Synthesis Optimization of Cadmium Carbonate Films as Potential Precursor to Produce CdSe, CdTe, and CdO Films
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
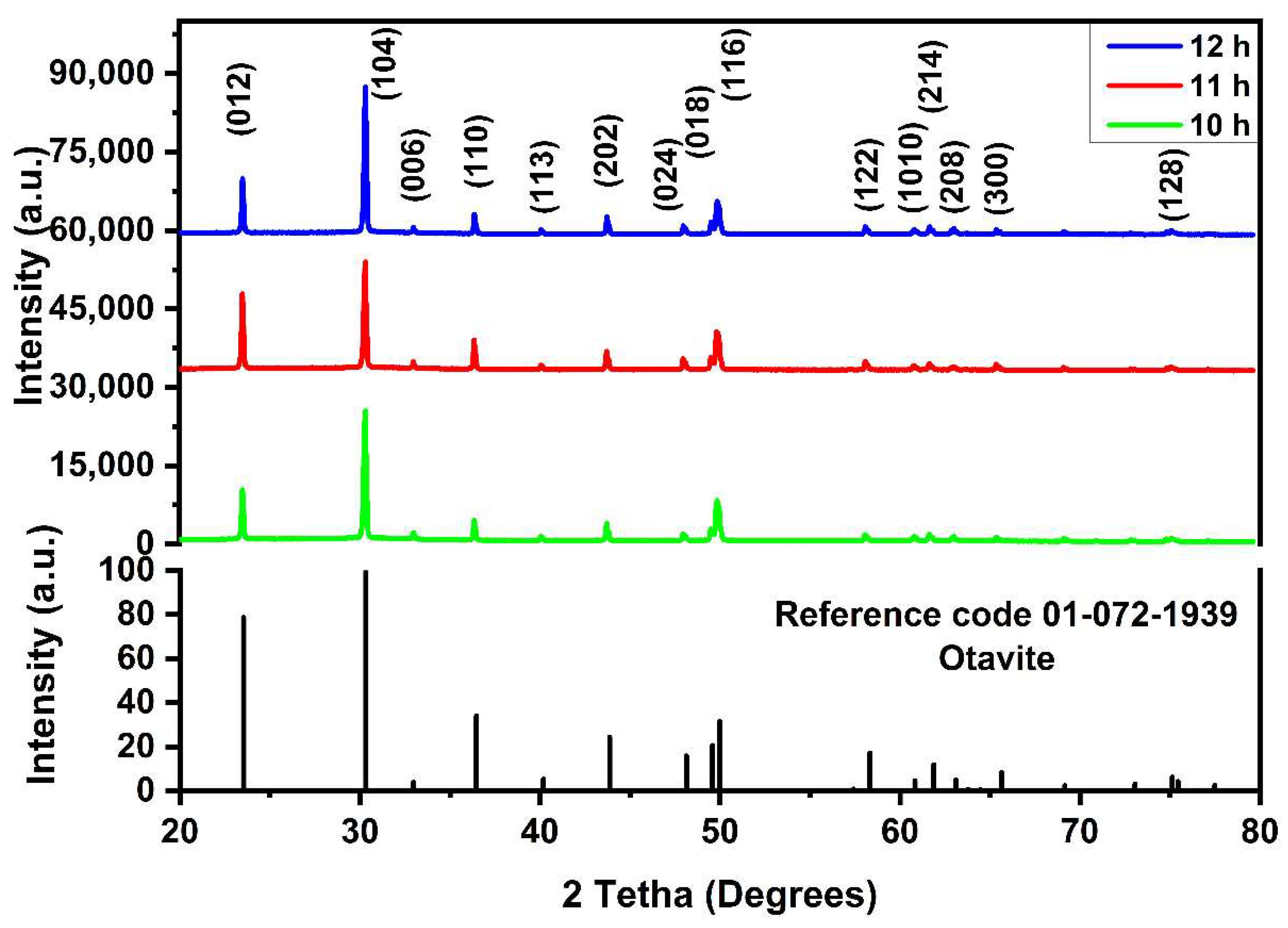
3.1. XRD Analysis
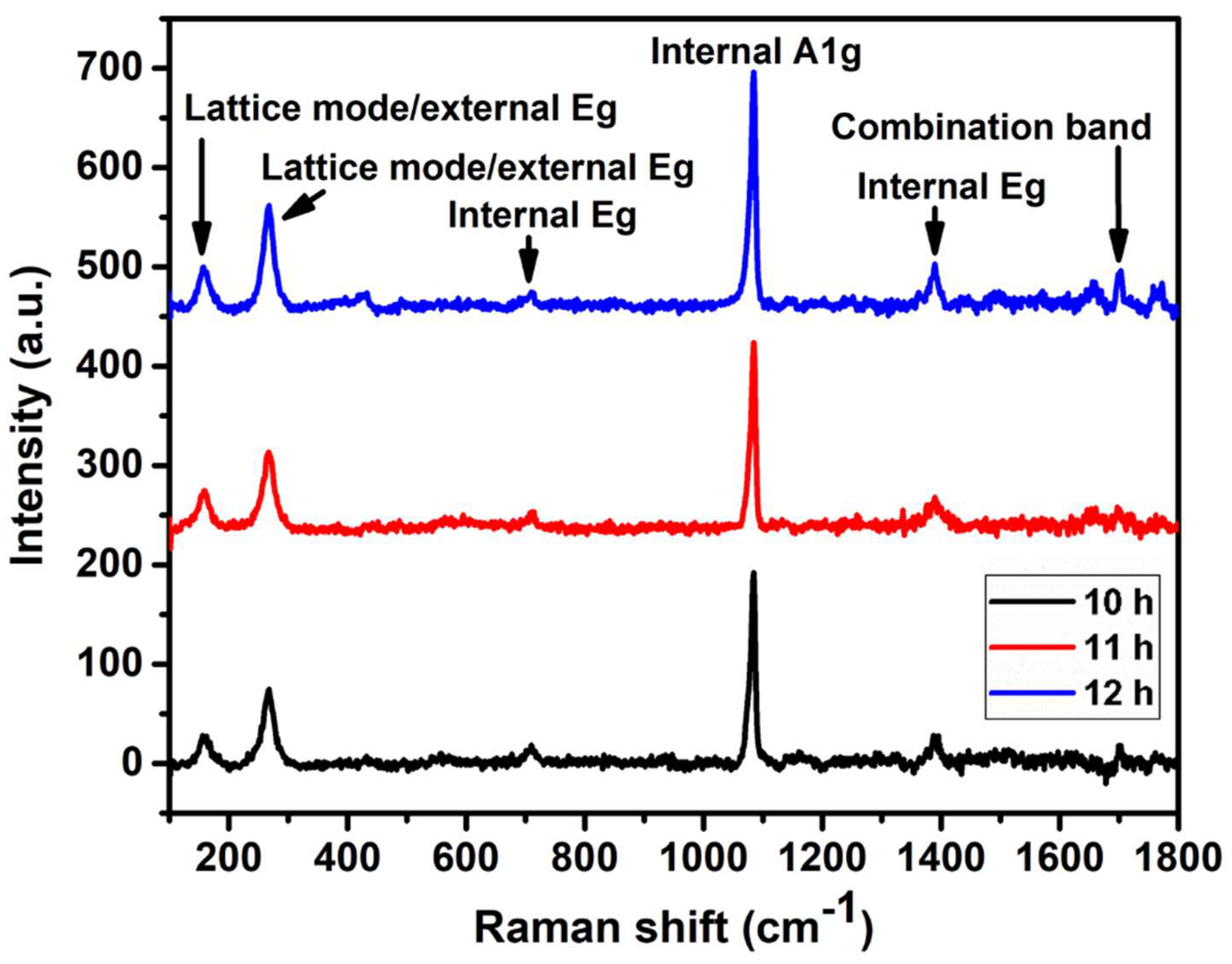
3.2. Raman Spectroscopy
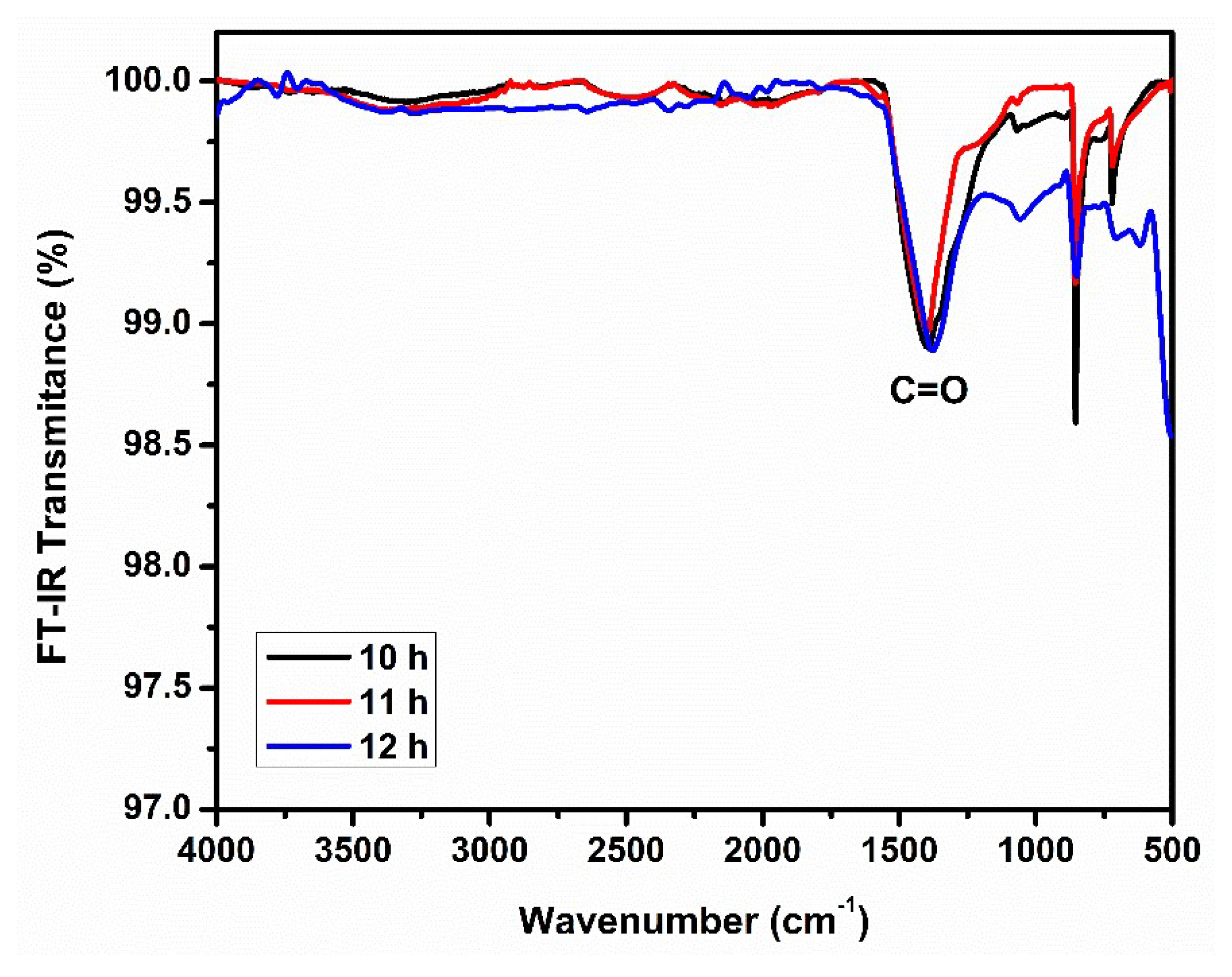
3.3. FTIR Spectroscopy
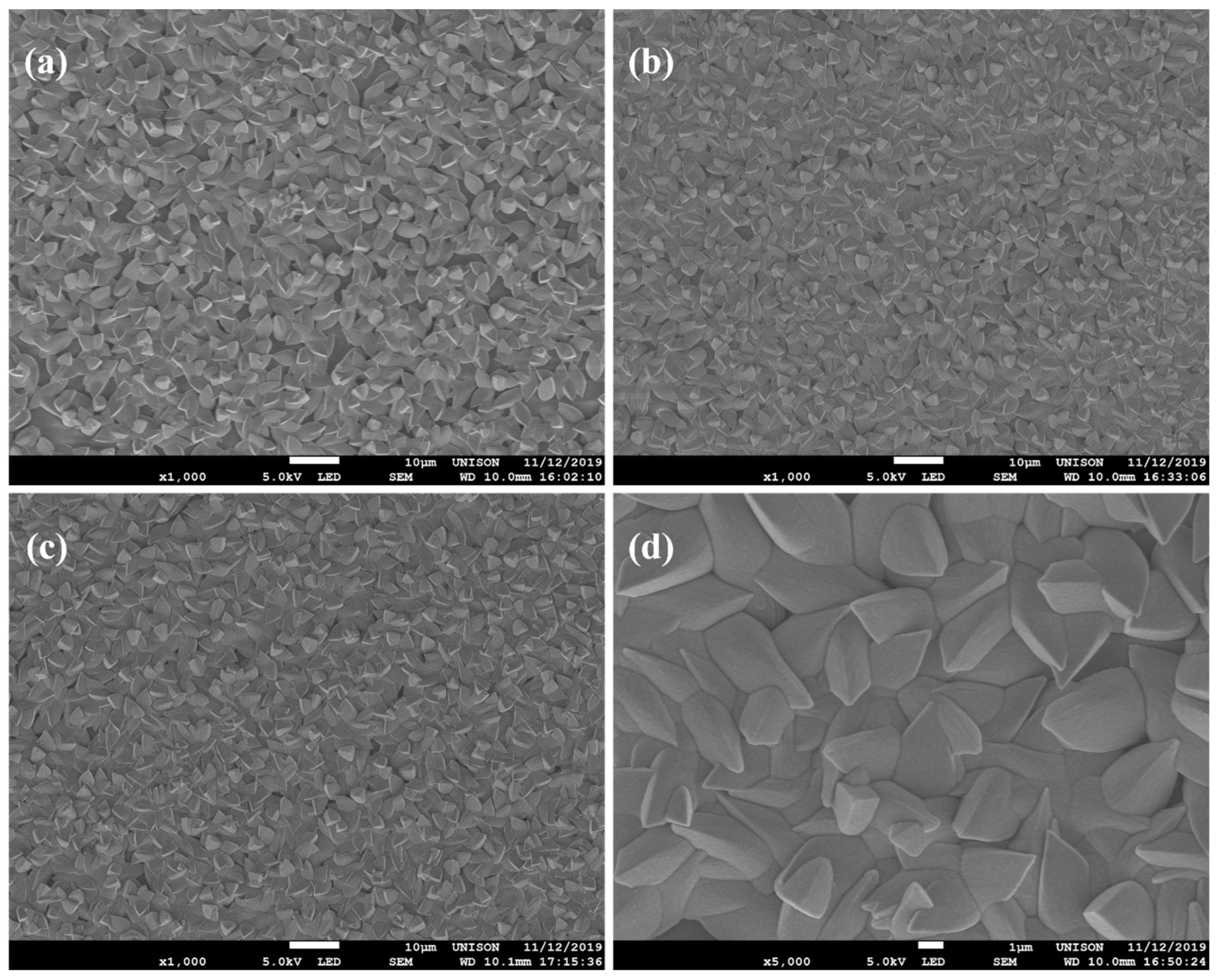
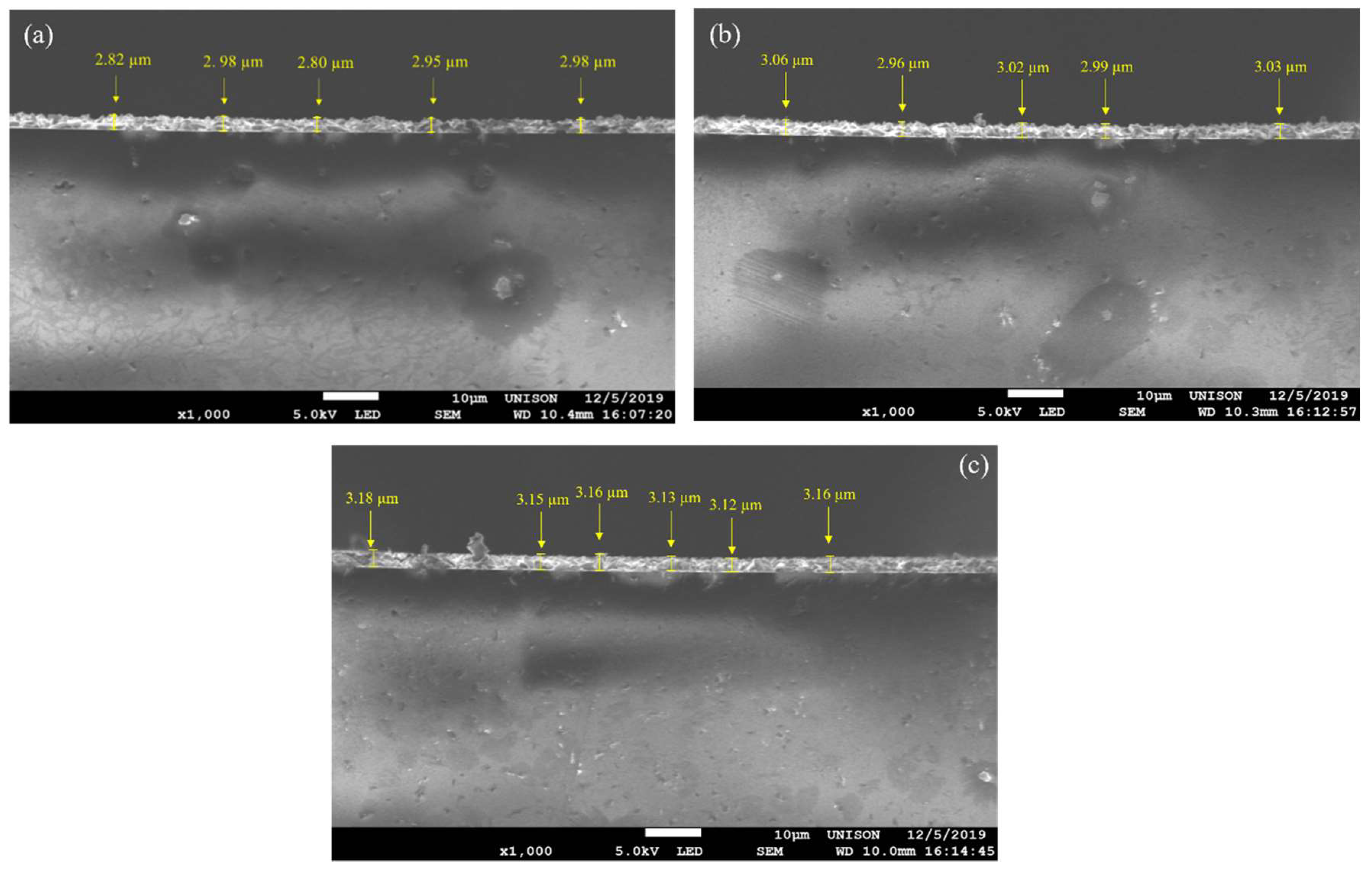
3.4. SEM and EDS
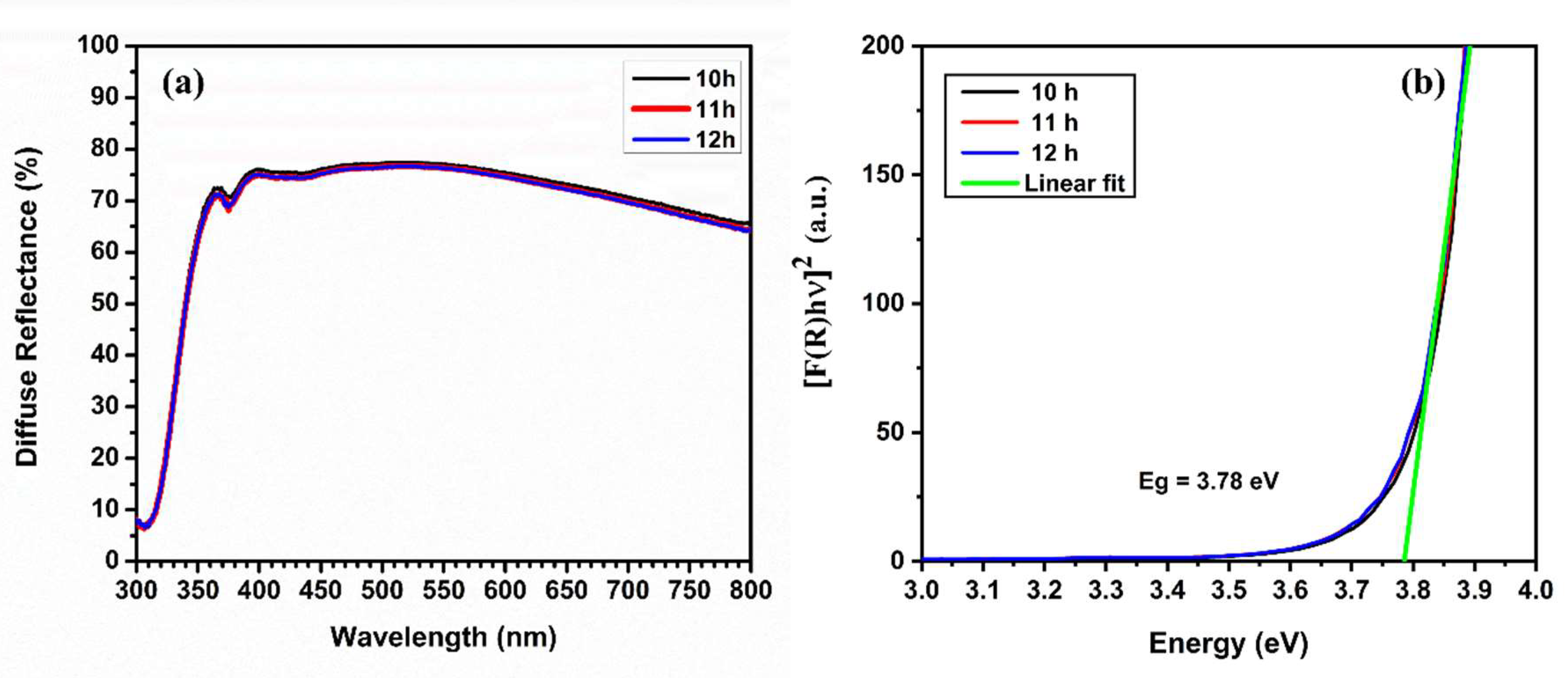
3.5. Reflectance and Direct Bandgap
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Hou, H.; Liu, G.; Zhang, X.; Lv, Q.; Yang, J.; Liu, J.; Qiao, G. Microstructures and optical properties of porous PbSe film prepared by ion exchange process. Mater. Sci. Semicond. Process. 2022, 147, 106741. [Google Scholar] [CrossRef]
