Evaluation of the Long-Term Performance of Marine and Offshore Coatings System Exposed on a Traditional Stationary Site and an Operating Ship and Its Correlation to Accelerated Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Painted Samples
2.2. Description of the Field Sites
2.3. Description of the Accelerated Tests
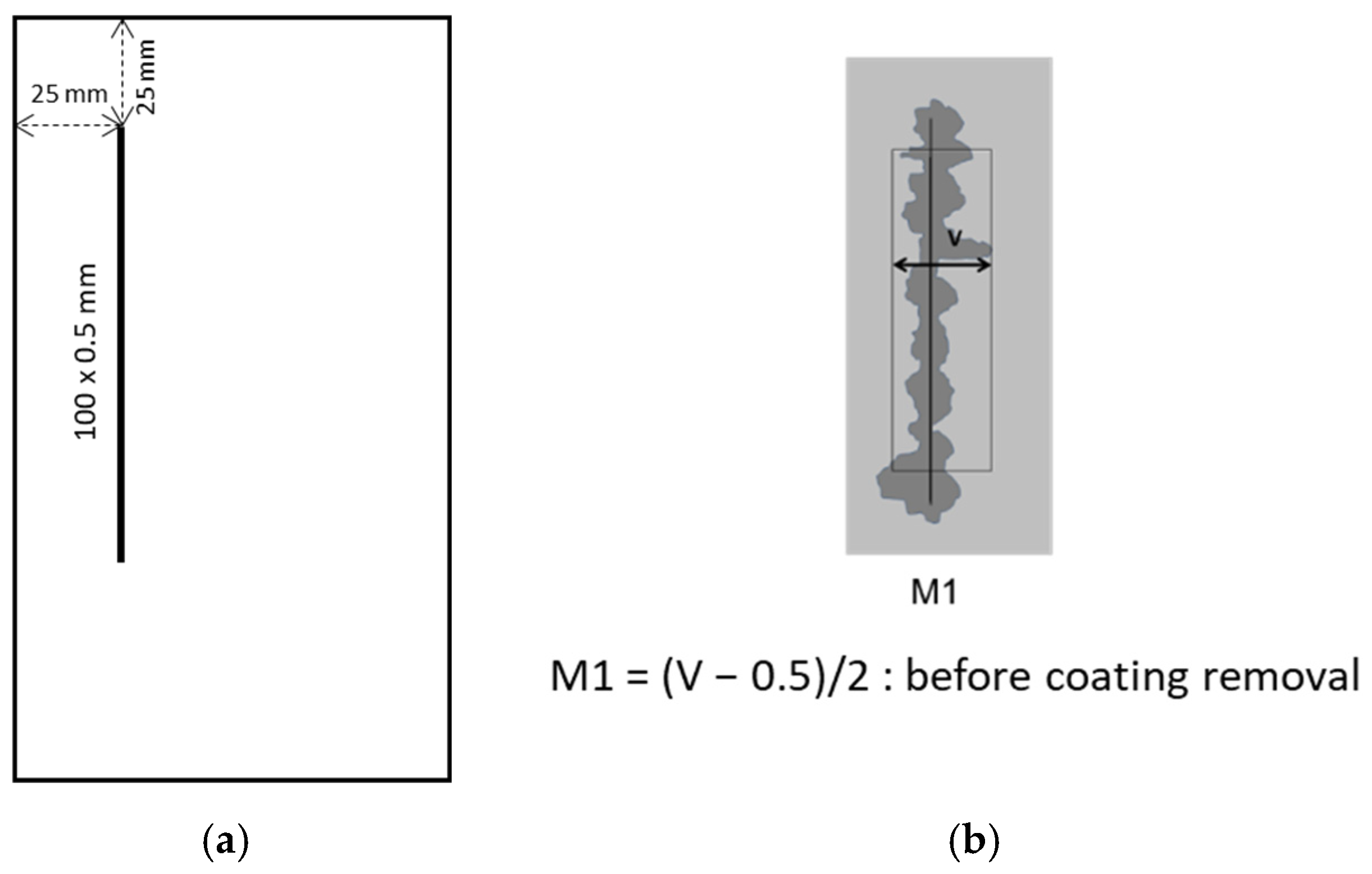
2.4. Sample Preparation and Evaluation
3. Results
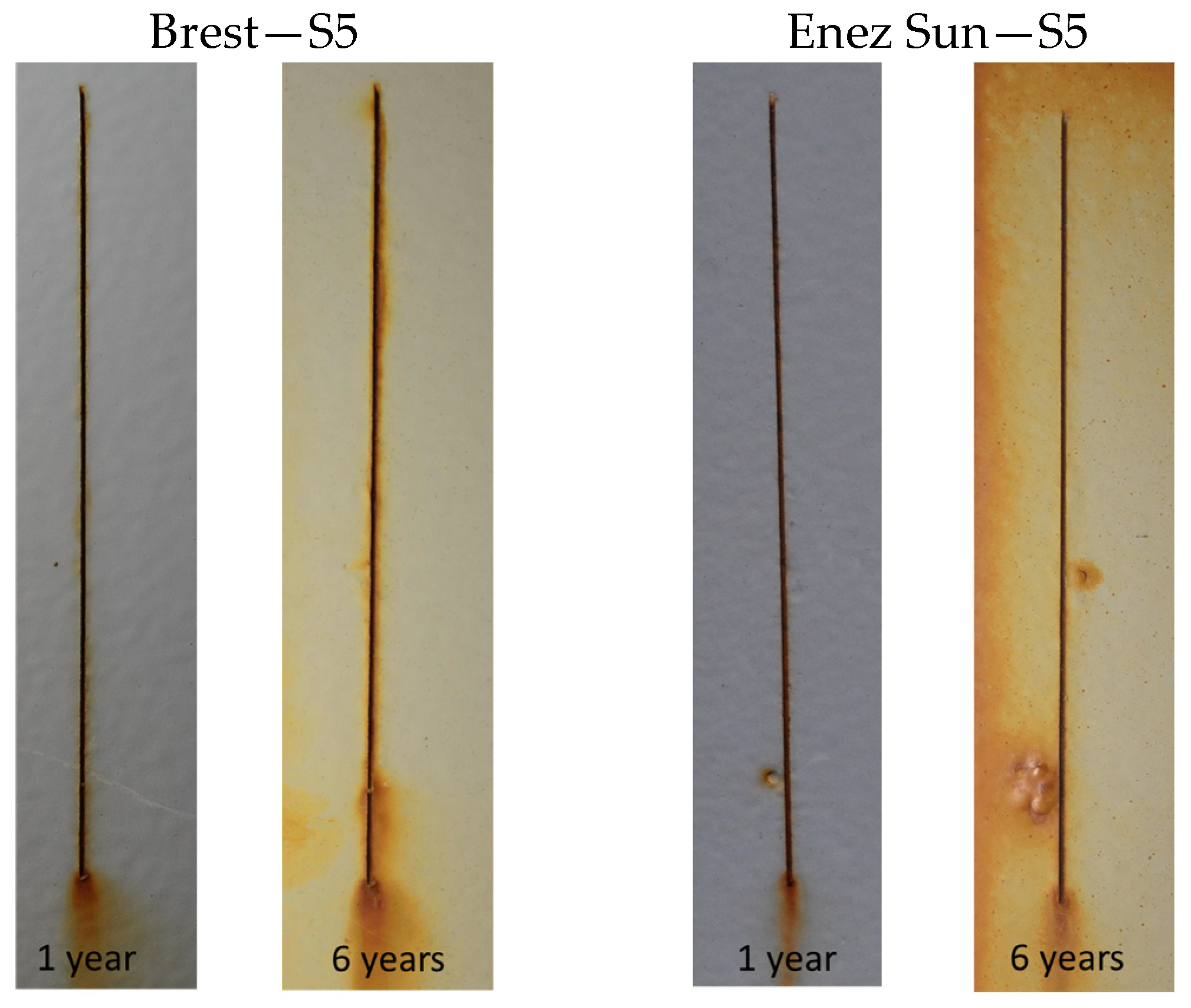
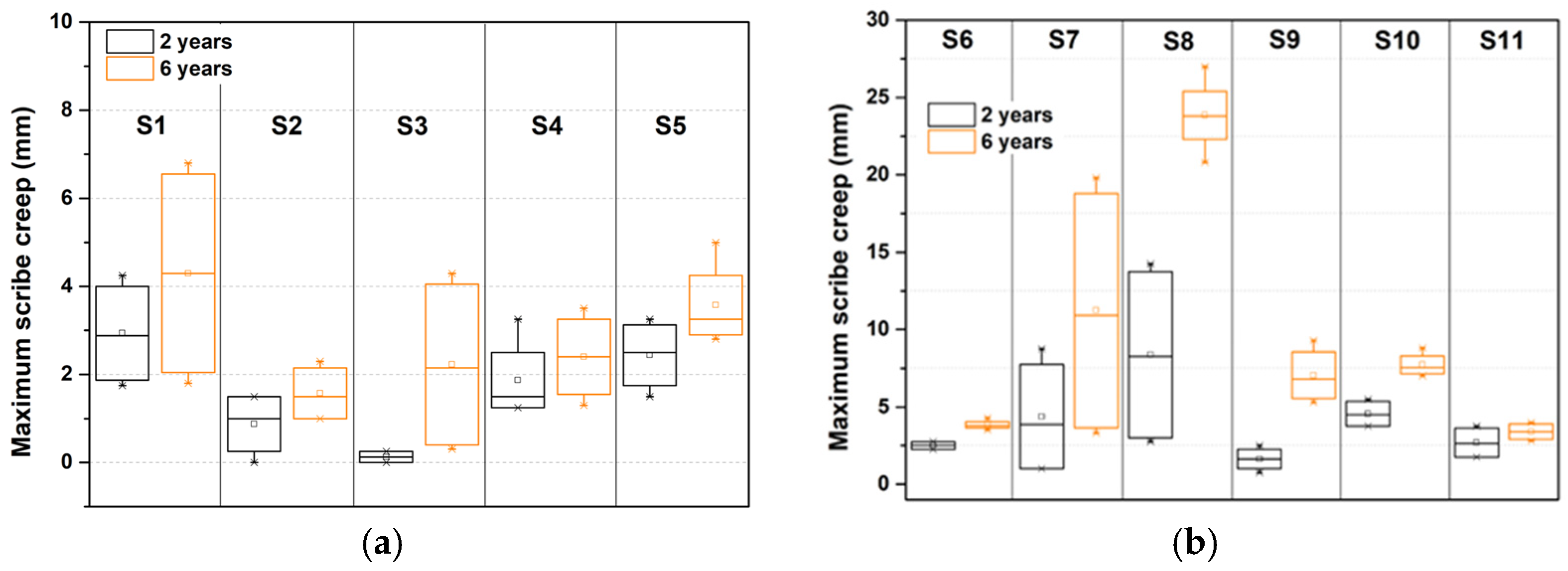
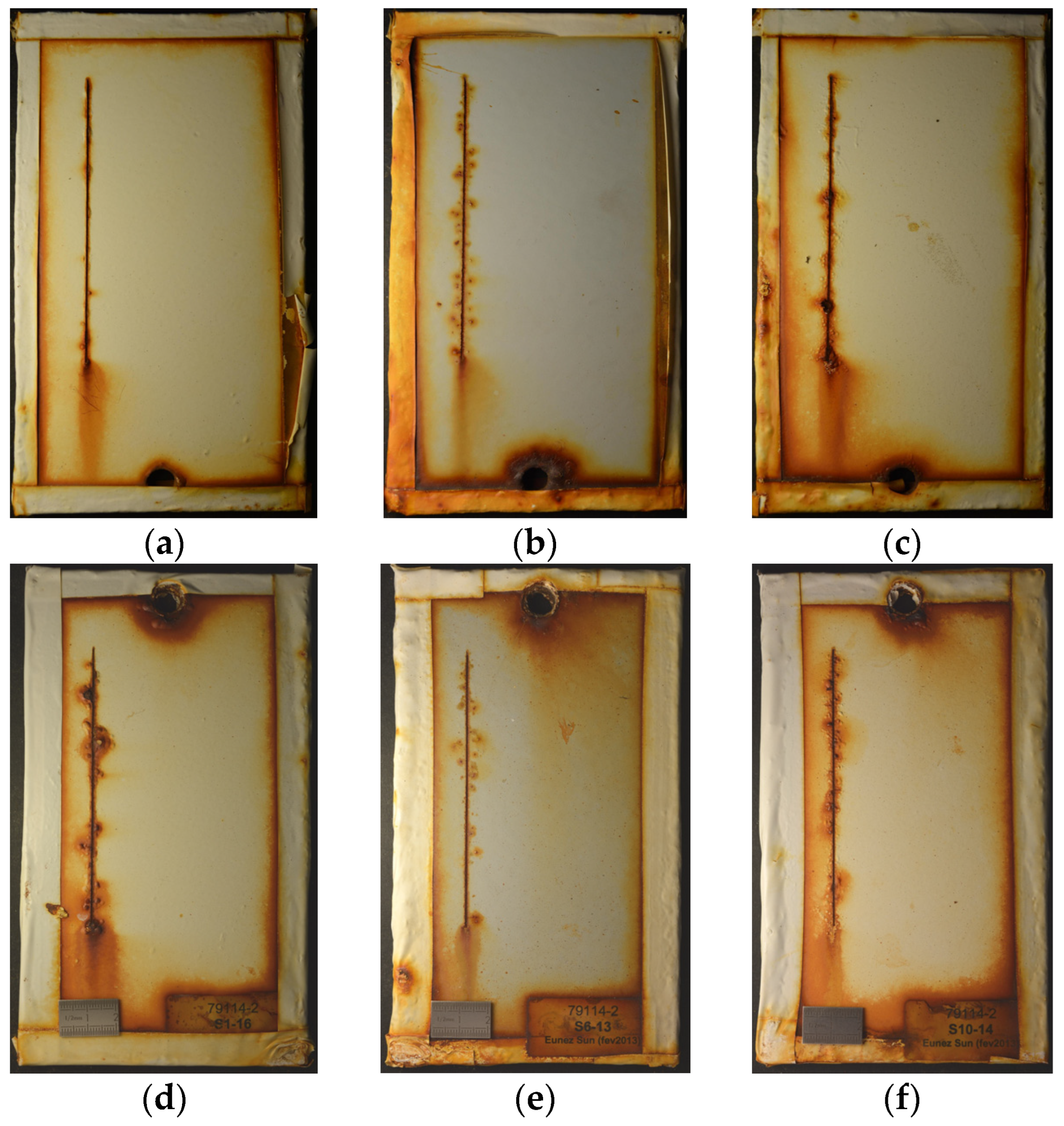
3.1. Field Exposures
3.1.1. Corrosivity
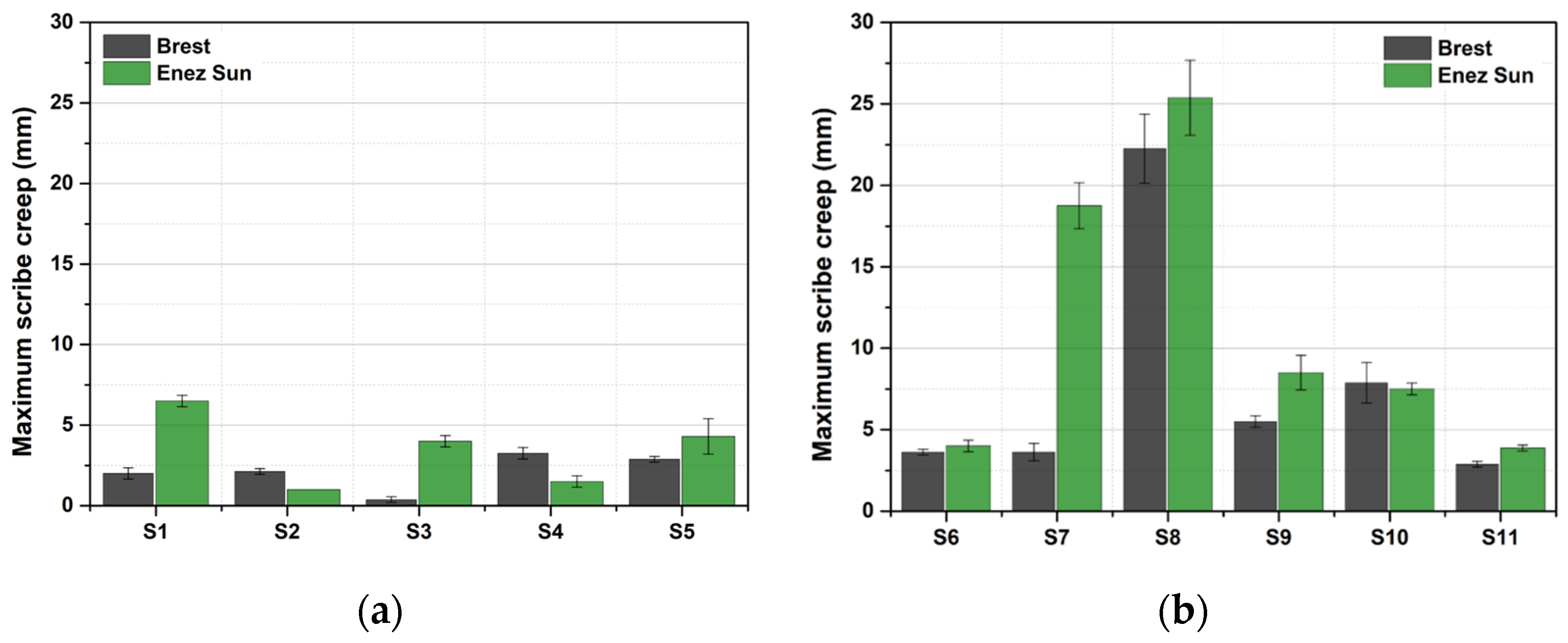
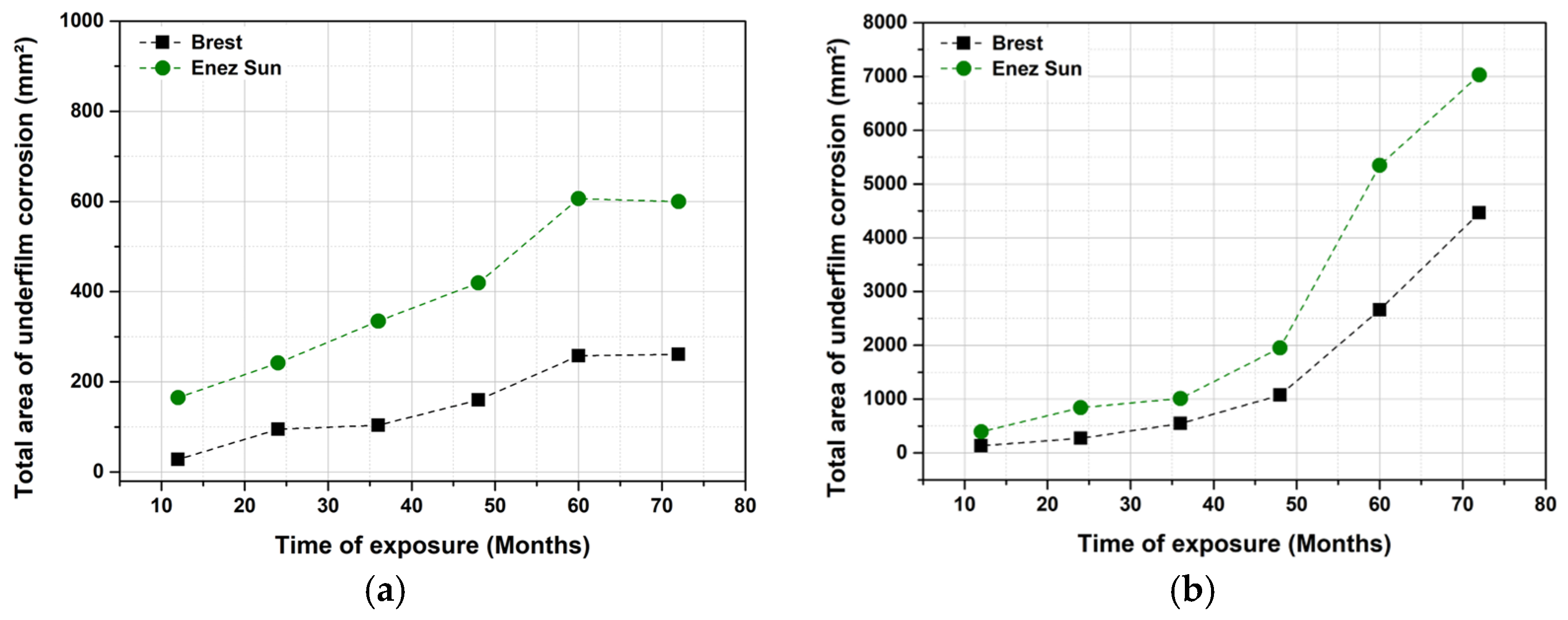
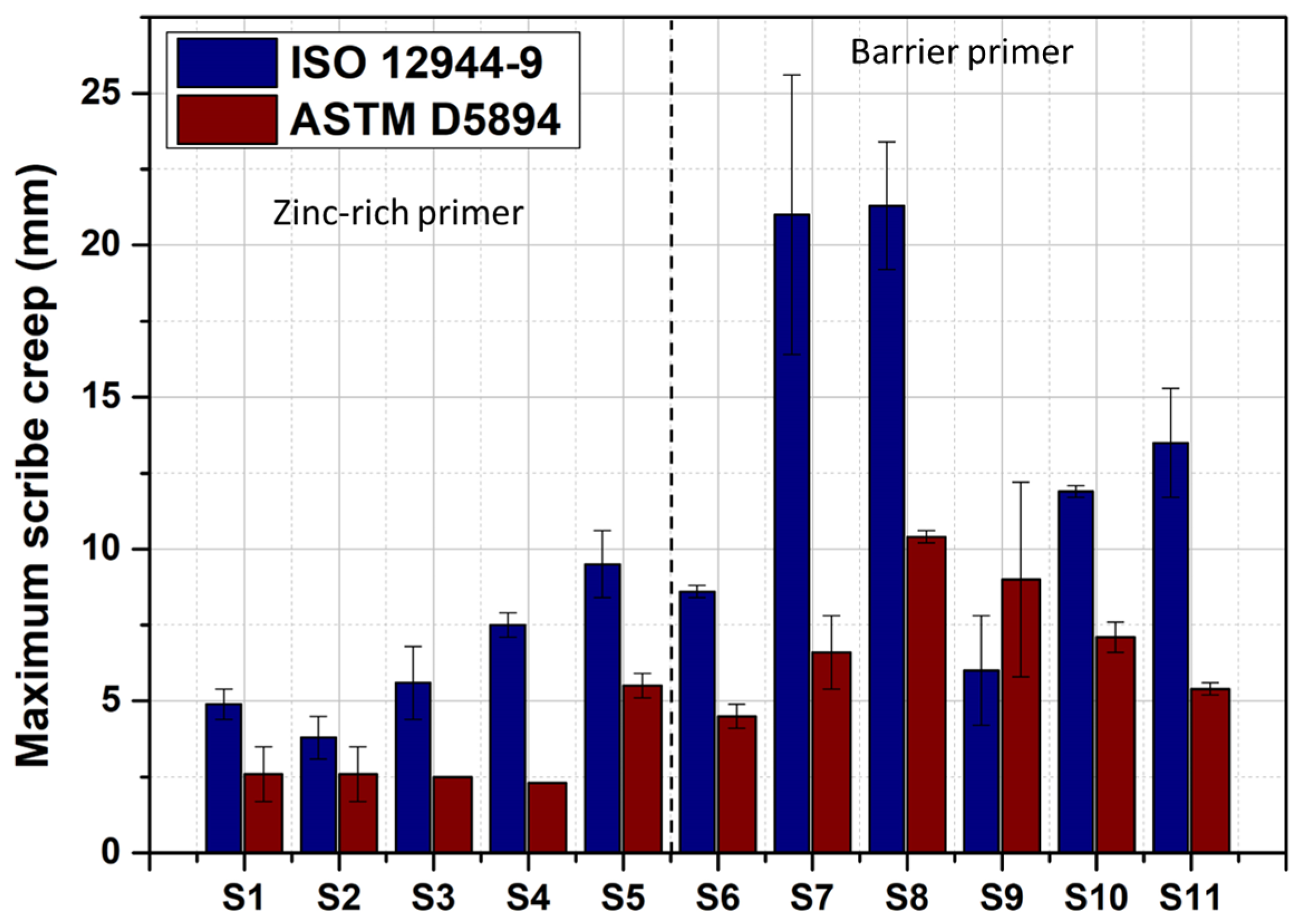
3.1.2. Site Comparison
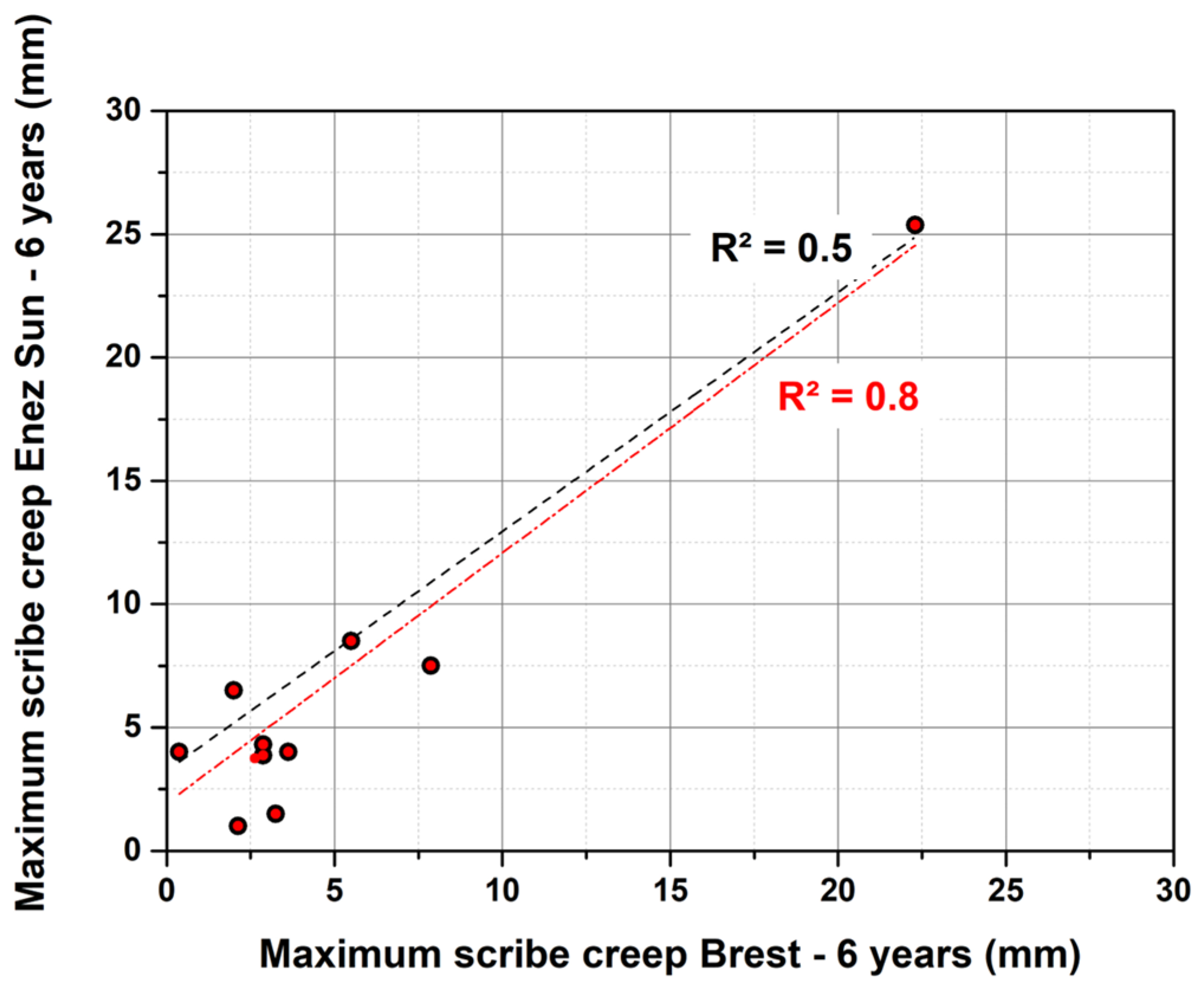
