Surface Modification of Titanium by Micro-Arc Oxidation in Promoting Schwann Cell Proliferation and Secretion of Neurotrophic Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. MAO-Modified Titanium Surface Preparation
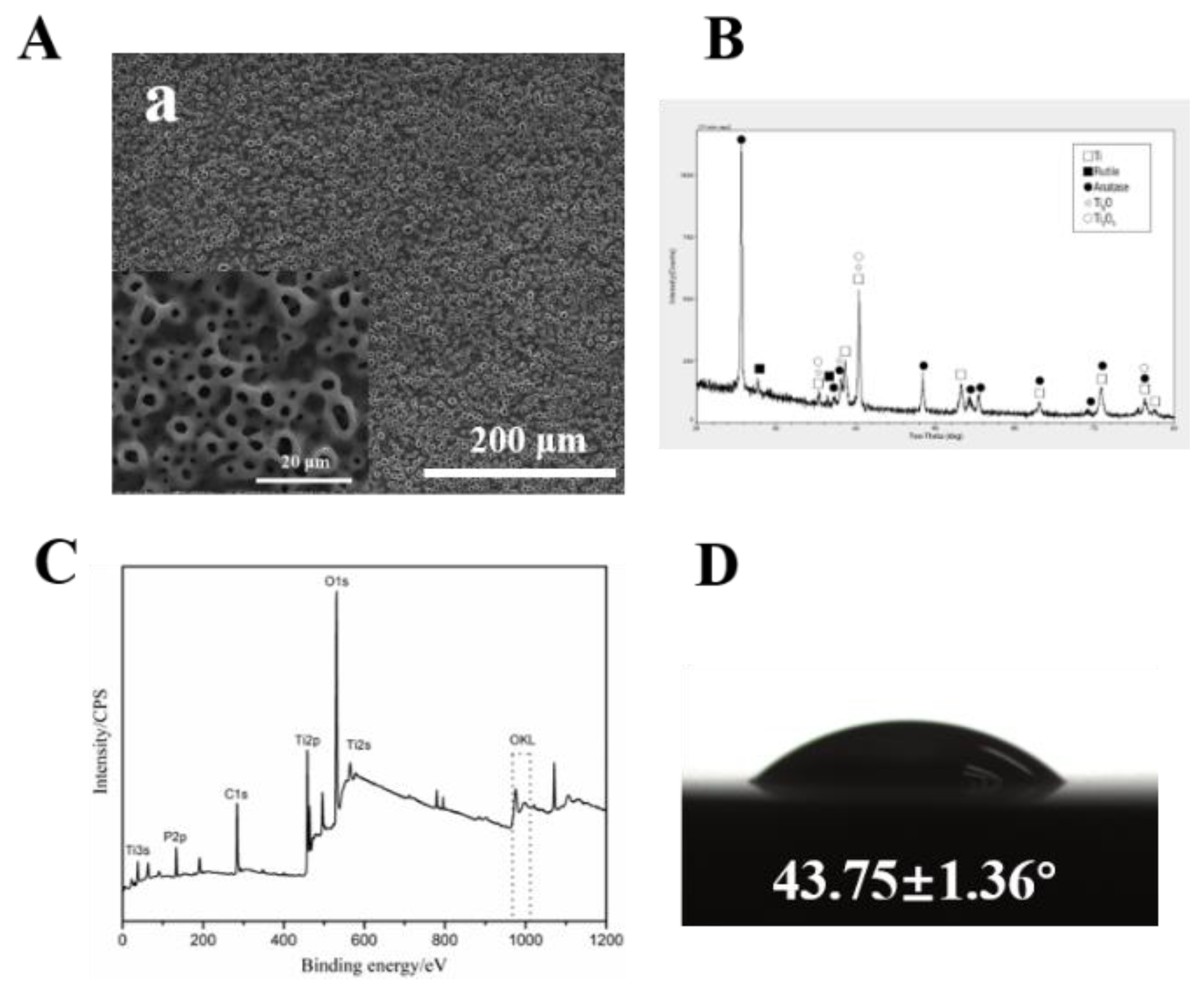
2.2. Surface Characterization
2.2.1. Surface Morphology Measurements
2.2.2. Chemical Composition and X-ray Diffraction Measurements
2.2.3. Wettability Measurements
2.3. Cell Assays
2.3.1. Cell Culture
2.3.2. Cell Viability Assays
2.3.3. Scanning Electron Microscope/Confocal Laser Scanning Microscope Assays
2.4. Molecule Assays
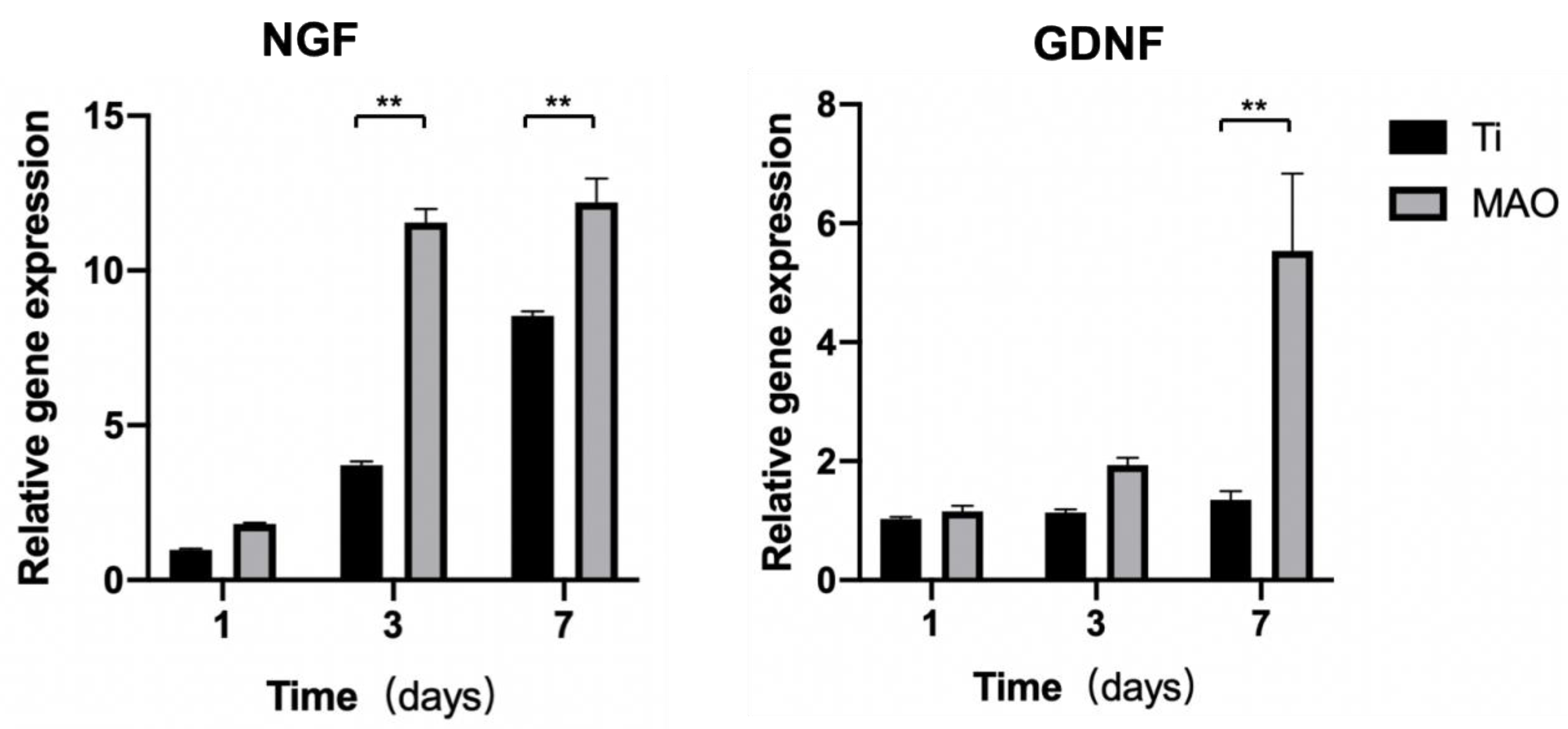
2.4.1. Real-Time PCR
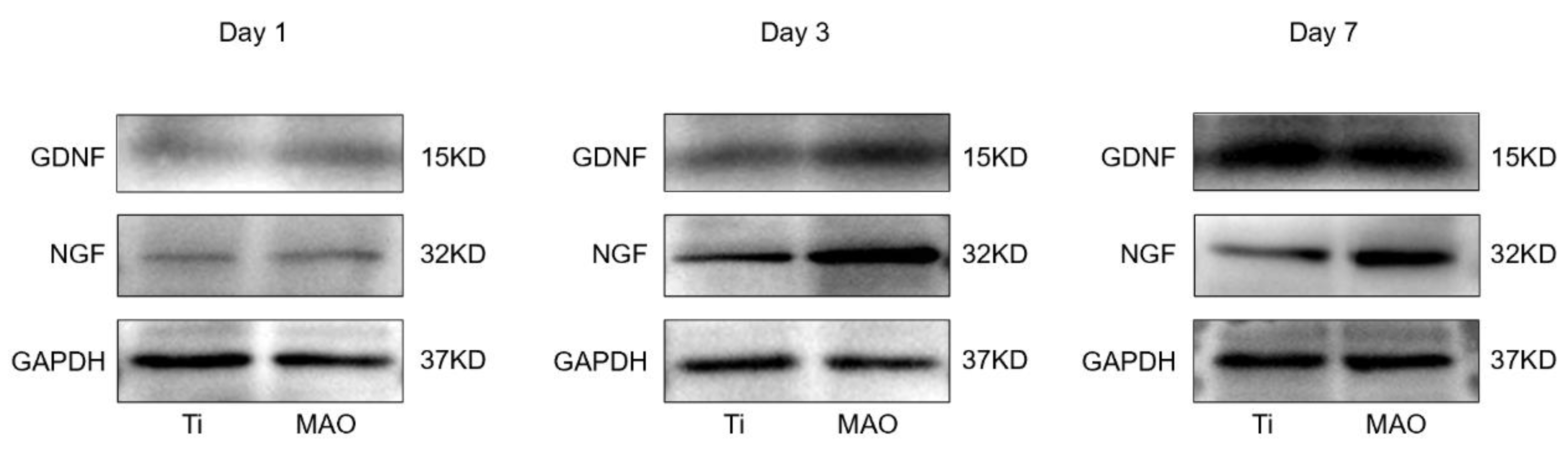
2.4.2. Western Blot
2.5. Statistical Analysis
3. Results
3.1. Surface Characterization
3.2. Cell Assays
3.2.1. Cell Viability and Scanning Electron Microscope Assays
3.2.2. Confocal Laser Scanning Microscope Assays
3.3. Molecules Assays
3.3.1. Real-Time PCR
3.3.2. Western Blot
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, J.; Huang, B.; Gong, P. Nerve Growth Factor-Chondroitin Sulfate/Hydroxyapatite-Coating Composite Implant Induces Early Osseointegration and Nerve Regeneration of Peri-Implant Tissues in Beagle Dogs. J. Orthop. Surg. Res. 2021, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Liang, X.; Zheng, H.; Shujaat, S.; Dessel, J.V.; Zhong, W.; Ma, G.; Lambrichts, I.; Jacobs, R. Peri-Implant Myelinated Nerve Fibers: Histological Findings in Dogs. J. Periodontal Res. 2020, 55, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Ge, X.-Y.; Hao, K.-Y.; Zhang, B.