- Fernández-Díaz, E.; Espinoza-Martinez, A.B.; Flores-Pacheco, A.; Ramírez-Bon, R.; Castillo, S.J.; Ochoa-Landin, R. Synthesis and Characterization of Silver Selenide Thin Films by Chemical Bath Deposition and Ionic Exchange. J. Electron. Mater. 2019, 48, 3405–3409. [Google Scholar] [CrossRef]
- Mattinen, M.; Hatanpää, T.; Sarnet, T.; Mizohata, K.; Meinander, K.; King, P.J.; Khriachtchev, L.; Räisänen, J.; Ritala, M.; Leskelä, M. Atomic Layer Deposition of Crystalline MoS2 Thin Films: New Molybdenum Precursor for Low-Temperature Film Growth. Adv. Mater. Interfaces 2017, 4, 1700123. [Google Scholar] [CrossRef]
- Urbiola, I.R.C.; Bon, R.R.; Vorobiev, Y.V. Lead Telluride Through Transformation of Plumbonacrite in Tellurium Atmosphere and Its Behavior as Part of PbTe–Si Photodiode. IEEE Sens. J. 2016, 16, 5875–5882. [Google Scholar] [CrossRef]
- Gujar, T.; Shinde, V.; Kim, W.-Y.; Jung, K.-D.; Lokhande, C.; Joo, O.-S. Formation of CdO films from chemically deposited Cd (OH) 2 films as a precursor. Appl. Surf. Sci. 2008, 254, 3813–3818. [Google Scholar] [CrossRef]
