Performance of BaCe0.8Y0.2O3-δ Proton Electrolyte Materials for Solid Oxide Fuel Cells by Compositing the Transition Metal Oxide NiO
Abstract
1. Introduction
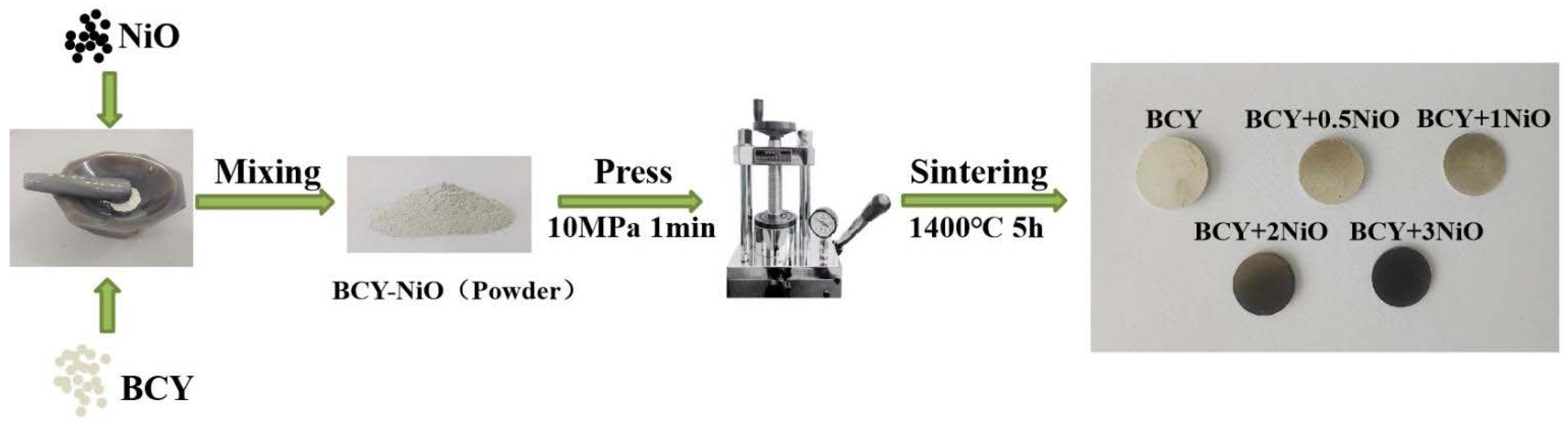
2. Experimental
2.1. Subtopic A: Experimental and Model of SOFC Set up
2.2. Subtopic B: Morphology Analysis
2.3. Subtopic C: Electrical Performance Analysis
3. Results and Discussion
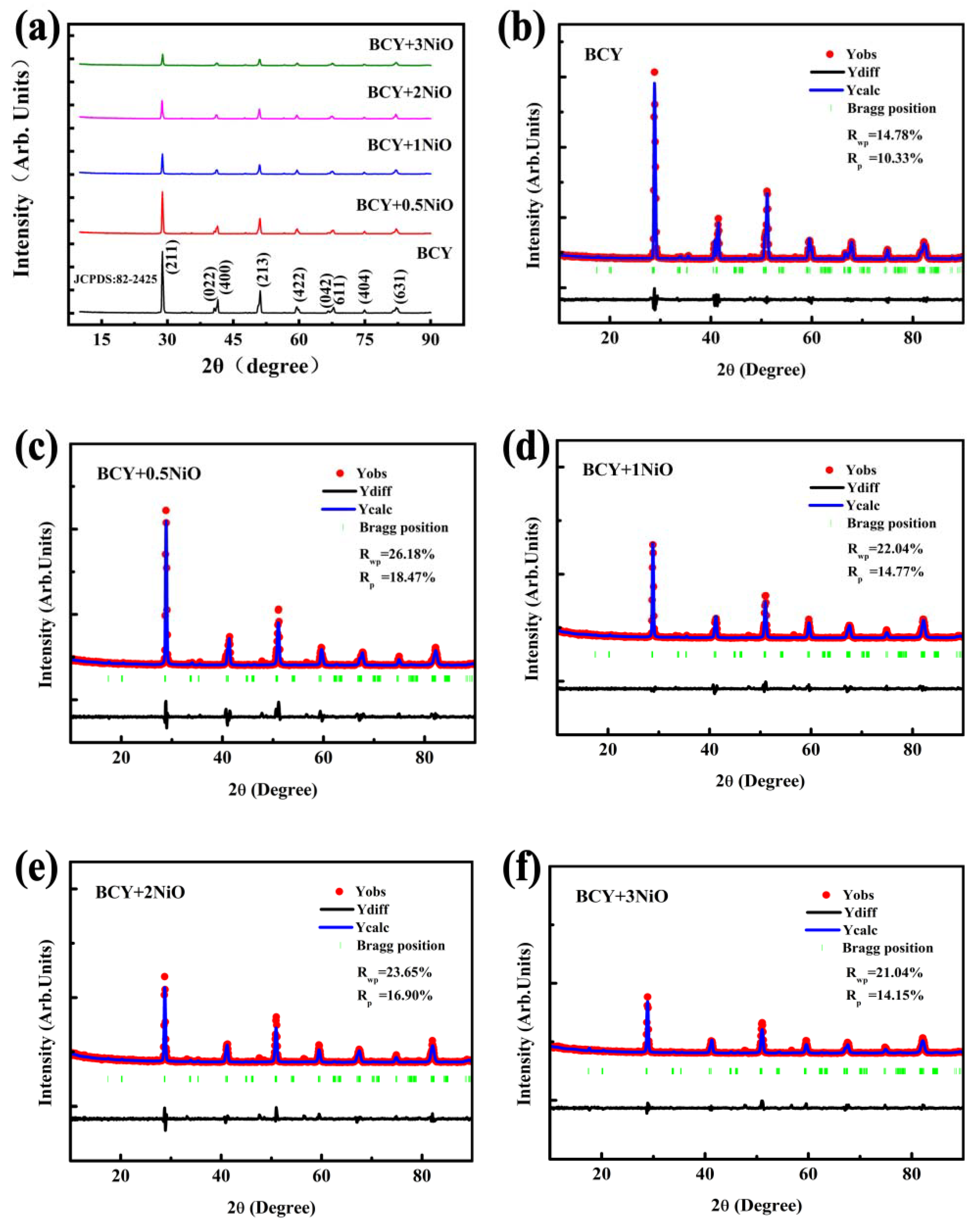
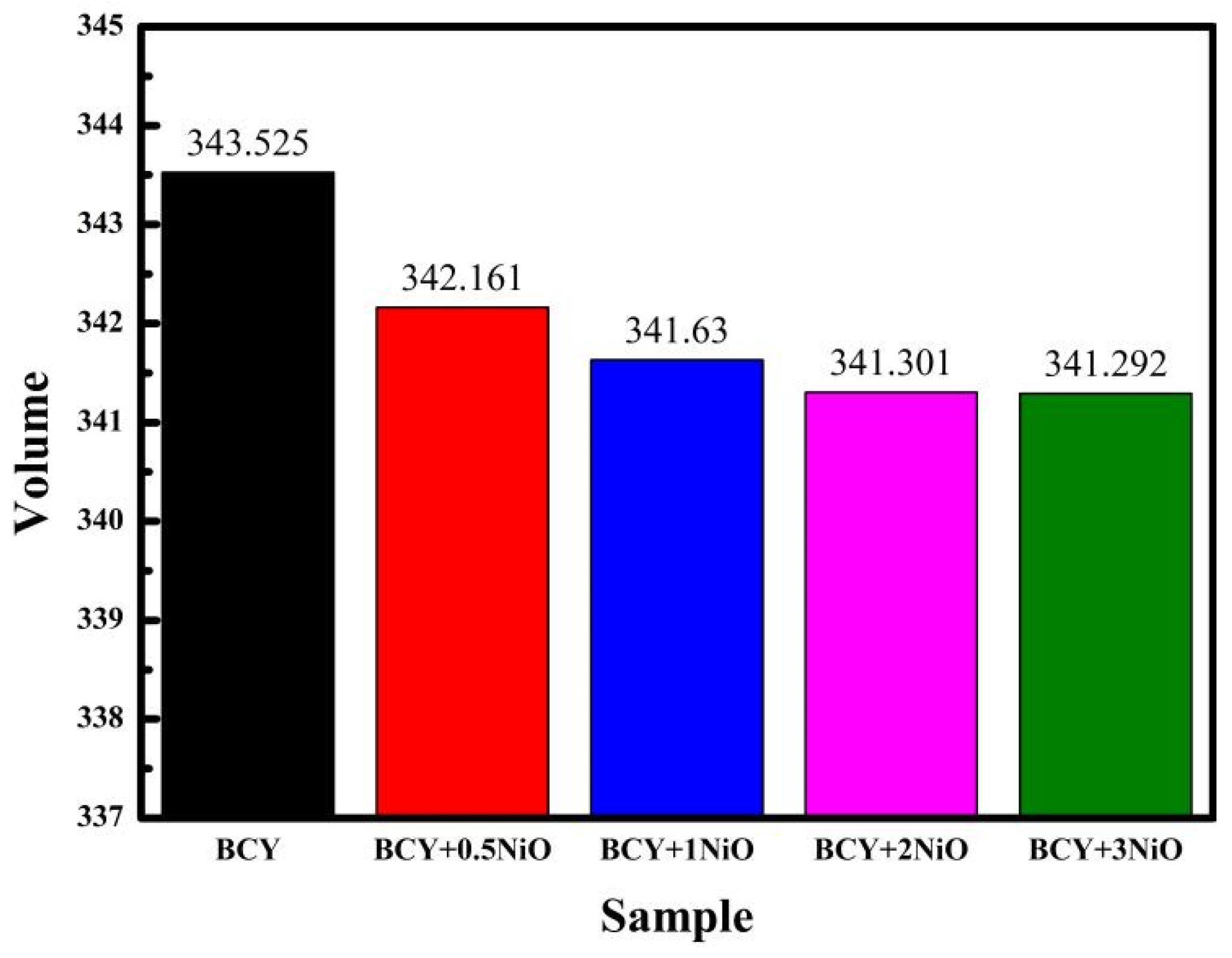
3.1. Morphological Analysis on BCY–NiO Powders
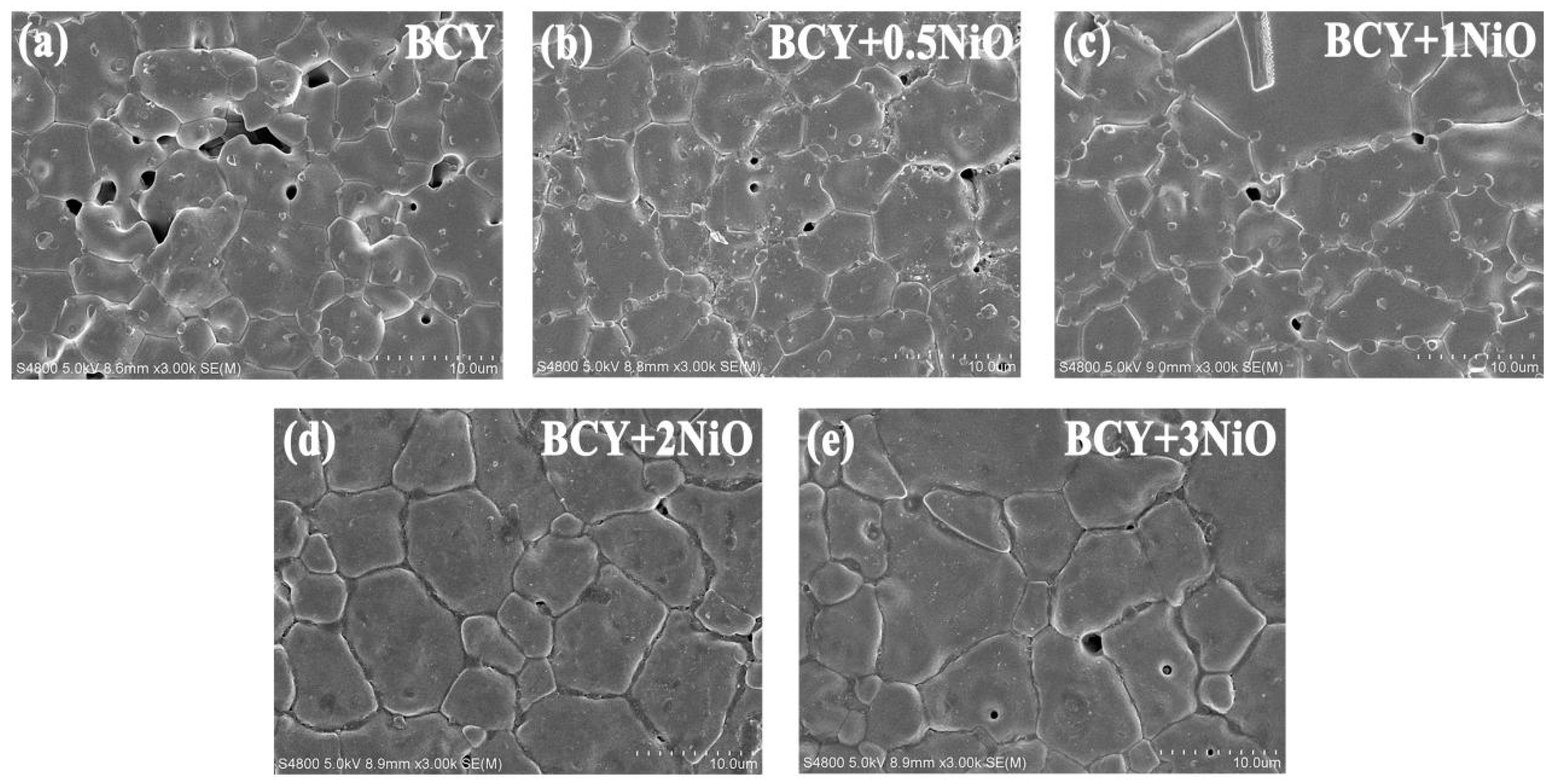
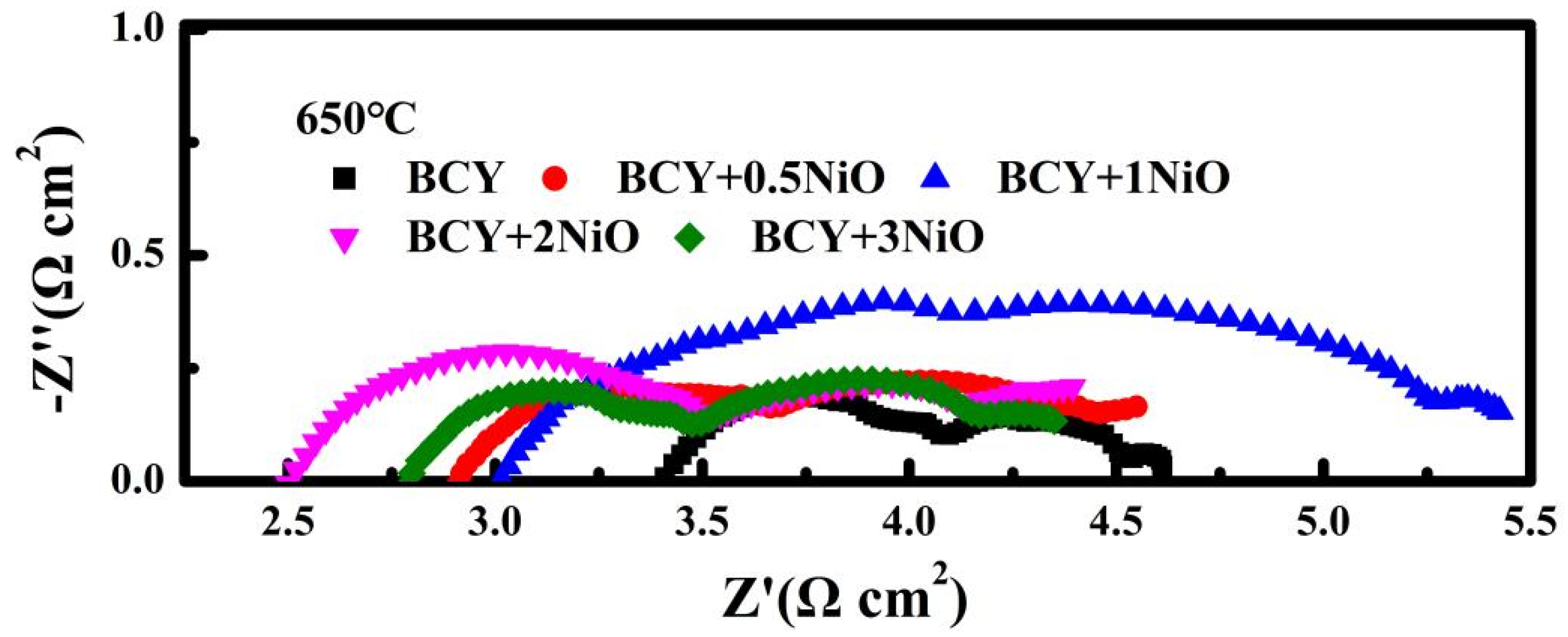
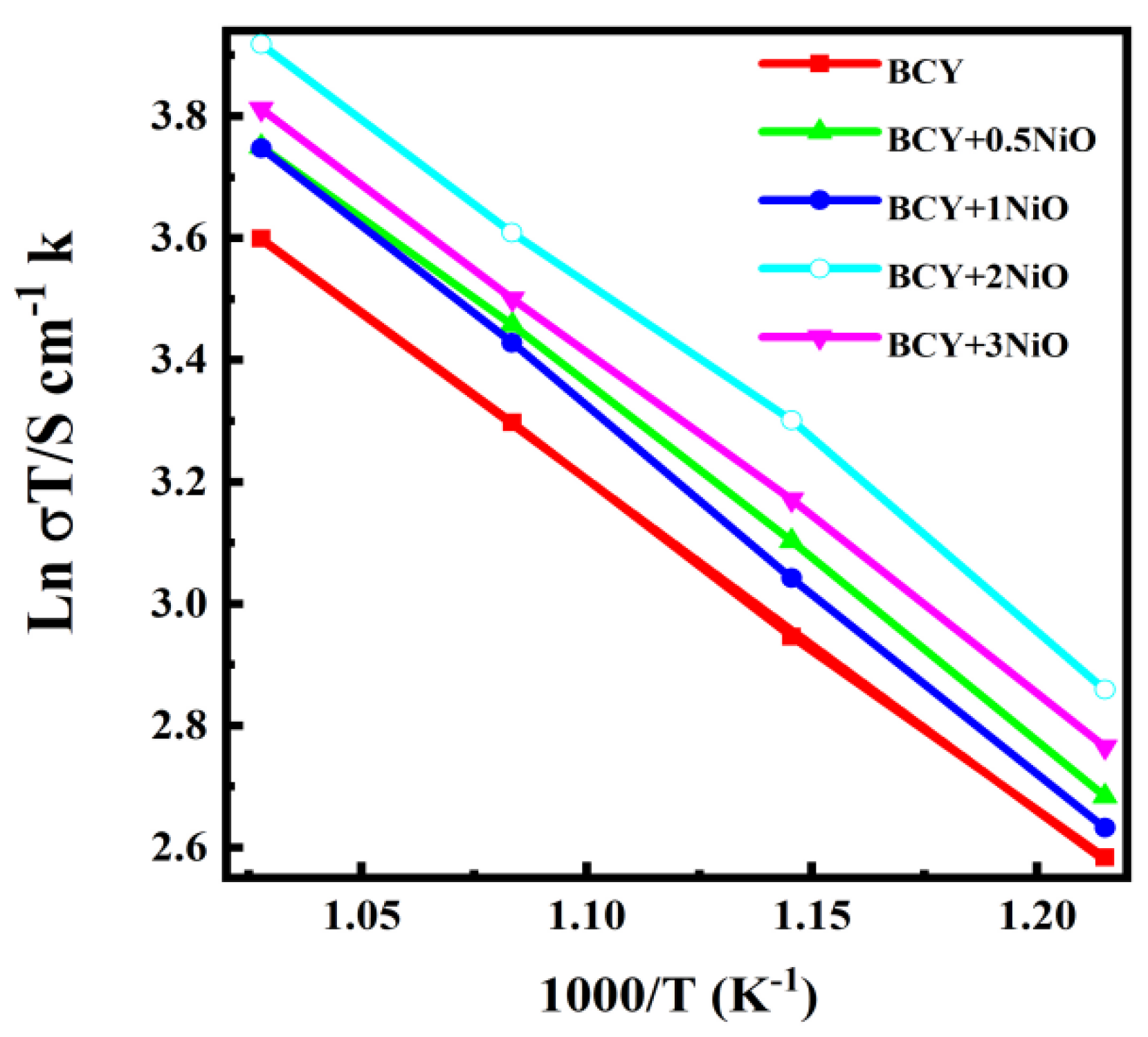
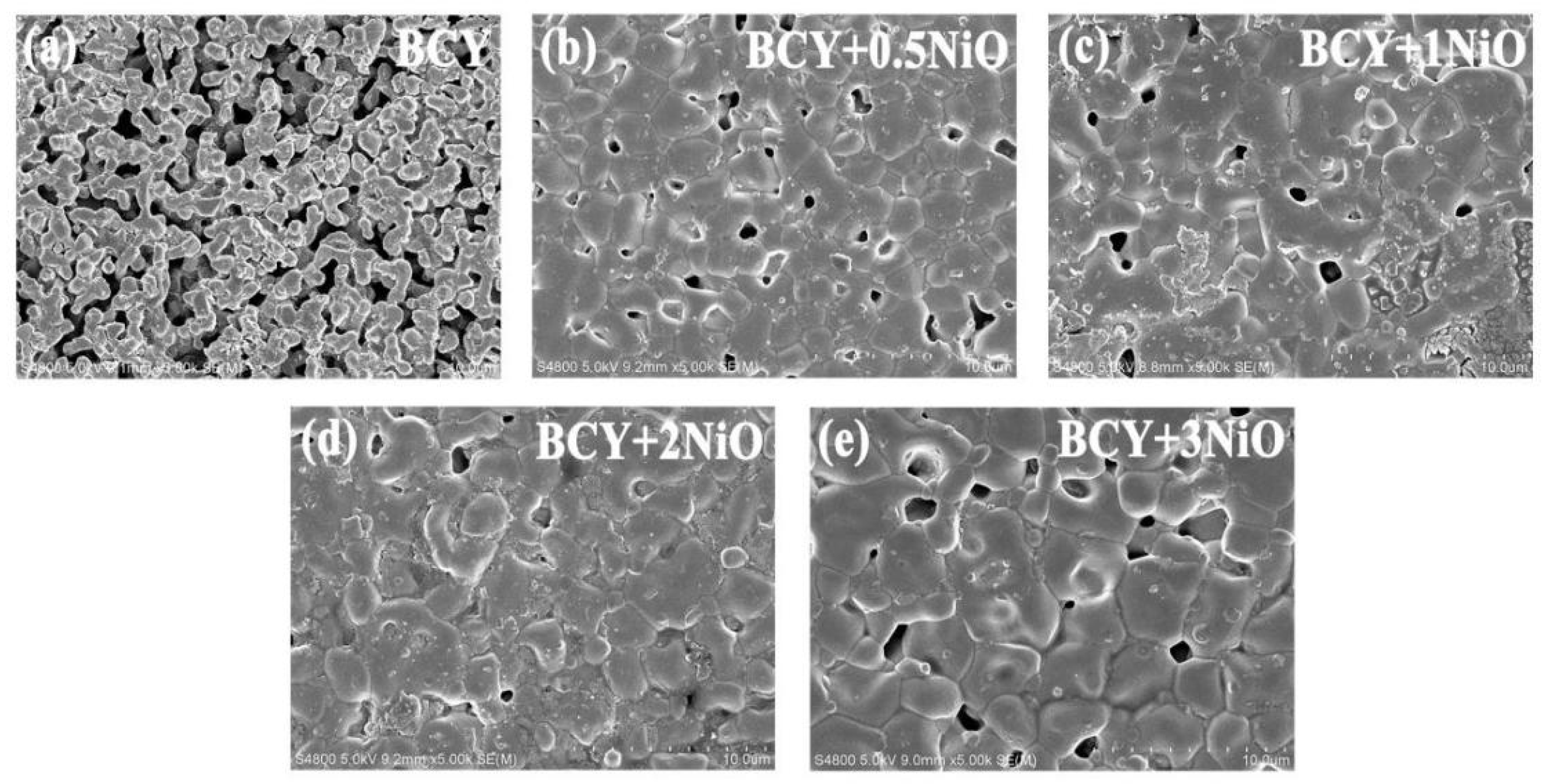
3.2. SEM Microstructure Analysis and Conductivity Tests on BCY–NiO Electrolytes
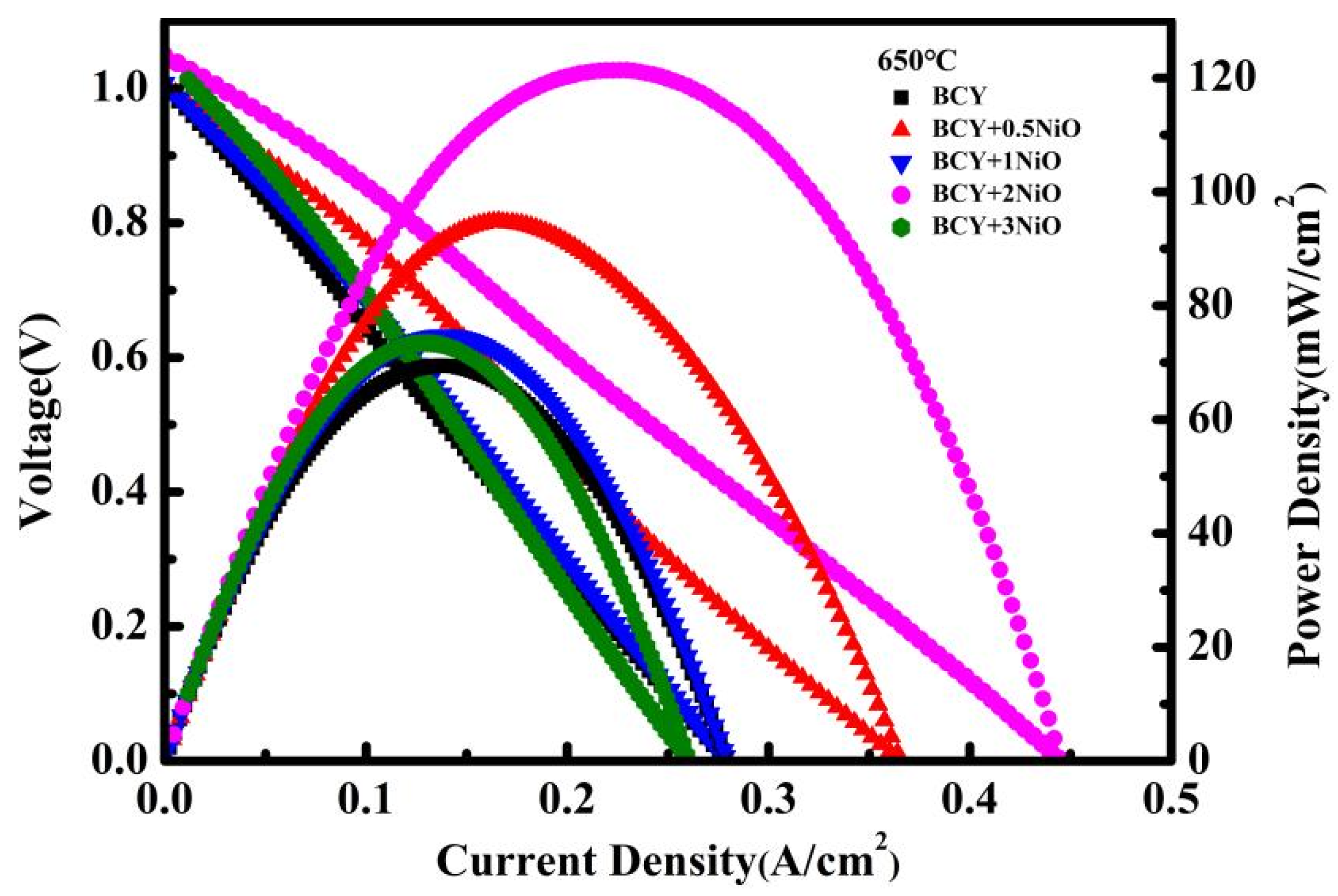
3.3. Full Cell Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsumoto, K.; Tachikawa, Y.; Lyth, S.M.; Matsuda, J.; Sasaki, K. Performance and durability of Ni–Co alloy cermet anodes for solid oxide fuel cells. Int. J. Hydrogen Energy 2022, 47, 29441–29455. [Google Scholar] [CrossRef]
- Ivers-Tiffée, E.; Weber, A.; Herbstritt, D. Materials and technologies for SOFC-components. J. Eur. Ceram. Soc. 2001, 21, 1805–1811. [Google Scholar] [CrossRef]
