Antibacterial Effect of Polymethyl Methacrylate Resin Base Containing TiO2 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Bacteria
2.1.2. PMMA/TiO2 NPs
2.2. Methods
2.2.1. Mechanical Properties of PMMA with and without TiO2 Added
2.2.2. Roughness Measurements
2.2.3. Contact Angle Measurements
2.2.4. Zeta Potential Measurements
2.2.5. Color Measurements
2.2.6. Bacterial Rate Measurement, SEM Micrographs and EDS Analysis
3. Results
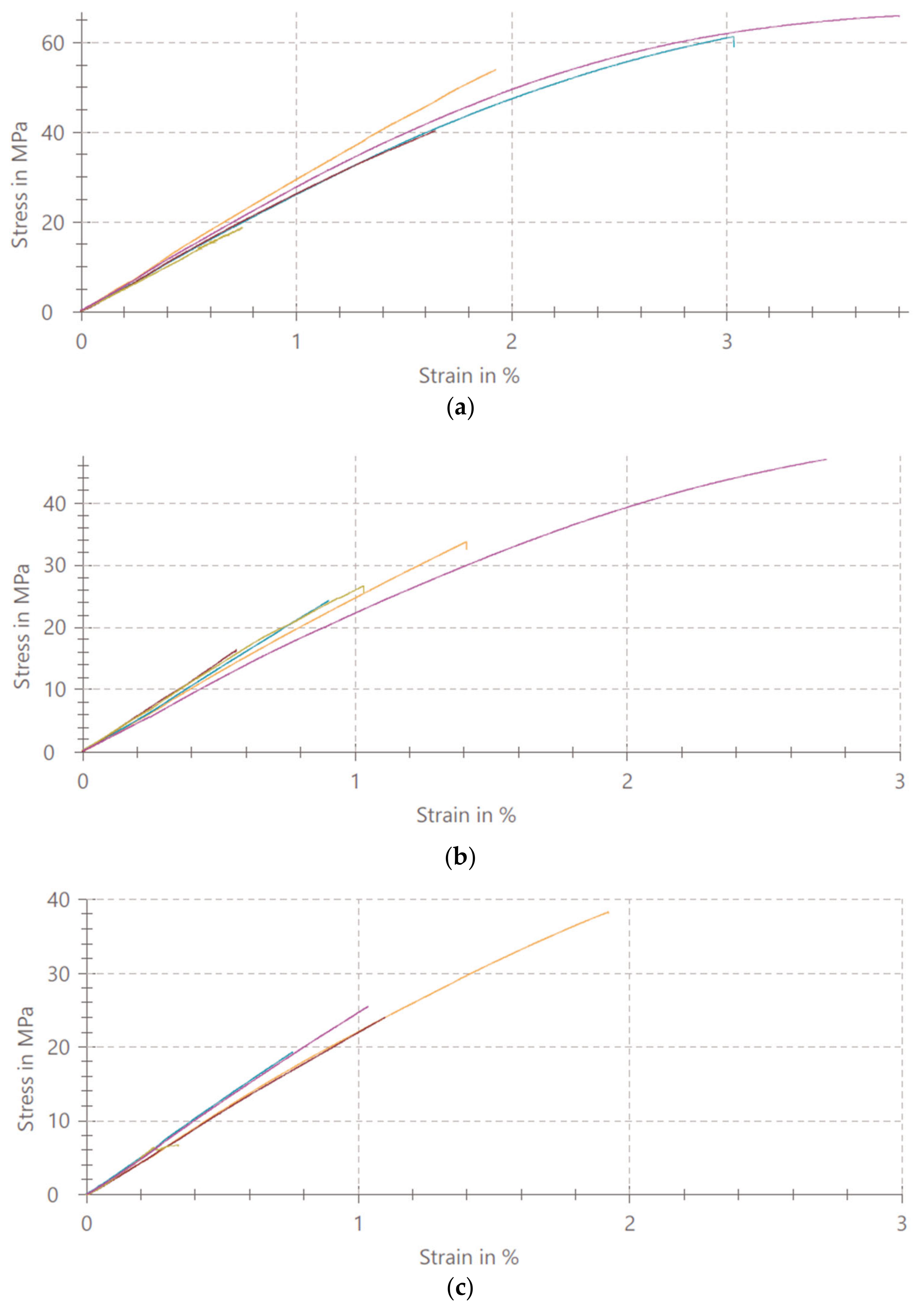
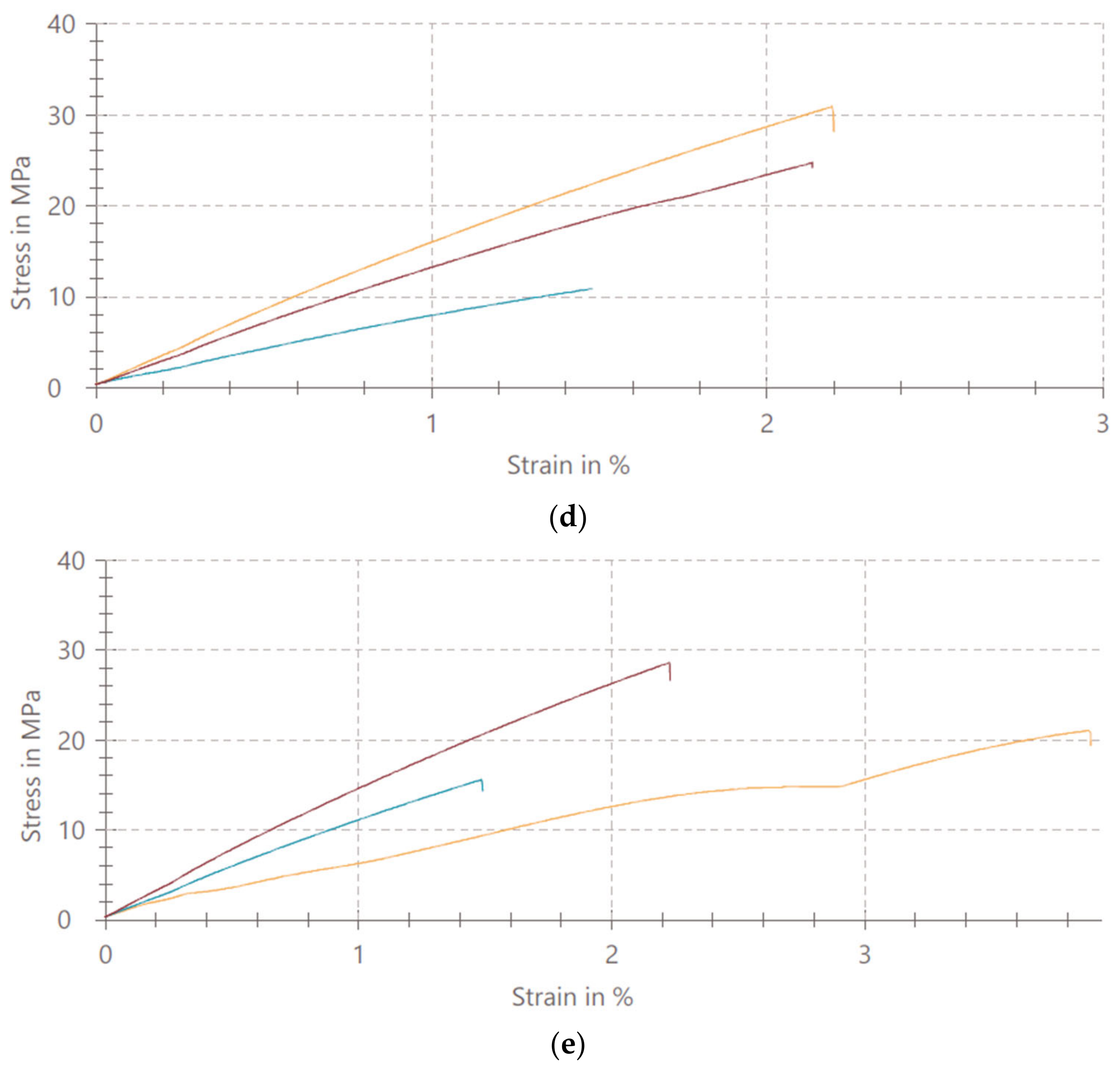
3.1. Mechanical Properties of PMMA/TiO2 NPs
3.2. Roughness
3.3. Contact Angle
3.4. Zeta Potential
3.5. Color Parameters
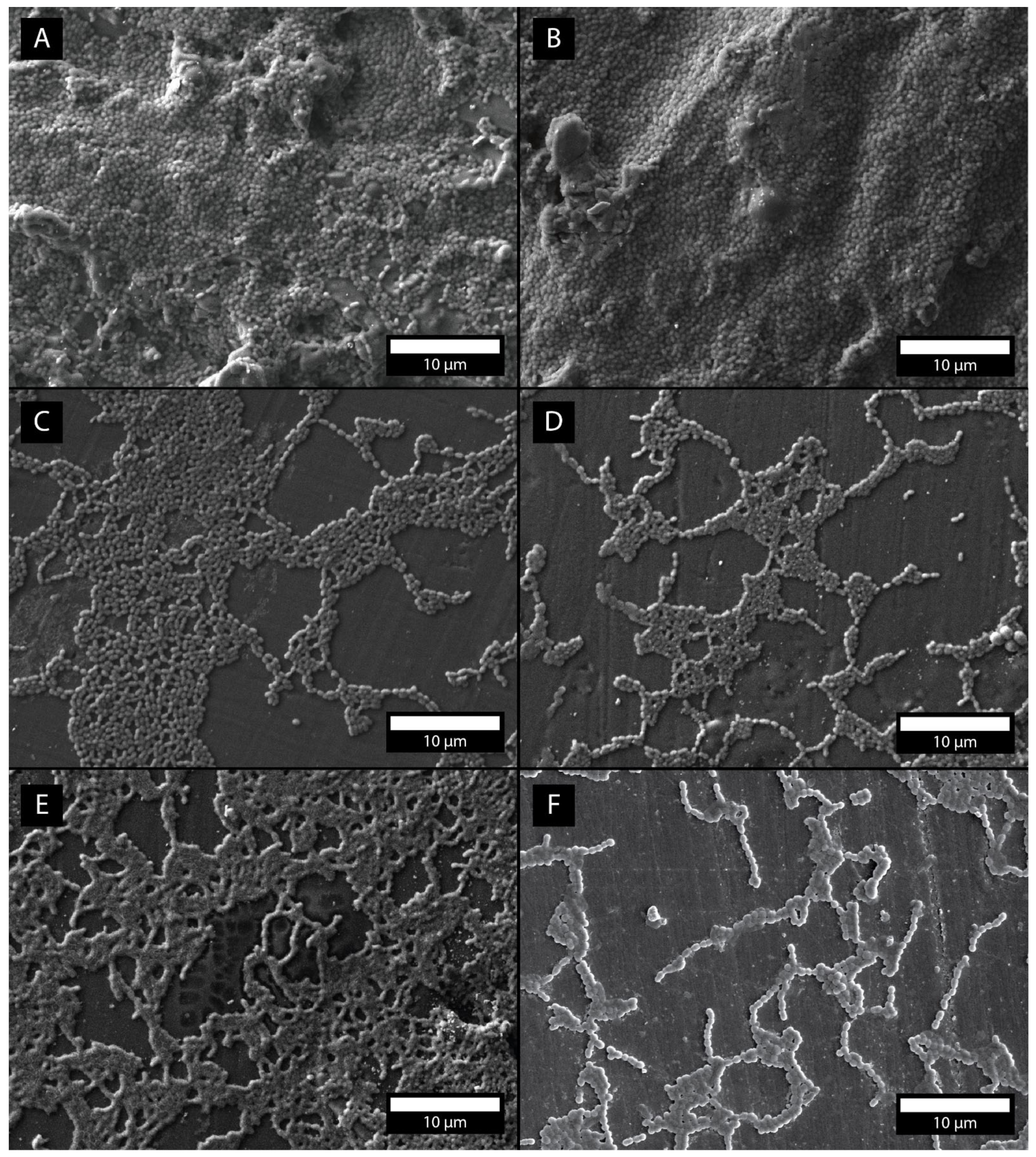
3.6. Bacterial Adhesion Rate Measurement, SEM Micrographs, EDS
4. Discussion
4.1. Mechanical Properties of PMMA/TiO2 NPs
4.2. Surface Roughness
4.3. Contact Angle
4.4. Zeta Potential
4.5. Color Parameters
4.6. Bacterial Adhesion Rate Measurement, SEM Micrographs
5. Conclusions
- The addition of TiO2 nanoparticles in concentrations of 1%, 5%, 10% and 20% affects the uniaxial tensile strength of denture base PMMA.