- Chávez Urbiola, I.R.; Ramírez Bon, R.; Vorobiev, Y.V. The transformation to cadmium oxide through annealing of cadmium oxide hydroxide deposited by ammonia-free SILAR method and the photocatalytic properties. Thin Solid Film. 2015, 592, 110–117. [Google Scholar] [CrossRef]
- Sotelo-Lerma, M.; Zingaro, R.A.; Castillo, S.J. Preparation of CdTe coatings using the chemical deposition method. J. Organomet. Chem. 2001, 623, 81–86. [Google Scholar] [CrossRef]
- Ochoa-Landín, R.; Castillo, S.J.; Ramírez-Bon, R. Growth from solution of CdTe films by conversion of chemically deposited cadmium oxide hydroxide films. Solar Energy 2012, 86, 3326–3330. [Google Scholar] [CrossRef]
- Chávez-Urbiola, I.R.; Chávez-Urbiola, E.A.; Ochoa-Landín, R.; Castillo, S.J.; Vorobiev, Y.V.; Ramírez-Bon, R. Cadmium selenide film through ammonia free thermal substitution reaction on cadmium oxide hydroxide films by chemical vapor deposition. Mater. Lett. 2014, 116, 254–257. [Google Scholar] [CrossRef]
- Balakhayeva, R.; Akilbekov, A.; Baimukhanov, Z.; Usseinov, A.; Giniyatova, S.; Zdorovets, M.; Vlasukova, L.; Popov, A.I.; Dauletbekova, A. CdTe Nanocrystal Synthesis in SiO2/Si Ion-Track Template: The Study of Electronic and Structural Properties. Phys. Status Solidi 2021, 218, 2000231. [Google Scholar] [CrossRef]
- Akilbekov, A.; Balakhayeva, R.; Zdorovets, M.; Baymukhanov, Z.; Komarov, F.F.; Karim, K.; Popov, A.I.; Dauletbekova, A. Ion track template technology for fabrication of CdTe and CdO nanocrystals. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 481, 30–34. [Google Scholar] [CrossRef]
- Karbovnyk, I.; Bolesta, I.; Rovetskyi, I.; Lesivtsiv, V.; Shmygelsky, Y.; Velgosh, S.; Popov, A.I. Long-term evolution of luminescent properties in CdI2 crystals. Low Temp. Phys. 2016, 42, 594–596. [Google Scholar] [CrossRef][Green Version]