-R.; Jiang, X.; Lin, Y.; Zhang, Y. Simple 3,4-Dihydroxy-L-Phenylalanine Surface Modification Enhances Titanium Implant Osseointegration in Ovariectomized Rats. Sci. Rep. 2017, 7, 17849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-Derived Magnesium Induces Local Neuronal Production of CGRP to Improve Bone-Fracture Healing in Rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef]
- Guglielmotti, M.B.; Olmedo, D.G.; Cabrini, R.L. Research on Implants and Osseointegration. Periodontol 2000 2019, 79, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Negahdari, R.; Ghavimi, M.; Ghanizadeh, M.; Bohlouli, S. Active Tactile Sensibility of Three-Unit Implant-Supported FPDs versus Natural Dentition. J. Clin. Exp. Dent. 2019, 11, e636–e641. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Shujaat, S.; Politis, C.; Orhan, K.; Jacobs, R. Osseoperception Following Dental Implant Treatment: A Systematic Review. J. Oral Rehabil. 2021, 49, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Zaminy, A.; Shokrgozar, M.A.; Sadeghi, Y.; Noroozian, M.; Heidari, M.H.; Piryaei, A. Mesenchymal stem cells as an alternative for Schwann cells in rat spinal cord injury. Iran. Biomed. J. 2013, 17, 113–122. [Google Scholar]
- Li, J.; Xue, S.; Liu, Z.; Yao, D.; Jiang, T. Distribution of Mature and Newly Regenerated Nerve Fibres after Tooth Extraction and Dental Implant Placement: An Immunohistological Study. J. Oral Rehabil. 2022, 49, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; van Dessel, J.; Martens, W.; Lambrichts, I.; Zhong, W.-J.; Ma, G.