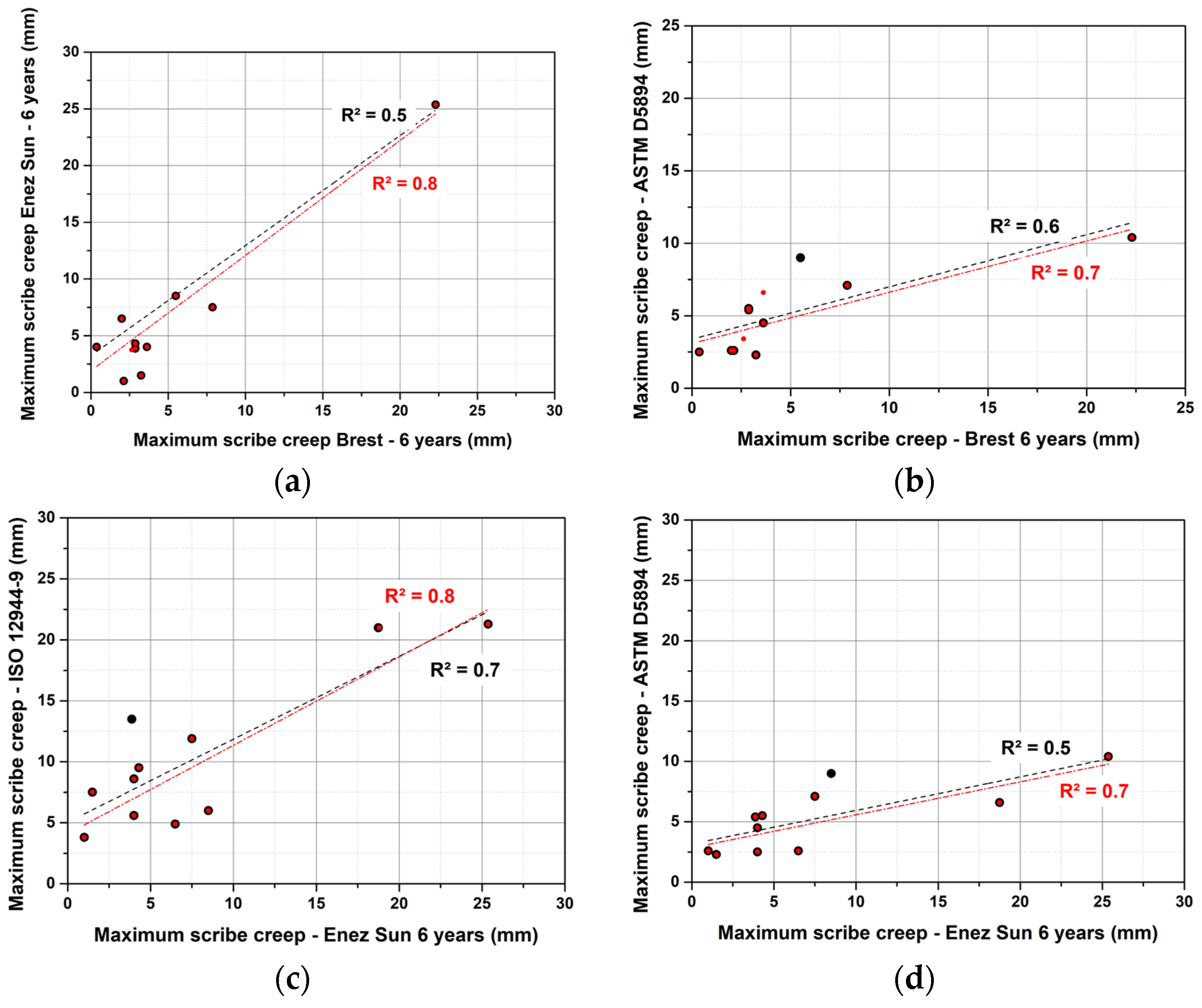
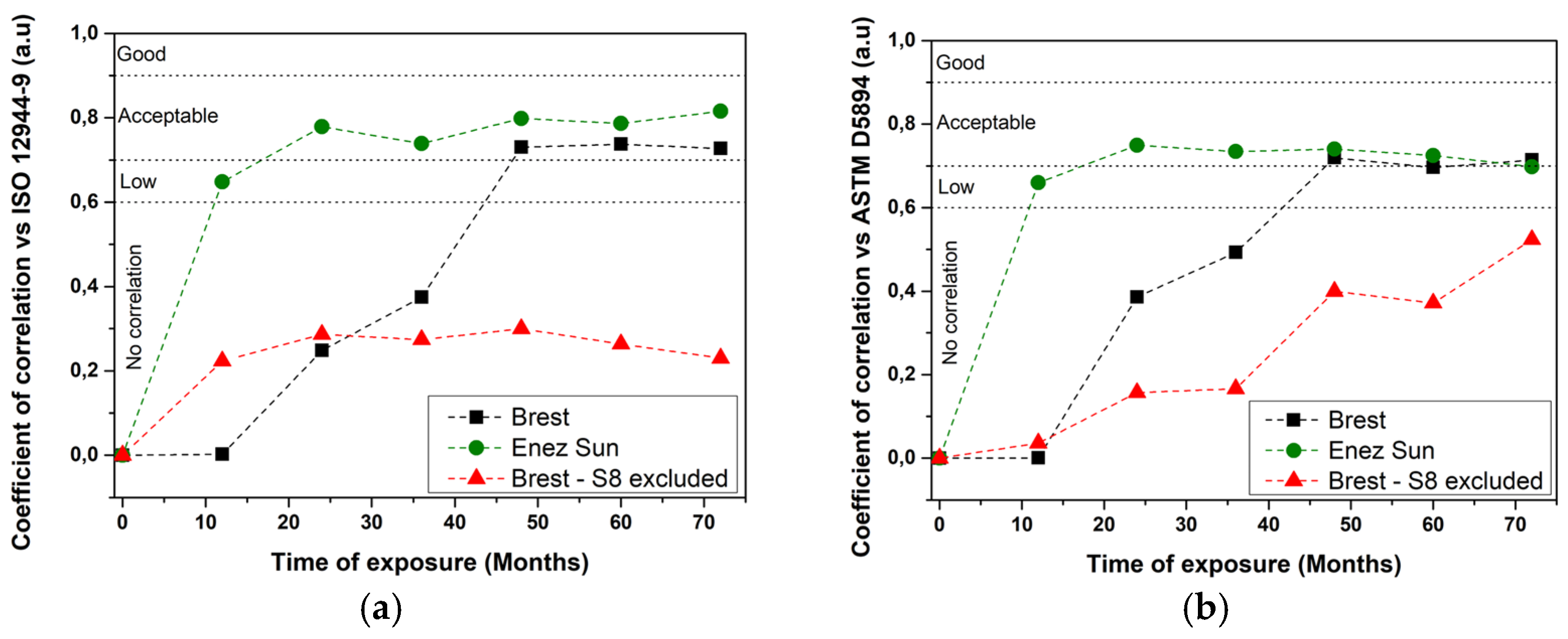
3.2. Correlation between Accelerated Tests and Field Exposures
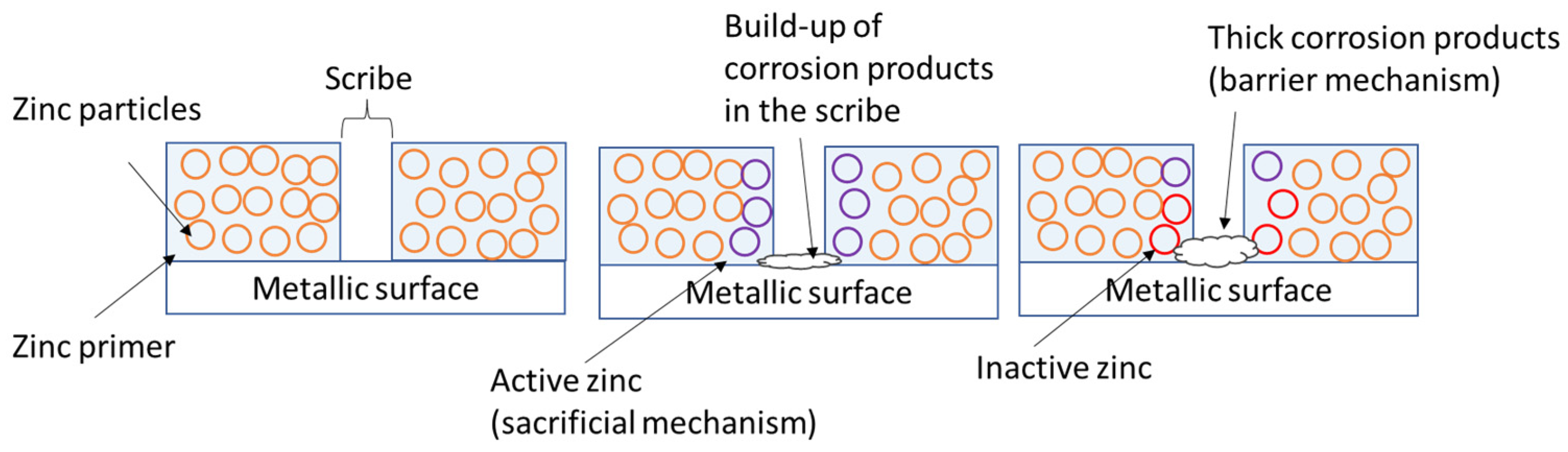
4. Discussion
5. Conclusions
- •
- The operating ship was more aggressive than the stationary site when considering carbon steel mass loss (CX vs. C5), whereas the corrosivity class for zinc was the same for the two sites, with the operating ship showing more corrosion. Coating systems exposed on the operating ship were more degraded than for those on the stationary site. This observation was especially true for coating systems based on the barrier-protection mechanism.
- •