- Yang, G.; Su, C.; Shi, H.; Zhu, Y.; Song, Y.; Zhou, W.; Shao, Z. Towards Reducing Operation Temperature of Solid Oxide Fuel Cells: Our Past Fifteen Years of Efforts in Cathode Development. Energy Fuels 2020, 34, 15169–15194. [Google Scholar] [CrossRef]
- Malik, V.; Srivastava, S.; Bhatnagar, M.K.; Vishnoi, M. Comparative study and analysis between Solid Oxide Fuel Cells (SOFC) and Proton Exchange Membrane (PEM) fuel cell—A review. Mater. Today Proc. 2021, 47, 2270–2275. [Google Scholar] [CrossRef]
- Lee, S.; Song, H.S.; Hyun, S.H.; Kim, J.; Moon, J. Interlayer-free nanostructured La0.58Sr0.4Co0.2Fe0.8O3−δ cathode on scandium stabilized zirconia electrolyte for intermediate-temperature solid oxide fuel cells. J. Power Sources 2008, 187, 74–79. [Google Scholar] [CrossRef]
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Wen, J.; Song, C.; Liu, T.; Deng, Z.; Niu, S.; Zhang, Y.; Liu, L.; Liu, M. Fabrication of Dense Gadolinia-Doped Ceria Coatings via Very-Low-Pressure Plasma Spray and Plasma Spray–Physical Vapor Deposition Process. Coatings 2019, 9, 717. [Google Scholar] [CrossRef]
- Goodenough, J.B. Oxide-Ion Electrolytes. Annu. Rev. Mater. Res. 2003, 12, 357–364. [Google Scholar] [CrossRef]
- Xie, D.; Ling, A.; Yan, D.; Jia, L.; Chi, B.; Pu, J.; Li, J. A comparative study on the composite cathodes with proton conductor and oxygen ion conductor for proton-conducting solid oxide fuel cell. Electrochim. Acta 2020, 344, 136143. [Google Scholar] [CrossRef]