- Heredia-Cancino, J.A.; Mendivil-Reynoso, T.; Ochoa-Landin, R.; Ramírez-Bon, R.; Castillo, S.J. Optical and structural properties of PbSe films obtained by ionic exchange of lead oxyhydroxicarbonate in a selenium-rongalite solution. Mater. Sci. Semicond. Process. 2016, 56, 90–93. [Google Scholar] [CrossRef]
- Heredia-Cancino, J.A.; Salcido, O.; Britto-Hurtado, R.; Ruvalcaba-Manzo, S.G.; Ochoa-Landín, R.; Castillo, S.J. CdS/PbSe Heterojunction Made via Chemical Bath Deposition and Ionic Exchange Processes to Develop Low-Cost and Scalable Devices. Appl. Sci. 2021, 11, 914. [Google Scholar] [CrossRef]
- Ruvalcaba-Manzo, S.G.; Castillo, S.J.; Ochoa-Landín, R.; Flores-Acosta, M.; Ramírez-Bon, R. Optical, structural, and morphological characterization of cadmium carbonate thin films by CBD two formulations. Opt. Mater. 2020, 109, 110295. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S.; Wu, X.; Wang, Z.; Wei, Q. Self-assemble, growth mechanism, and optical properties of complex and oriented 3D CdCO3 pyramids consist of single crystal parallel tetrahedrons. Chem. Eng. J. 2012, 195–196, 15–21. [Google Scholar] [CrossRef]
- Bucca, M.; Dietzel, M.; Tang, J.; Leis, A.; Köhler, S.J. Nucleation and crystallization of otavite, witherite, calcite, strontianite, hydrozincite, and hydrocerussite by CO2 membrane diffusion technique. Chem. Geol. 2009, 266, 143–156. [Google Scholar] [CrossRef]
- Rutt, H.N.; Nicola, J.H. Raman spectra of carbonates of calcite structure. J. Phys. C Solid State Phys. 1974, 7, 4522. [Google Scholar] [CrossRef]
- Minch, R.; Seoung, D.-H.; Ehm, L.; Winkler, B.; Knorr, K.; Peters, L.; Borkowski, L.; Parise, J.; Lee, Y.; Dubrovinsky, L.; et al. High-pressure behavior of otavite (CdCO3). J. Alloys Compd. 2010, 508, 251–257. [Google Scholar] [CrossRef]