- The zinc primers exhibited a better performance for whatever site was considered. It was shown that zinc primers were more sensitive to environmental factors and that this was system-dependent, at least when considering scribe creep corrosion. Coating systems based on barrier primers exhibited more degradation, and their extent of degradation followed the corrosivity class of the sites (for carbon steel).
- •
- No correlation was observed between the ISO 12944-9 standard and the stationary site, whereas a satisfying correlation was obtained for the real structure. A rather correct correlation between the modified ASTM D5894 standard, which was seen for both types of sites. When looking at the evolution of the correlation over the years of exposure, it was noticed that at least 3 years of exposure were needed for the operating ship to acquire an acceptable correlation with the ISO 12944-9 standard or modified ASTM D5894 standard, whereas at least 5 to 7 years were needed for the stationary site and modified ASTM D5894.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study. NACE Int. 2016, 216, 2–3. [Google Scholar]
- Bierwagen, G.P. Reflections on Corrosion Control by Organic Coatings. Prog. Org. Coat. 1996, 28, 43–48. [Google Scholar] [CrossRef]
- Morales, R.; Hempel, P.; Prieto, A.; Hempel, J.L. Challenging the Performance Myth of Inorganic Zinc Rich vs Organic Zinc Rich Primers and Activated Zinc Primers. In Proceedings of the NACE International’s Corrosion Conference, New Orleans, LA, USA, 26–30 March 2017; p. 9127. [Google Scholar]