- The addition of TiO2 nanoparticles in concentrations of 1%, 5%, 10% and 20% affects the color change of the denture base PMMA.
- The addition of TiO2 nanoparticles in concentrations of 1%, 5%, 10% and 20% considerably affects the formation of S. mutans biofilm on the surface of the denture base PMMA; the single bacterial adhesion decays of 10% and 20% of added TiO2 amounts to 58% and 60%, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs Population Division. World Population Aging 2019. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf (accessed on 5 August 2022).
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe tooth loss: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 20S–28S. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Lassila, L.V.; Sasaki, H.; Takahashi, Y.; Vallittu, P.K. Effect of heat treatment of polymethyl methacrylate powder on mechanical properties of denture base resin. J. Mech. Behav. Biomed. Mater. 2014, 39, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Alp, G.; Johnston, W.M.; Yilmaz, B. Optical properties and surface roughness of prepolymerized poly(methyl methacrylate) denture base materials. J. Prosthet. Dent. 2019, 121, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Ali Sabri, B.; Satgunam, M.; Abreeza, N.; Abed, A.N. A review on enhancements of PMMA Denture Base Material with Different Nano-Fillers. Cogent. Eng. 2021, 8, 1875968. [Google Scholar] [CrossRef]
- Adamovic, T.; Veselinovic, V.; Trtic, N.; Hadzi-Mihailovic, M.; Atlagić, S.G.; Balaban, M.; Yoshiyuki, H.; Hironori, S.; Ivanič, A.; Rudolf, R. Mechanical properties of new denture base material modified with gold nanoparticles. J. Prosthodont. Res. 2021, 65, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ajaj-Alkordy, N.M.; Alsaadi, M.H. Elastic modulus and flexural strength comparisons of high-impact and traditional denture base acrylic resins. Saudi Dent. J. 2014, 26, 15–18. [Google Scholar] [CrossRef]
- De Castro, D.T.; Valente, M.L.; Agnelli, J.A.; Lavato da Silva, C.H.; Watanabe, E.; Siqueira, R.L.; Alves, O.L.; Holtz, R.D.; dos Reis, A.C. In vitro study of the antibacterial properties and impact strength of dental acrylic resins modified with a nanomaterial. J. Prosthet. Dent. 2016, 115, 238–246. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Näpänkangas, R.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef]
- Gad, M.M.; Al-Thobity, A.M.; Rahoma, A.; Abualsaud, R.; Al-Harbi, F.A.; Akhtar, S. Reinforcement of PMMA Denture Base Material with a Mixture of ZrO2 Nanoparticles and Glass Fibers. Int. J. Dent. 2019, 2019, 2489393. [Google Scholar] [CrossRef]
- Mahmood, W.S. The effect of incorporating carbon nanotubes on impact, transverse strength, hardness, and roughness to high impact denture base material. J. Baghdad Coll. Dent. 2015, 27, 96–99. [Google Scholar] [CrossRef]
- Hameed, H.K.; Rahman, H.A. The effect of addition nano particle ZrO2 on some properties of autoclave processed heat-cured acrylic denture base material. J. Baghdad Coll. Dent. 2015, 27, 32–39. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Rahoma, A.; Al-Thobity, A.M.; Al-Abidi, K.S.; Akhtar, S. Effect of zirconium oxide nanoparticles addition on the optical and tensile properties of polymethyl methacrylate denture base material. Int. J. Nanomed. 2018, 9, 283–292. [Google Scholar] [CrossRef]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Charman, K.M.; Fernandez, P.; Loewy, Z.; Middleton, A.M. Attachment of Streptococcus oralis on acrylic substrates of varying roughness. Lett. Appl. Microbiol. 