- Yadav, A.; Pyare, R.; Goodenough, J.B.; Singh, P. KTa1−x−yTixGeyO3−δ: A High κ Relaxor Dielectric and Superior Oxide-Ion Electrolyte for IT-SOFC. ACS Appl. Energy Mater. 2020, 3, 3205–3211. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.J.; Zhu, H.; Karakaya, C.; Chen, Y.; Ricote, S.; Jarry, A.; Crumlin, E.J.; Hook, D.; Braun, R.; et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 2018, 557, 217–222. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kawasaki, Y.; Ito, N.; Enoki, M.; Ishihara, T. Relation Between Electrical Conductivity and Chemical Stability of BaCeO3. Electrochem. Solid State Lett. 2007, 10, B77–B80. [Google Scholar] [CrossRef]
- Kim, H.S.; Bae, H.B.; Jung, W.; Chung, S.Y. Manipulation of Nanoscale Intergranular Phases for High Proton Conduction and Decomposition Tolerance in BaCeO3 Polycrystals. Nano Lett. 2018, 18, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hua, B.; Luo, J.-L.; Jiang, S.P.; Pu, J.; Chi, B.; Jian, L. Carbon-tolerant Ni-based cermet anodes modified by proton conducting yttrium- and ytterbium-doped barium cerates for direct methane solid oxide fuel cells. J. Mater. Chem. A 2015, 3, 21609–21617. [Google Scholar] [CrossRef]