- Pélissier, K.; Thierry, D. Powder and High-Solid Coatings as Anticorrosive Solutions for Marine and Offshore Applications? A Review. Coatings 2020, 10, 916. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive Coatings: A Review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Kunce, I.; Królikowska, A.; Komorowski, L. Accelerated Corrosion Tests in Quality Labels for Powder Coatings on Galvanized Steel—Comparison of Requirements and Experimental Evaluation. Materials 2021, 14, 6547. [Google Scholar] [CrossRef]
- Stojanović, I.; Šimunović, V.; Alar, V.; Kapor, F. Experimental Evaluation of Polyester and Epoxy-Polyester Powder Coatings in Aggressive Media. Coatings 2018, 8, 98. [Google Scholar] [CrossRef]
- López-Ortega, A.; Areitioaurtena, O.; Alves, S.A.; Goitandia, A.M.; Elexpe, I.; Arana, J.L.; Bayón, R. Development of a Superhydrophobic and Bactericide Organic Topcoat to Be Applied on Thermally Sprayed Aluminum Coatings in Offshore Submerged Components. Prog. Org. Coat. 2019, 137, 5376. [Google Scholar] [CrossRef]
- Almeida, E.; Santos, D.; Uruchurtu, J. Corrosion Performance of Waterborne Coatings for Structural Steel. Prog. Org. Coat. 1999, 37, 131–140. [Google Scholar] [CrossRef]
- López-Ortega, A.; Bayón, R.; Arana, J.L. Evaluation of Protective Coatings for High-Corrosivity Category Atmospheres in Offshore Applications. Materials 2019, 12, 1325. [Google Scholar] [CrossRef]
- ISO 12944-6; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems—Part 6: Laboratory Performance Test Methods. ISO Standard: Brussels, Belgium, 1998.