2009, 48, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.D.; Wilson, M. The effects of surface roughness and type of denture acrylic on biofilm formation by Streptococcus oralis in a constant depth film fermentor. J. Appl. Microbiol. 2001, 91, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Pinke, K.H.; Freitas, P.; Viera, N.A.; Honorio, H.M.; Porto, V.C.; Lara, V.S. Decreased production of proinflammatory cytokines by monocytes from individuals presenting Candida-associated denture stomatitis. Cytokine 2016, 77, 145–151. [Google Scholar] [CrossRef]
- Lee, H.E.; Li, C.Y.; Chang, H.W.; Yang, Y.H.; Wu, J.H. Effects of different denture cleaning methods to remove Candida albicans from acrylic resin denture based material. J. Dent. Sci. 2011, 6, 216–220. [Google Scholar] [CrossRef]
- Garcia, A.A.M.N.; Sugio, C.Y.C.; de Azevedo-Silva, L.J.; Gomes, A.C.G.; Batista, A.U.D.; Porto, V.C.; Soares, S.; Neppelenbroek, K.H. Nanoparticle-modified PMMA to prevent denture stomatitis: A systematic review. Arch. Microbiol. 2021, 204, 75. [Google Scholar] [CrossRef]
- Giti, R.; Zomorodian, K.; Firouzmandi, M.; Zareshahrabadi, Z.; Rahmannasab, S. Antimicrobial Activity of Thermocycled Polymethyl Methacrylate Resin Reinforced with Titanium Dioxide and Copper Oxide Nanoparticles. Int. J. Dent. 2021, 2021, 6690806. [Google Scholar] [CrossRef]
- Bacali, C.; Baldea, I.; Moldovan, M.; Carpa, R.; Olteanu, D.E.; Filip, G.A.; Nastase, V.; Lascu, L.; Badea, M.; Constantiniuc, M.; et al. Flexural strength, biocompatibility, and antimicrobial activity of a polymethyl methacrylate denture resin enhanced with graphene and silver nanoparticles. Clin. Oral Investig. 2020, 24, 2713–2725. [Google Scholar] [CrossRef] [PubMed]
- Abdulrazzaq Naji, S.; Jafarzadeh Kashi, T.S.; Pourhajibagher, M.; Behroozibakhsh, M.; Masaeli, R.; Bahador, A. Evaluation of Antimicrobial Properties of Conventional Poly(Methyl Methacrylate) Denture Base Resin Materials Containing Hydrothermally Synthesised Anatase TiO2 Nanotubes against Cariogenic Bacteria and Candida albicans. Iran. J. Pharm. Res. 2018, 17, 161–172. [Google Scholar] [PubMed]
- Abualsaud, R.; Aleraky, D.M.; Akhtar, S.; Khan, S.Q.; Gad, M.M. Antifungal Activity of Denture Base Resin Containing Nanozirconia: In Vitro Assessment of Candida albicans Biofilm. Sci. World J. 2021, 2021, 5556413. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R. Behavior of PMMA Denture Base Materials Containing Titanium Dioxide Nanoparticles: A Literature Review. Int. J. Biomater. 2019, 2019, 6190610. [Google Scholar] [CrossRef]
- Alrahlah, A.; Fouad, H.; Hashem, M.; Niazy, A.A.; AlBadah, A. Titanium Oxide (TiO2)/Polymethylmethacrylate (PMMA) Denture Base Nanocomposites: Mechanical, Viscoelastic and Antibacterial Behavior. Materials 2018, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Bangera, M.K.; Kotian, R.N.R. Effect of titanium dioxide nanoparticle reinforcement on flexural strength of denture base resin: A systematic review and meta-analysis. Jpn. Dent. Sci. Rev. 2020, 56, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Alwan, S.A.; Alameer, S.S. The effect of the addition of silanized nano titania fillers on some physical and mechanical properties of heat cured acrylic denture base materials. J. Baghdad Coll. Dent. 2015, 27, 86–91. [Google Scholar] [CrossRef]