- Triviño-Peláez, Á.; Pérez-Coll, D.; Mosa, J.; Ritter, C.; Amador, U.; Mather, G.C. Enhanced proton conductivity and stability of Ba-deficient BaCe0.8Y0.2O3−δ. J. Power Sources 2021, 493, 229691. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.H. Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: From materials to devices. Energy Sci. Eng. 2021, 9, 984–1011. [Google Scholar] [CrossRef]
- Fop, S. Solid oxide proton conductors beyond perovskites. J. Mater. Chem. A 2021, 9, 18836–18856. [Google Scholar] [CrossRef]
- Exner, J.; Nazarenus, T.; Kita, J.; Moos, R. Dense Y-doped ion conducting perovskite films of BaZrO3, BaSnO3, and BaCeO3 for SOFC applications produced by powder aerosol deposition at room temperature. Int. J. Hydrogen Energy 2020, 45, 10000–10016. [Google Scholar] [CrossRef]
- Tomita, A.; Hibino, T.; Suzuki, M.; Sano, M. Proton conduction at the surface of Y-doped BaCeO3 and its application to an air/fuel sensor. J. Mater. Sci. 2004, 39, 2493–2497. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Ma, G. Proton conduction in BaxCe0.8Y0.2O3−α + 0.04ZnO at intermediate temperatures and its application in ammonia synthesis at atmospheric pressure. J. Mater. Sci. 2011, 46, 4690–4694. [Google Scholar] [CrossRef]