-W.; Lin, D.; Liang, X.; Jacobs, R. Sensory Innervation around Immediately vs. Delayed Loaded Implants: A Pilot Study. Int. J. Oral Sci. 2015, 7, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, G.; Liu, L.; Wang, Z.; Wang, Y.; Chen, Q.; Luo, E. Inhibition of Osteogenesis Surrounding the Titanium Implant by CGRP Deficiency. Connect. Tissue Res. 2018, 59, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Titanium-Tissue Interface Reaction and Its Control with Surface Treatment. Front. BioEng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, J.; Yan, T.; Han, Y. Fibroblast responses and antibacterial activity of Cu and Zn co-doped TiO2 for percutaneous implants. Appl. Surf. Sci. 2018, 434, 633–642. [Google Scholar] [CrossRef]
- Kang, B.; Lan, D.; Liu, L.; Dang, R.; Yao, C.; Liu, P.; Ma, F.; Qi, S.; Chen, X. Antibacterial Activity and Bioactivity of Zn-Doped TiO2 Coating for Implants. Coatings 2022, 12, 1264. [Google Scholar] [CrossRef]
- Dubový, P.; Klusáková, I.; Hradilová-Svíženská, I.; Joukal, M. Expression of Regeneration-Associated Proteins in Primary Sensory Neurons and Regenerating Axons After Nerve Injury—An Overview. Anat. Rec. 2018, 301, 1618–1627. [Google Scholar] [CrossRef]
- Hopf, A.; Schaefer, D.J.; Kalbermatten, D.F.; Guzman, R.; Madduri, S. Schwann Cell-Like Cells: Origin and Usability for Repair and Regeneration of the Peripheral and Central Nervous System. Cells 2020, 9, 1990. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Liao, D.; Yang, X.; Li, X.; Wei, N.; Tan, Z.; Gong, P. Effect of Implant Surface Microtopography on Proliferation, Neurotrophin Secretion, and Gene Expression of Schwann Cells. J. Biomed. Mater. Res. A 2010, 93, 381–388. [Google Scholar] [CrossRef] [PubMed]
- A, L.; Xu, W.; Zhao, J.; Li, C.; Qi, M.; Li, X.; Wang, L.; Zhou, Y. Surface Functionalization of TiO2 Nanotubes with Minocycline and Its in Vitro Biological Effects on Schwann Cells. Biomed. Eng. Online 2018, 17, 88. [Google Scholar] [CrossRef]