- Giti, R.; Firouzmandi, M.; Zare Khafri, N.; Ansarifard, E. Influence of different concentrations of titanium dioxide and copper oxide nanoparticles on water sorption and solubility of heat-cured PMMA denture base resin. Clin. Exp. Dent. Res. 2022, 8, 287–293. [Google Scholar] [CrossRef]
- Chen, R.; Han, Z.; Huang, Z.; Karki, J.; Wang, C.; Zhu, B.; Zhang, X. Antibacterial activity, cytotoxicity and mechanical behavior of nano-enhanced denture base resin with different kinds of inorganic antibacterial agents. Dent. Mater. J. 2017, 36, 693–699. [Google Scholar] [CrossRef]
- Totu, E.E.; Nechifor, A.C.; Nechifor, G.; Aboul-Enein, H.Y.; Cristache, C.M. Poly(methyl methacrylate) with TiO2 nanoparticles inclusion for stereolitographic complete denture manufacturing—The fututre in dental care for elderly edentulous patients. J. Dent. 2017, 59, 68–77. [Google Scholar] [CrossRef]
- Onwubu, S.C.; Vahed, A.; Singh, S.; Kanny, K.M. Reducing the surface roughness of dental acrylic resins by using an eggshell abrasive material. J. Prosthet. Dent. 2017, 117, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Cenci, T.; Pereira, T.; Cury, A.A.; Cenci, M.S.; Rodrigues-Garcia, R.C.M. In vitro Candida colonization on acrylic resins and denture liners: Influence of surface free energy, roughness, saliva, and adhering bacteria. Int. J. Prosthodont. 2007, 20, 308–310. [Google Scholar] [PubMed]
- Dos Santos, R.L.O.; Sarra, G.; Lincopan, N.; Petri, D.F.S.; Aliaga, J.; Marques, M.M.; Dias, R.B.; Coto, N.P.; Sugaya, N.N.; Paula, C.R. Preparation, antimicrobial properties, and cytotoxicity of acrylic resins containing poly(diallyldimethylammonium chloride). Int. J. Prosthodont. 2020, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sakai, T.; Moroi, R.; Moroi, R.; Nakagawa, M.; Sakai, H.; Ogata, T.; Terada, Y. Self-cleaning ability of a photocatalyst-containing denture base material. Dent. Mater. J. 2008, 27, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.J.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Hengzhuang, W.; Wu, H.; Ciofu, O.; Song, Z.J.; Høiby, N. In vivo pharmacokinetics/pharmacodynamics of colistin and imipenem in Pseudomonas aeruginosa biofilm infection. Antimicrob. Agents Chemother. 2012, 56, 2683–2690. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by sur-709 face modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Ho, D.K.; Ghinea, R.; Herrera, L.J.; Angelov, N.; Paravina, R.D.J.S.R. Color range and color distribution of healthy human gingiva: A prospective clinical study. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Arunachalam, A.; Dhanapandian, S.; Manoharan, C.; Sivakumar, G. Physical properties of Zn doped TiO2 thin films with spray pyrolysis technique and its effects in antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A. Properties improvement of PMMA using nano TiO2. J. Appl. Polym. Sci. 2010, 118, 2890–2897. [Google Scholar] [CrossRef]
- Shirkavand, S.; Moslehifard, E. Effect of TiO2 Nanoparticles on Tensile Strength of Dental Acrylic Resins. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 197–203. [Google Scholar]
- Sun, J.; Forster, A.M.; Johnson, P.M.; Eidelman, N.; Quinn, G.; Schumacher, G. Improving performance of dental resins by adding titanium dioxide nanoparticles. Dent. Mater. 2011, 27, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Moslehifard, E.; Robati Anaraki, M.; Shirkavand, S. Effect of adding TiO2 nanoparticles on the SEM morphology and mechanical properties of conventional heat-cured acrylic resin. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Mosalman, S.; Rashahmadi, S.; Hasanzadeh, R. The effect of TiO2 nanoparticles on mechanical properties of poly methyl methacrylate nanocomposites. Int. J. Eng. Trans. B Appl. 2017, 30, 807–813. [Google Scholar]