- Presto, S.; Viviani, M. Effect of CuO on microstructure and conductivity of Y-doped BaCeO3. Solid State Ion. 2016, 295, 111–116. [Google Scholar] [CrossRef]
- Costa, R.; Grünbaum, N.; Berger, M.H.; Dessemond, L.; Thorel, A. On the use of NiO as sintering additive for BaCe0.9Y0.1O3−α. Solid State Ion. 2009, 180, 891–895. [Google Scholar] [CrossRef]
- Zhang, T.S.; Ma, J.; Kong, L.B.; Chan, S.H.; Hing, P.; Kilner, J.A. Iron oxide as an effective sintering aid and a grain boundary scavenger for ceria-based electrolytes. Solid State Ion. 2004, 167, 203–207. [Google Scholar] [CrossRef]
- Zhang, T.S.; Ma, J.; Leng, Y.J.; Chan, S.H.; Hing, P.; Kilner, J.A. Effect of transition metal oxides on densification and electrical properties of Si-containing Ce0.8Gd0.2O2−δ ceramics. Solid State Ion. 2004, 168, 187–195. [Google Scholar] [CrossRef]
- Zhang, T.; Kong, L.; Zeng, Z.; Huang, H.; Hing, P.; Xia, Z.; Kilner, J. Sintering behavior and ionic conductivity of Ce0.8Gd0.2O1.9 with a small amount of MnO2 doping. J. Solid State Electrochem. 2003, 7, 348–354. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Liu, M.; Tang, Z.; Liu, M. Enhanced sinterability of BaZr0.1Ce0.7Y0.1Yb0.1O3−δ by addition of nickel oxide. J. Power Sources 2011, 196, 9980–9984. [Google Scholar] [CrossRef]
