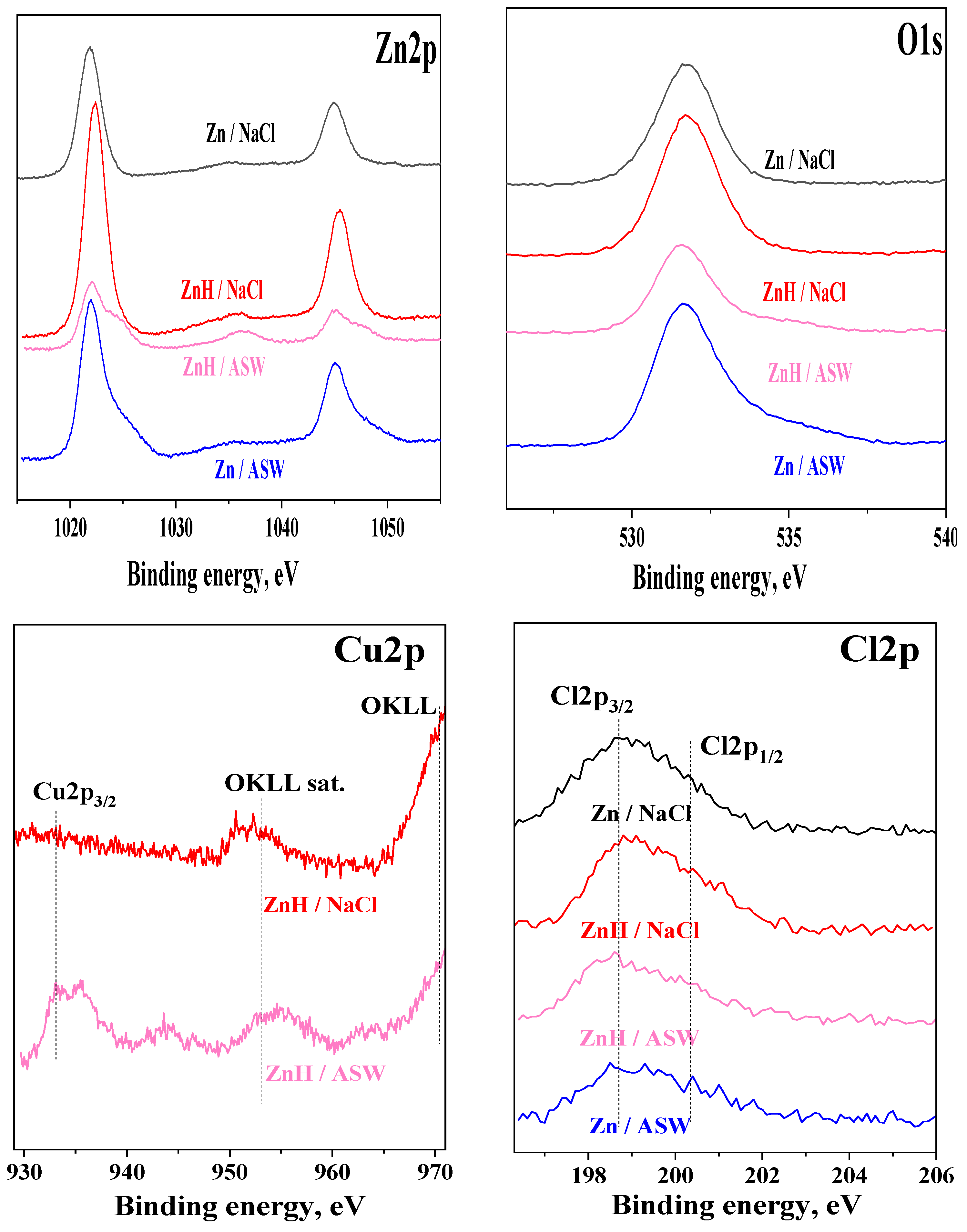
3.6. XPS Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piola, R.F.; Dafforn, K.; Johnston, E. The influence of antifouling practices on marine invasions. Biofouling 2009, 25, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Kruk, T.; Szczepanowicz, K.; Stefańska, J.; Socha, R.P.; Warszyński, P. Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Colloids Surf. B Biointerfaces 2015, 128, 17–22. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Abdoli, L.; Li, H. Participation of copper ions in formation of alginate conditioning layer: Evolved structure and regulated microbial adhesion. Colloids Surf. B Biointerfaces 2018, 162, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Rui, D.; Xiangbo, L.; Jia, W.; Likun, X. Electrochemical corrosion and mathematical model of cold spray Cu-Cu2O coating in NaCl solution—part I: Tafel polarization region model. Int. J. Electrochem. Sci. 2013, 8, 5902–5924. [Google Scholar]
- Maniam, K.; Paul, S. Corrosion Performance of Electrodeposited Zinc and Zinc-Alloy Coatings in Marine Environment. Corros. Mater. Degrad. 2021, 2, 163–189. [Google Scholar] [CrossRef]
- Popoola, P.A.; Malatji, N.; Fayomi, O.S. Fabrication and Properties of Zinc Composite Coatings for Mitigation of Corrosion in Coastal and Marine Zone. In Applied Studies of Coastal and Marine Environments; InTech: London, UK, 2016; pp. 137–144. [Google Scholar] [CrossRef]
- Aruoja, V.; Dubourguier, H.-C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Sengupta, S.; Murmu, M.; Murmu, N.C. Copper oxide as a corrosion inhibitor. Inorg. Anticorros. Mater. 2022, 211–229. [Google Scholar] [CrossRef]
- Deepa, K.; Nayaka, Y.A. Synthesis of CuO micro and nanoparticles as composite additives for corrosion resistant Zn-composite coatings on mild steel. Inorg. Nano-Metal Chem. 2020, 50, 354–360. [Google Scholar] [CrossRef]
- Walsh, F.; Wang, S.; Zhou, N. The electrodeposition of composite coatings: Diversity, applications and challenges. Curr. Opin. Electrochem. 2020, 20, 8–19. [Google Scholar] [CrossRef]
- Finšgar, M.; Fassbender, S.; Nicolini, F.; Milošev, I. Polyethyleneimine as a corrosion inhibitor for ASTM 420 stainless steel in near-neutral saline media. Corros. Sci. 2009, 51, 525–533. [Google Scholar] [CrossRef]
- Sun, Y.; Ata, M.S.; Zhitomirsky, I. Electrophoretic deposition of linear polyethylenimine and composite films. Surf. Eng. 2013, 29, 495–499. [Google Scholar] [CrossRef]