- Chávez Portillo, M.; Chaltel Lima, L.A.; Peña Rosas, U.; Hernández Téllez, G.; Gutiérrez Pérez, R.; Portillo Moreno, O. Shape- and size controlled synthesis of CdCO3 in situ-doped-Er3+. Mater. Lett. 2014, 120, 130–132. [Google Scholar] [CrossRef]
- Chávez Portillo, M.; Sosa Sánchez, A.; Juárez Díaz, G.; Chaltel Lima, L.A.; Cruz Cruz, S.; Gutierrez Pérez, R.; Hernández Téllez, G.; Portillo Moreno, O. Optical, structural and morphological properties of CdS-CdCO3 films. Rev. Mex. Fis. 2015, 61, 83–87. [Google Scholar]
- Nevins, N.; Allinger, N.L. Molecular mechanics (MM4) vibrational frequency calculations for alkenes and conjugated hydrocarbons. J. Comput. Chem. 1996, 17, 730–746. [Google Scholar] [CrossRef]
- Ashoka, S.; Nagaraju, G.; Thipperudraiah, K.V.; Chandrappa, G.T. Controlled synthesis of cadmium carbonate nanowires, nanoribbons, nanorings and sphere like architectures via hydrothermal method. Mater. Res. Bull. 2010, 45, 1736–1740. [Google Scholar] [CrossRef]