- Ghahremani, L.; Shirkavand, S.; Akbari, F.; Sabzikari, N. Tensile strength and impact strength of color modified acrylic resin reinforced with titanium dioxide nanoparticles. J. Clin. Exp. Dent. 2017, 9, 661–665. [Google Scholar] [CrossRef]
- Ahmed, M.A.; El-Shennawy, M. Effect of titanium dioxide nano particles incorporation on mechanical and physical properties on two different types of acrylic resin denture base. World J. Nano Sci. Eng. 2016, 6, 111. [Google Scholar] [CrossRef]
- Bohinc, K.; Dražić, G.; Fink, R.; Oder, M.; Jevšnik, M.; Nipič, D.; Godič Torkar, K.; Raspor, P. Available surface dictates microbial adhesion capacity. Int. J. Adhes. Adhes. 2014, 50, 265–272. [Google Scholar] [CrossRef]
- Bohinc, K.; Dražić, G.; Abram, A.; Jevšnik, M.; Jeršek, B.; Nipič, D.; Kurinčič, M.; Raspor, P. Metal surface characteristics dictate bacterial adhesion capacity. Int. J. Adhes. Adhes. 2016, 68, 39–46. [Google Scholar] [CrossRef]
- Janovak, L.; Deak, A.; Tallosy, S.P.; Sebok, D.; Csapo, E.; Bohinc, K.; Abram, A.; Palinko, A.; Dekany, I. Hydroxyapatite-enhanced structural, photocatalytic and antibacterial properties of photoreactive TiO2/HAp/polyacrylate hybrid thin films. Surf. Coat. Technol. 2017, 326, 316–326. [Google Scholar] [CrossRef]
- Hengzhuang, W.; Wu, H.; Ciofu, O.; Song, Z.; Høiby, N. Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2011, 55, 4469–4474. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.; Al Rez, M.F.; Fouad, H. Infuence of titanium oxide nanoparticles on the physical and thermomechanical behavior of poly methyl methacrylate (pmma): A denture base resin. Sci. Adv. Mater. 2017, 9, 938–944. [Google Scholar] [CrossRef]
- Andreotti, A.M. Influence of nanoparticles on color stability, microhardness, and flexural strength of acrylic resins specific for ocular prosthesis. Int. J. Nanomed. 2014, 9, 5779–5787. [Google Scholar]
- Chang, J.; Da Silva, J.D.; Sakai, M.; Kristiansen, J.; Ishikawa Nagai, S. The optical effect of composite luting cement on all ceramic crowns. J. Dent. 2009, 37, 937–943. [Google Scholar] [CrossRef]
- Chu, S.J.; Devigus, A.; Mieleszko, A.J. Fundamentals of Color: Shade Matching and Communication in Esthetic Dentistry; Quintessence Publishing Co., Inc.: Batavia, IL, USA, 2004. [Google Scholar]
- Gómez-Polo, C.; Montero, J.; Gómez-Polo, M.; Martín Casado, A.M. Clinical study on natural gingival color. Odontology 2019, 107, 80–89. [Google Scholar] [CrossRef]
- Aziz, H.K. TiO-Nanofillers Effects on Some Properties of Highly-Impact Resin Using Different Processing Techniques. Open Dent. J. 2018, 12, 202–212. [Google Scholar] [CrossRef]
- Acosta-Torres, L.S.; López-Marín, L.M.; Nunez-Anita, R.E.; Hernández-Padrón, G.; Castaño, V.M. Biocompatible Metal Oxide Nanoparticles: Nanotechnology Improvement of Conventional Prosthetic Acrylic Resins. J. Nanomater. 2011, 2011, 941561. [Google Scholar] [CrossRef]
- Ihab, N.S.; Hassanen, K.A.; Ali, N.A. Assessment of zirconium oxide nano-fillers incorporation and silanation on impact, tensile strength and color alteration of heat polymerized acrylic resin. J. Baghdad Coll. Dent. 2012, 24, 36–42. [Google Scholar]