- ISO 12944-2; Paint and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems—Part 2: Classification of Environments. ISO Standard: Brussels, Belgium, 2017.
- Lebozec, N.; Blandin, N.; Thierry, D. Accelerated Corrosion Tests in the Automotive Industry: A Comparison of the Performance towards Cosmetic Corrosion. Mater. Corros. 2008, 59, 889–894. [Google Scholar] [CrossRef]
- LeBozec, N.; Thierry, D.; le Calvé, P.; Favennec, C.; Pautasso, J.P.; Hubert, C. Performance of Marine and Offshore Paint Systems: Correlation of Accelerated Corrosion Tests and Field Exposure on Operating Ships. Mater. Corros. 2015, 66, 215–225. [Google Scholar] [CrossRef]
- Lebozec, N.; Thierry, D.; le Calvé, P.; Pautasso, J.-P. Influence of Climatic Factors (UV, Salt Concentration, Wet-Dry Cycle) in Cyclic Corrosion Tests Used for Marine Paint Systems. In Proceedings of the Eurocorr, Stockholm, Sweden, 4–8 September 2011; p. 4630. [Google Scholar]
- ISO 12944-9; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems—Part 9: Protective Paint Systems and Laboratory Performance Test Methods for Offshore and Related Structures. ISO Standard: Brussels, Belgium, 2018.
- Knudsen, O.O.; Steinsmo, U.; Bjordal, M.; Nijjer, S. Correlation between Four Accelerated Tests and Five Years of Offshore Field Testing. J. Prot. Coat. Linings 2001, 12, 52–56. [Google Scholar]
- Ward, D. Correlation of Accelerated Corrosion Testing with Natural Exposure after 6+ Years in a Costal Environment. In Proceedings of the NACE International’ s Corrosion Conference, New Orleans, LA, USA, 16–20 March 2008; p. 08003. [Google Scholar]
- LeBozec, N.; Thierry, D.; Pelissier, K. A New Accelerated Corrosion Test for Marine Paint Systems Used for Ship’s Topsides and Superstructures. Mater. Corros. 2018, 69, 447–459. [Google Scholar] [CrossRef]
- LeBozec, N.; Carter, J.; Scholz, T.; Knudsen, O.O.; Flogård, A. Round-Robin Evaluation of ISO 20340 Annex A Test Method. In Proceedings of the NACE International’s Corrosion Conference, Vancouver, BC, Canada, 6–10 March 2016; p. 6991. [Google Scholar]
- ISO 8503-1; Surface Roughness Characteristics of Blast-Cleaned Steel Substrates. ISO Standard: Brussels, Belgium, 1998.
- Lebozec, N.; Pautasso, J.-P.; Le Calve, P.; Thierry, D. Assessment of the Aggressiveness and Environmental Conditions on Ships. In Proceedings of the Eurocorr, Nice, France, 6–10 September 2009; p. 7745. [Google Scholar]
- ISO 8407; Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens. ISO Standard: Brussels, Belgium, 2021.
- Lebozec, N.; Thierry, D. Performance of Marine Paint Systems on Operating Ships and Outdoor Marine Sites. In Proceedings of the Eurocorr, Stockholm, Sweden, 4–8 September 2011; p. 4629. [Google Scholar]
- ASTM D5894; Standard Practice for Cyclic Salt FogUV Exposure of Painted Metal, Alternating Exposures in a FogDry Cabinet and a UVCondensation Cabinet. ASTM Standard: West Conshohocken, PA, USA, 2010.
- NACE TM 0304; Offshore Platform Atmospheric and Splash Zone Maintenance Coating System Evaluation. NACE International: Houston, TX, USA, 2004.
- NACE TM 0404; Offshore Platform Atmospheric and Splash Zone New Construction Coating System Evaluation. NACE International: Houston, TX, USA, 2004.
- ASTM D1141; Standard Practice for the Preparation of Substitute Ocean Water. ASTM Standard: West Conshohocken, PA, USA, 2008.
- ISO 4628-2; Paints and Varnishes—Evaluation of Degradation of Coatings—Blistering. ISO Standard: Brussels, Belgium, 2016.
- ISO 4628-3; Paints and Varnishes—Evaluation of Degradation of Coatings—Rusting. ISO Standard: Brussels, Belgium, 2016.
- ISO 4628-4; Paints and Varnishes—Evaluation of Degradation of Coatings—Cracking. ISO Standard: Brussels, Belgium, 2016.
- ISO 4628-6; Paints and Varnishes—Evaluation of Degradation of Coatings—Chalking Tape. ISO Standard: Brussels, Belgium, 2011.
- ISO 12944-1; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems—Part 1 General Introduction. ISO Standard: Brussels, Belgium, 2017.