- Zhang, L.; Lan, R.; Kraft, A.; Wang, M.; Tao, S. Cost-effective solid oxide fuel cell prepared by single step co-press-firing process with lithiated NiO cathode. Electrochem. Commun. 2010, 12, 1589–1592. [Google Scholar] [CrossRef]
- Sharma, D.; Jha, R. Transition metal (Co, Mn) co-doped ZnO nanoparticles: Effect on structural and optical properties. J. Alloys Compd. 2017, 698, 532–538. [Google Scholar] [CrossRef]
- Harrington, R.; Miles, G.C.; West, A.R. Crystallography of Ni-doped Zn7Sb2O12 and phase equilibria in the system ZnOSb2O5NiO. J. Eur. Ceram. Soc. 2005, 26, 2307–2311. [Google Scholar] [CrossRef]
- Zhang, T.S.; Ma, J.; Chan, S.H.; Kilner, J.A. Grain boundary conduction of Ce0.9Gd0.1O2−δ ceramics derived from oxalate coprecipitation: Effects of Fe loading and sintering temperature. Solid State Ion. 2005, 176, 377–384. [Google Scholar] [CrossRef]
- Zhang, T.S.; Ma, J.; Leng, Y.J.; He, Z.M. Sintering, microstructure and grain growth of Fe-doped Ce0.9Gd0.1O2−δ ceramics derived from oxalate coprecipitation. J. Cryst. Growth 2005, 274, 603–611. [Google Scholar] [CrossRef]
- Jadhav, S.T.; Dubal, S.U.; Jamale, A.P.; Patil, S.P.; Bhosale, C.H.; Puri, V.R.; Jadhav, L.D. Structural, morphological and electrical studies of BaCe0.8Y0.2O3−δ synthesized by solution combustion method. Ionics 2015, 21, 1295–1300. [Google Scholar] [CrossRef]