- Mishra, S.K.; Chowdhary, R.; Chrcanovic, B.R.; Brånemark, P.-I. Osseoperception in Dental Implants: A Systematic Review. J. Prosthodont. 2016, 25, 185–195. [Google Scholar] [CrossRef]
- Habre-Hallage, P.; Dricot, L.; Jacobs, R.; van Steenberghe, D.; Reychler, H.; Grandin, C.B. Brain Plasticity and Cortical Correlates of Osseoperception Revealed by Punctate Mechanical Stimulation of Osseointegrated Oral Implants during FMRI. Eur. J. Oral Implantol. 2012, 5, 175–190. [Google Scholar]
- Yan, C.; Ye, L.; Zhen, J.; Ke, L.; Gang, L. Neuroplasticity of Edentulous Patients with Implant-Supported Full Dentures. Eur. J. Oral Sci. 2012, 116, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Diggins, N.H.; Gunderson, Z.J.; Fehrenbacher, J.C.; White, F.A.; Kacena, M.A. No Pain, No Gain? The Effects of Pain-Promoting Neuropeptides and Neurotrophins on Fracture Healing. Bone 2020, 131, 115109. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Zhang, W.; Bai, Y.; Liu, Y.; Meng, J.; Zhang, L. A Magnesium-Incorporated Nanoporous Titanium Coating for Rapid Osseointegration. Int. J. Nanomed. 2020, 15, 6593–6603. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.; Denzer, A.; Cochran, D.; Hoffmann, B.; Lussi, A.; Steinemann, S. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, X.; Zhao, Q.; Xie, Y.; Yao, X.; Wang, M.; Cao, F. Nanofibrous Bicomponent Scaffolds for the Dual Delivery of NGF and GDNF: Controlled Release of Growth Factors and Their Biological Effects. J. Mater. Sci. Mater. Med. 2021, 32, 9. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pu, P.; Su, Z.; Zhang, X.; Nie, L.; Chang, Y. Schwann Cell-derived exosomes promote bone regeneration and repair by enhancing the biological activity of porous Ti6Al4V scaffolds. Biochem. Biophys. Res. Commun. 2020, 531, 559–565. [Google Scholar] [CrossRef] [PubMed]