- Turlybekuly, A.; Pogrebnjak, A.D.; Sukhodub, L.F.; Sukhodub, L.B.; Kistaubayeva, A.S.; Savitskaya, I.S.; Shokatayeva, D.H.; Bondar, O.V.; Shaimardanov, Z.K.; Plotnikov, S.V.; et al. Synthesis, characterization, in vitro biocompatibility and antibacterial properties study of nanocomposite materials based on hydroxyapatite-biphasic ZnO micro- and nanoparticles embedded in Alginate matrix. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109965. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Adeleye, A.S.; Page, H.M.; Kui, L.; Lenihan, H.S.; Keller, A.A. Nano and traditional copper and zinc antifouling coatings: Metal release and impact on marine sessile invertebrate communities. J. Nanopart. Res. 2020, 22, 129–144. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Radeva, T. Hybrid Zinc Coating with CuO Nanocontainers Containing Corrosion Inhibitor for Combined Protection of Mild Steel from Corrosion and Biofouling. Coatings 2022, 12, 1254. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Radeva, T.S. Composite coatings with polymeric modified ZnO nanoparticles and nanocontainers with inhibitor for corrosion protection of low carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125741. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Atanassova, G.; Radeva, T.S. Hybrid zinc coatings for corrosion protection of steel using polyelectrolyte nanocontainers loaded with benzotriazole. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 243–250. [Google Scholar] [CrossRef]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and Aggregation of Metal Oxide Nanoparticles in Natural Aqueous Matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Shen, C.; Zheng, S.; Yang, W.; Hu, H.; Liu, J.; Shi, J. Transformation of CuO Nanoparticles in the Aquatic Environment: Influence of pH, Electrolytes and Natural Organic Matter. Nanomaterials 2017, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.S.; Teixeira, M.R. Aggregation kinetics and surface charge of CuO nanoparticles: The influence of pH, ionic strength and humic acids. Environ. Chem. 2013, 10, 313–322. [Google Scholar] [CrossRef]
- Gomes, T.; Pinheiro, J.; Cancio, I.; Pereira, C.; Cardoso, C.; Bebianno, M. Effects of copper nanoparticles exposure in the mussel Mytilus galloprovinciallis. Environ. Sci. Technol. 2011, 45, 9356–9362. [Google Scholar] [CrossRef]
- Shehayeb, S.; Deschanels, X.; Lautru, J.; Ghannam, L.; Odorico, M.; Karamé, I.; Toquer, G. Thin polymeric CuO film from EPD designed for low temperature photothermal absorbers. Electrochim. Acta 2019, 305, 295–303. [Google Scholar] [CrossRef]
- ISO 10289; 2006 Methods for Corrosion Testing of Metallic and Other Inorganic Coatings on Metallic Substrates—Rating of Test Specimens and Manufactured Articles Subjected to Corrosion Tests; CP 401-1214. International Organization for Standardization: Geneva, Switzerland, 2006.