- Bi, L.; Traversa, E. A chemically stable electrolyte with a novel sandwiched structure for proton-conducting solid oxide fuel cells (SOFCs). Electrochem. Commun. 2013, 36, 42–45. [Google Scholar] [CrossRef]
- Yang, N.; Tebano, A.; Di Castro, D.; Balestrino, G.; D’Epifanio, A.; Licoccia, S.; Di Bartolomeo, E. Deposition and electrochemical characterization of Yttrium doped Barium cerate and zirconate heterostructures. Thin Solid Films 2014, 562, 264–268. [Google Scholar] [CrossRef]
- Lima, C.G.; Santos, T.H.; Grilo, J.P.; Dutra, R.P.; Nascimento, R.M.; Rajesh, S.; Fonseca, F.C.; Macedo, D.A. Synthesis and properties of CuO-doped Ce0.9Gd0.1O2−δ electrolytes for SOFCs. Ceram. Int. 2015, 41, 4161–4168. [Google Scholar] [CrossRef]
- Tian, N.; Qu, Y.; Men, H.; Yu, J.; Wang, X.; Zheng, J. Properties of Ce0.85Sm0.15O2-δ-CuO electrolytes for intermediate-temperature solid oxide fuel cells. Solid State Ion. 2020, 351, 115331. [Google Scholar] [CrossRef]
- Yu, J.; Tian, N.; Deng, Y.; Li, G.; Liu, L.; Cheng, L.; Gao, P.; Pan, Q.; Wang, Y.; Chen, X. Fabrication and characterization of BaCe0.8Y0.2O2.9-Ce0.85Sm0.15O1.925 composite electrolytes for IT-SOFCs. Sci. China Chem. 2015, 58, 473–477. [Google Scholar] [CrossRef]