| Primer | Sequence(5′-3′) |
|---|---|
| GAPDH-F | GGCACAGTCAAGGCTGAGAATG |
| GAPDH-R | ATGGTGGTGAAGACGCCAGTA |
| NGF-F | TCAACAGGACTCACAGGAGCA |
| NGF-R | GGTCTTATCTCCAACCCACACAC |
| GDNF-F | CAGAGGGAAAGGTCGCAGAG |
| GDNF-R | ATCAGTTCCTCCTTGGTTTCGTAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, C.; Xue, S.; Kang, B.; Zhang, X.; Zhong, Q.; Chen, X.; Qi, S. Surface Modification of Titanium by Micro-Arc Oxidation in Promoting Schwann Cell Proliferation and Secretion of Neurotrophic Factors. Coatings 2022, 12, 1797. https://doi.org/10.3390/coatings12121797
Dong C, Xue S, Kang B, Zhang X, Zhong Q, Chen X, Qi S. Surface Modification of Titanium by Micro-Arc Oxidation in Promoting Schwann Cell Proliferation and Secretion of Neurotrophic Factors. Coatings. 2022; 12(12):1797. https://doi.org/10.3390/coatings12121797
Chicago/Turabian StyleDong, Cong, Shenghao Xue, Binbin Kang, Xinyuan Zhang, Qun Zhong, Xiaohong Chen, and Shengcai Qi. 2022. "Surface Modification of Titanium by Micro-Arc Oxidation in Promoting Schwann Cell Proliferation and Secretion of Neurotrophic Factors" Coatings 12, no. 12: 1797. https://doi.org/10.3390/coatings12121797
APA StyleDong, C., Xue, S., Kang, B., Zhang, X., Zhong, Q., Chen, X., & Qi, S. (2022). Surface Modification of Titanium by Micro-Arc Oxidation in Promoting Schwann Cell Proliferation and Secretion of Neurotrophic Factors. Coatings, 12(12), 1797. https://doi.org/10.3390/coatings12121797