- Singh, J.K.; Mandal, S.; Adnin, R.J.; Lee, H.S.; Yang, H.M. Role of coating processes on the corrosion kinetics and mechanism of zinc in artificial seawater. Materials 2021, 14, 7464. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, L.; Zhang, D.; Dong, C.; Yan, Y.; Volinsky, A.A.; Wang, L.N. Initial formation of corrosion products on pure zinc in saline solution. Bioact. Mater. 2019, 4, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Boshkov, N.; Petrov, K.; Kovacheva, D.; Vitkova, S.; Nemska, S. Influence of the alloying component on the protective ability of some zinc galvanic coatings. Electrochim. Acta 2005, 51, 77–84. [Google Scholar] [CrossRef]
- Boshkov, N.; Petrov, K.; Vitkova, S.; Nemska, S.; Raichevski, G. Composition of the corrosion products of galvanic coatings Zn-Co and their influence on the protective ability. Surf. Coat. Technol. 2002, 157, 171–178. [Google Scholar] [CrossRef]
- Noli, F.; Misaelides, P.; Hatzidimitriou, A.; Pavlidou, E.; Pogrebnjak, A.D. Investigation of the characteristics and corrosion resistance of Al2O3/TiN coatings. Appl. Surf. Sci. 2006, 252, 8043–8049. [Google Scholar] [CrossRef]


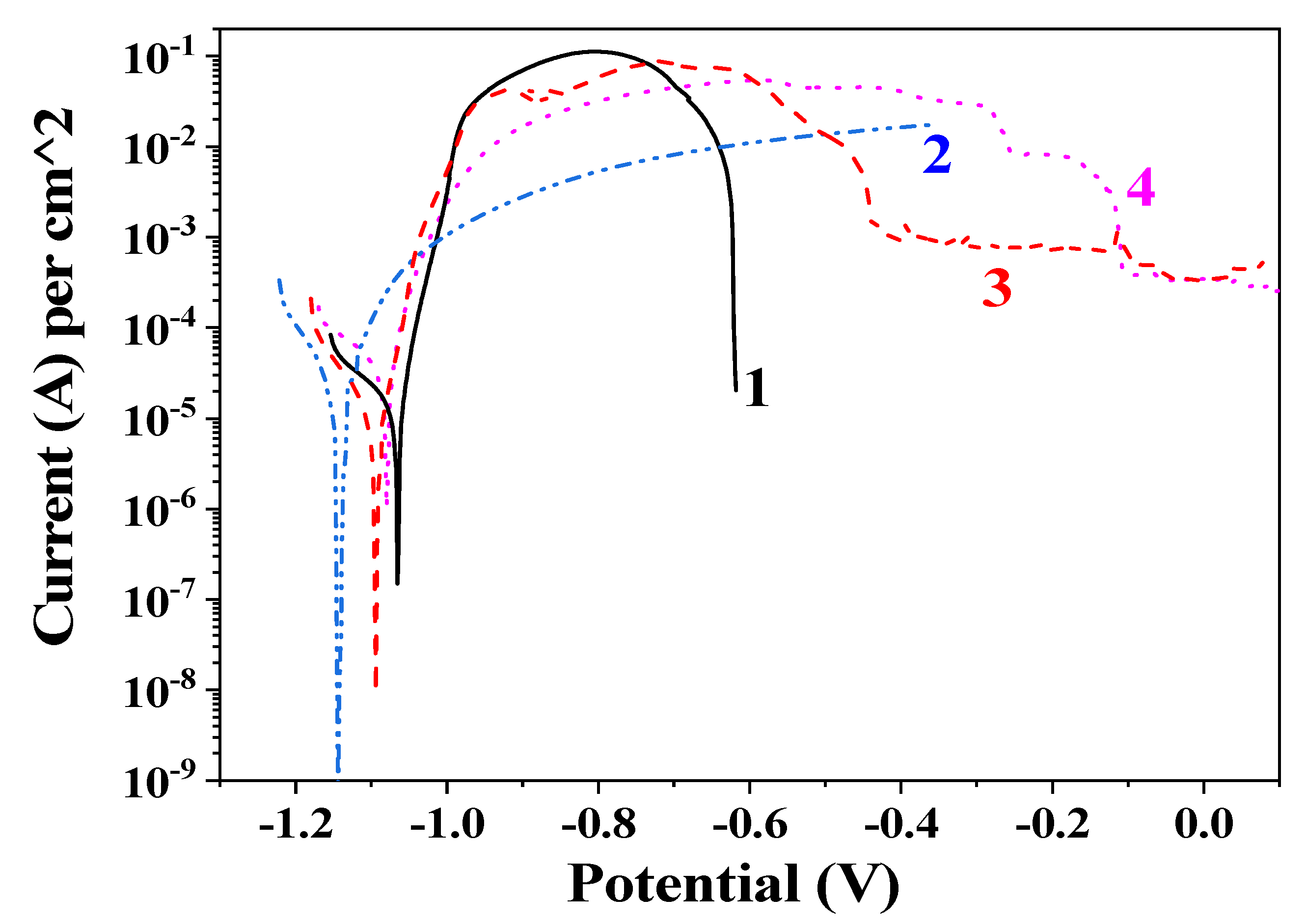

| No. | Sample/Medium | Icorr, A·cm−2 | Ecorr, V | Ipass, A·cm−2 |
|---|---|---|---|---|
| 1 | Zn/5% NaCl | 1.8 × 10−5 | −1.07 | - |