- Cierech, M.; Szerszeń, M.; Wojnarowicz, J.; Łojkowski, W.; Kostrzewa-Janicka, J.; Mierzwińska-Nastalska, E. Preparation and Characterisation of Poly(methyl metacrylate)-Titanium Dioxide Nanocomposites for Denture Bases. Polymers 2020, 12, 2655. [Google Scholar] [CrossRef]
- Manerat, C.; Hayata, Y. Antifungal activity of TiO2 photocatalysis against Penicillium expansum in vitro and in fruit tests. Int. J. Food Microbiol. 2006, 107, 99–103. [Google Scholar] [CrossRef]
- Sagsoz, N.P.; Yanıkoglu, N.; Ulu, H.; Bayındır, F. Color changes of polyamid and polymetyhl methacrylate denture base materials. Open J. Stomatol. 2014, 4, 489. [Google Scholar] [CrossRef]
- Dimitrova, M.; Corsalini, M.; Kazakova, R.; Vlahova, A.; Barile, G.; Dell’Olio, F.; Tomova, Z.; Kazakov, S.; Capodiferro, S. Color Stability Determination of CAD/CAM Milled and 3D Printed Acrylic Resins for Denture Bases: A Narrative Review. J. Compos. Sci. 2022, 6, 201. [Google Scholar] [CrossRef]
- Kotanidis, A.; Kontonasaki, E.; Koidis, P. Color alterations of a PMMA resin for fixed interim prostheses reinforced with silica nanoparticles. J. Adv. Prosthodont. 2019, 11, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef]
- Sodagar, A.; Khalil, S.; Kassaee, M.Z.; Shahroudi, A.S.; Pourakbari, B.; Bahador, A. Antimicrobial properties of poly(methyl methacrylate) acrylic resins incorporated with silicon dioxide and titanium dioxide nanoparticles on cariogenic bacteria. J. Orthod. Sci. 2016, 5, 7–13. [Google Scholar]




| σM (MPa) | ε M (%) | |
|---|---|---|
| PMMA | 48.1 ± 19.0 | 2.2 ± 1.2 |
| PMMA/TiO2 NPs (1%) | 29.6 ± 11.5 | 1.3 ± 0.8 |
| PMMA/TiO2 NPs (5%) | 22.7 ± 11.4 | 1.0 ± 0.6 |
| PMMA/TiO2 NPs (10%) | 22.2 ± 10.3 | 1.9 ± 0.4 |
| PMMA/TiO2 NPs (20%) | 21.7 ± 6.5 | 2.5 ± 1.2 |
| L* | a* | b* | ΔE | |
|---|---|---|---|---|
| PMMA | 57.6 ± 0.38 | 22.2 ± 0.42 | 14.4 ± 0.22 | 4.85 |
| PMMA + TiO2 (1%) | 72.0 ± 0.44 | 16.7 ± 0.26 | 8.4 ± 0.43 | 21.23 |
| PMMA + TiO2 (5%) | 78.9 ± 0.37 | 14.1 ± 0.44 | 5.9 ± 0.37 | 29.01 |
| PMMA + TiO2 (10%) | 81.3 ± 0.28 | 7.8 ± 0.87 | 3.4 ± 0.44 | 34.34 |
| PMMA + TiO2 (20%) | 85.0 ± 0.29 | 6.1 ± 0.22 | 2.7 ± 0.10 | 38.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zore, A.; Abram, A.; Učakar, A.; Godina, I.; Rojko, F.; Štukelj, R.; Škapin, A.S.; Vidrih, R.; Dolic, O.; Veselinovic, V.; et al. Antibacterial Effect of Polymethyl Methacrylate Resin Base Containing TiO2 Nanoparticles. Coatings 2022, 12, 1757. https://doi.org/10.3390/coatings12111757
Zore A, Abram A, Učakar A, Godina I, Rojko F, Štukelj R, Škapin AS, Vidrih R, Dolic O, Veselinovic V, et al. Antibacterial Effect of Polymethyl Methacrylate Resin Base Containing TiO2 Nanoparticles. Coatings. 2022; 12(11):1757. https://doi.org/10.3390/coatings12111757
Chicago/Turabian StyleZore, Anamarija, Anže Abram, Aleksander Učakar, Ivo Godina, Franc Rojko, Roman Štukelj, Andrijana Sever Škapin, Rajko Vidrih, Olivera Dolic, Valentina Veselinovic, and et al. 2022. "Antibacterial Effect of Polymethyl Methacrylate Resin Base Containing TiO2 Nanoparticles" Coatings 12, no. 11: 1757. https://doi.org/10.3390/coatings12111757
APA StyleZore, A., Abram, A., Učakar, A., Godina, I., Rojko, F., Štukelj, R., Škapin, A. S., Vidrih, R., Dolic, O., Veselinovic, V., & Bohinc, K. (2022). Antibacterial Effect of Polymethyl Methacrylate Resin Base Containing TiO2 Nanoparticles. Coatings, 12(11), 1757. https://doi.org/10.3390/coatings12111757