- Knudsen, O.; Skilbred, A.W.B.; Løken, A.; Daneshian, B.; Höche, D. Correlations between Standard Accelerated Tests for Protective Organic Coatings and Field Performance. Mater. Today Commun. 2022, 31, 103729. [Google Scholar] [CrossRef]
- Akbarinezhad, E.; Faridi, H.R.; Ghanbarzadeh, A. Evaluation of Zinc Rich Ethyl Silicate Primer by Applying Electrochemical Methods. Surf. Eng. 2009, 25, 163–166. [Google Scholar] [CrossRef]
- Knudsen, O.Ø.; Steinsmo, U.; Bjordal, M. Zinc-Rich Primers—Test Performance and Electrochemical Properties. Prog. Org. Coat. 2005, 54, 224–229. [Google Scholar] [CrossRef]
- Nazarov, A.; le Bozec, N.; Thierry, D. Scanning Kelvin Probe Assessment of Steel Corrosion Protection by Marine Paints Containing Zn-Rich Primer. Prog. Org. Coat. 2018, 125, 61–72. [Google Scholar] [CrossRef]
- Morcillo, M.; Barajas, R.; Feliu, S.; Bastidas, J.M. A SEM Study on the Galvanic Protection of Zinc-Rich Paints. J. Mater. Sci. 1990, 25, 2441–2446. [Google Scholar] [CrossRef]
- Renaud, A.; Pommier, V.; Garnier, J.; Frappart, S.; Florimond, L.; Koch, M.; Grolleau, A.-M.; Puente-Lelièvre, C. Touzain Sébastien Aggressiveness of Different Ageing Conditions for Three Thick Marine Epoxy Systems. Corros. Mater. Degrad. 2021, 2, 721–742. [Google Scholar] [CrossRef]
- Abreu, C.M.; Izquierdo, M.; Merino, P.; Nóvoa, X.R.; Pérez, C. A New Approach to the Determination of the Cathodic Protection Period in Zinc-Rich Paints. Corros. Eng. Sect. 1999, 55, 1173–1181. [Google Scholar] [CrossRef]
- Nazarov, A.; le Bozec, N.; Thierry, D. Assessment of Steel Corrosion and Deadhesion of Epoxy Barrier Paint by Scanning Kelvin Probe. Prog. Org. Coat. 2018, 114, 123–134. [Google Scholar] [CrossRef]
- Nazarov, A.; Le Bozec, N.; Thierry, D.; Le Calvé, P.; Pautasso, J.-P. Scanning Kelvin Probe Investigation of Corrosion Under Thick Marine Paint Systems Applied on Carbon Steel. Corrosion 2012, 68, 720–729. [Google Scholar] [CrossRef]
- Binder, G.J. Determine Usable Coating Systems—Results of Laboratory Tests versus Long Term Trials in Nature. In Proceedings of the NACE International’s Corrosion Conference, New Orleans, LA, USA, 16–20 March 2008; p. 08001. [Google Scholar]
- Lebozec, N.; Hall, C.; Melot, D. Comparison of Accelerated Ageing Tests as per ISO 20340 Annex A and NACE SP0108 Standards. In Proceedings of the NACE International’s Corrosion Conferece, San Antonio, TX, USA, 9–13 March 2014; p. 3762. [Google Scholar]
- Saeedikhani, M.; Wijesinghe, S.; Blackwood, D.J. Revisiting Corrosion Protection Mechanisms of a Steel Surface by Damaged Zinc-Rich Paints. Corrosion 2019, 75, 756–770. [Google Scholar] [CrossRef]
- Amirudin, A.; Thierry, D. Application of Electrochemical Impedance Spectroscopy to Study the Degradation of Polymer-Coated Metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Pommersheim, J.M.; Nguyen, T. Prediction of Blistering in Coating Systems. ACS Symp. Ser. 1998, 689, 137–150. [Google Scholar] [CrossRef]

| Carbon | Manganese | Phosphorous | Silicon | Sulfur |
|---|---|---|---|---|
| 0.18% | 0.9%–1.6% | 0.04% | 0.1%–0.5% | 0.035% |
| System | Type of Layer | Thickness (µm) | Type of Protection |
|---|---|---|---|
| S1 | Zinc ethyl silicate (76%) | 85 | Sacrificial |
| Micaceous iron oxide-pigmented epoxy | 40 | ||
| High-build epoxy | 150 | ||
| Aliphatic acrylic polyurethane | 50 | ||
| S2 | Epoxy zinc rich (81%) | 75 | Sacrificial |
| Epoxy with micaceous iron oxide | 150 | ||
| High-build epoxy | 75 | ||
| S3 | Epoxy zinc rich (80%) | 75 | Sacrificial |
| Epoxy aluminium | 200 | ||
| Polyamine epoxy | 75 | ||
| High solid silicone alkyd | 50 | ||
| S4 | H2O epoxy zinc rich (≈25%) | 50 | Sacrificial |
| H2O epoxy zinc rich | 120 | ||
| Waterborne acrylic | 80 | ||
| S5 | Epoxy zinc rich (80%) | 60 | Sacrificial |
| High-solid polyamide epoxy | 100 | ||
| High-solid polyamide epoxy | 100 | ||
| Aliphatic polyurethane with zinc phosphate | 50 | ||
| S6 | Epoxy aluminium | 200 | Barrier |
| Vinylic epoxy | 100 | ||
| Acrylic | 50 | ||
| S7 | Epoxy aluminium | 200 | Barrier |
| Epoxy aluminium | 200 | ||
| Polyamine epoxy | 50 | ||
| Polyurethane | 50 | ||
| S8 | Epoxy with glass flakes | 325 | Barrier |
| Epoxy with glass flakes | 300 | ||
| Aliphatic polyurethane with zinc phosphate | 60 | ||
| S9 | Polyamide epoxy with zinc phosphate | 50 | Barrier |
| Polyamide epoxy with glass flakes | 400 | ||
| S10 | Polyamine epoxy | 60 | Barrier |
| High-build polyamide epoxy | 100 | ||
| Aliphatic acrylic polyurethane | 50 | ||
| S11 | Epoxy aluminium | 150 | Barrier |
| Epoxy aluminium | 150 | ||
| Aliphatic polyurethane with zinc phosphate | 50 |
| Max T °C * | Min T °C * | Mean T °C ** | Min RH% *** | Max RH% *** | Mean RH% ** | Chloride Deposition (mg/m2,d) ** |
|---|---|---|---|---|---|---|
| 15.5 | 8.5 | 12.4 | 41.2 | 98.5 | 82.3 | 2535 |
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
|---|---|---|---|---|---|---|
| UV/Condensation–ISO 16474−3 4 h of UVA−340 at 0.83 W/m2/mm at 60 °C 4 h of condensation at 50 °C | Neutral Salt Spray Test–ISO 9227 5% NaCl | Freezing Phase (−20 ± 2) °C | ||||
| Week 1 | Week 2 |
|---|---|
| UVA 340 nm 60 °C 4 h/condensation 50 °C 4 h | 1 h salt spray (synthetic seawater) at 35 °C 1 h dry off at 35 °C |
| Site | C-Steel Mass Loss (g·m−2) | Corrosivity Class for C-Steel | Zinc Mass Loss (g·m−2) | Corrosivity Class for Zinc |
|---|---|---|---|---|
| Brest | 660 ± 15 | C5 | 6.0 ± 0.8 | C3 |
| Enez Sun | 1560.3 ± 233.3 | CX | 11.8 ± 1.5 | C3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pélissier, K.; Le Bozec, N.; Thierry, D.; Larché, N. Evaluation of the Long-Term Performance of Marine and Offshore Coatings System Exposed on a Traditional Stationary Site and an Operating Ship and Its Correlation to Accelerated Test. Coatings 2022, 12, 1758. https://doi.org/10.3390/coatings12111758
Pélissier K, Le Bozec N, Thierry D, Larché N. Evaluation of the Long-Term Performance of Marine and Offshore Coatings System Exposed on a Traditional Stationary Site and an Operating Ship and Its Correlation to Accelerated Test. Coatings. 2022; 12(11):1758. https://doi.org/10.3390/coatings12111758
Chicago/Turabian StylePélissier, Krystel, Nathalie Le Bozec, Dominique Thierry, and Nicolas Larché. 2022. "Evaluation of the Long-Term Performance of Marine and Offshore Coatings System Exposed on a Traditional Stationary Site and an Operating Ship and Its Correlation to Accelerated Test" Coatings 12, no. 11: 1758. https://doi.org/10.3390/coatings12111758
APA StylePélissier, K., Le Bozec, N., Thierry, D., & Larché, N. (2022). Evaluation of the Long-Term Performance of Marine and Offshore Coatings System Exposed on a Traditional Stationary Site and an Operating Ship and Its Correlation to Accelerated Test. Coatings, 12(11), 1758. https://doi.org/10.3390/coatings12111758