| 2 | Zn/ASW | 2.1 × 10−5 | −1.14 | - |
| 3 | ZnH/5% NaCl | 9.5 × 10−6 | −1.09 | 7.2 × 10−4 |
| 4 | ZnH/ASW | 3.2 × 10−5 | −1.08 | 3.2 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boshkova, N.; Kamburova, K.; Radeva, T.; Simeonova, S.; Grozev, N.; Shipochka, M.; Boshkov, N. Comparative Corrosion Characterization of Hybrid Zinc Coatings in Cl−-Containing Medium and Artificial Sea Water. Coatings 2022, 12, 1798. https://doi.org/10.3390/coatings12121798
Boshkova N, Kamburova K, Radeva T, Simeonova S, Grozev N, Shipochka M, Boshkov N. Comparative Corrosion Characterization of Hybrid Zinc Coatings in Cl−-Containing Medium and Artificial Sea Water. Coatings. 2022; 12(12):1798. https://doi.org/10.3390/coatings12121798
Chicago/Turabian StyleBoshkova, Nelly, Kamelia Kamburova, Tsetska Radeva, Silviya Simeonova, Nikolay Grozev, Maria Shipochka, and Nikolai Boshkov. 2022. "Comparative Corrosion Characterization of Hybrid Zinc Coatings in Cl−-Containing Medium and Artificial Sea Water" Coatings 12, no. 12: 1798. https://doi.org/10.3390/coatings12121798
APA StyleBoshkova, N., Kamburova, K., Radeva, T., Simeonova, S., Grozev, N., Shipochka, M., & Boshkov, N. (2022). Comparative Corrosion Characterization of Hybrid Zinc Coatings in Cl−-Containing Medium and Artificial Sea Water. Coatings, 12(12), 1798. https://doi.org/10.3390/coatings12121798