- Itagaki, Y.; Yamamoto, Y.; Aono, H.; Yahiro, H. Anode-supported SOFC with thin film of proton-conducting BaCe0.8Y0.2O3−α by electrophoretic deposition. J. Ceram. Soc. Jpn. 2017, 125, 528–532. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Yu, J.; Tian, N.; Zheng, J.; Qu, Y.; Tan, W.; Luo, Y. Performance of BaCe0.8Y0.2O3-δ Proton Electrolyte Materials for Solid Oxide Fuel Cells by Compositing the Transition Metal Oxide NiO. Coatings 2022, 12, 1692. https://doi.org/10.3390/coatings12111692
Huang Y, Yu J, Tian N, Zheng J, Qu Y, Tan W, Luo Y. Performance of BaCe0.8Y0.2O3-δ Proton Electrolyte Materials for Solid Oxide Fuel Cells by Compositing the Transition Metal Oxide NiO. Coatings. 2022; 12(11):1692. https://doi.org/10.3390/coatings12111692
Chicago/Turabian StyleHuang, Yongtao, Ji Yu, Ning Tian, Jie Zheng, Yanmei Qu, Wenzhu Tan, and Yinxian Luo. 2022. "Performance of BaCe0.8Y0.2O3-δ Proton Electrolyte Materials for Solid Oxide Fuel Cells by Compositing the Transition Metal Oxide NiO" Coatings 12, no. 11: 1692. https://doi.org/10.3390/coatings12111692
APA StyleHuang, Y., Yu, J., Tian, N., Zheng, J., Qu, Y., Tan, W., & Luo, Y. (2022). Performance of BaCe0.8Y0.2O3-δ Proton Electrolyte Materials for Solid Oxide Fuel Cells by Compositing the Transition Metal Oxide NiO. Coatings, 12(11), 1692. https://doi.org/10.3390/coatings12111692