- Moreno, O.P.; Pérez, R.G.; Portillo, M.C.; García, M.E.A.; Moreno, G.E.; Cruz, S.C.; Rosas, E.R.; Lascano, M.H. Morphological, optical and structural analysis in CdS, CdS-CdCO3 and CdCO3 thin solid films grown by chemical bath. Optik 2018, 157, 388–399. [Google Scholar] [CrossRef]
- Anthony, J.W. Handbook of Mineralogy; Mineral Data Pub.: Tucson, AZ, USA, 1990. [Google Scholar]
- Abdullahi, S.S.; Güner, S.; Musa, Y.; Adamu, B.I.; Abdulhamid, M.I. Sımple method for the determınatıon of band gap of a nanopowdered sample usıng Kubelka Munk theory. NAMP J. 2016, 35, 241–246. [Google Scholar]
- Alcaraz de la Osa, R.; Iparragirre, I.; Ortiz, D.; Saiz, J.M. The extended Kubelka–Munk theory and its application to spectroscopy. ChemTexts 2020, 6, 2. [Google Scholar] [CrossRef]
- Brik, M.; Srivastava, A.; Popov, A.I. A few common misconceptions in the interpretation of experimental spectroscopic data. Opt. Mater. 2022, 127, 112276. [Google Scholar] [CrossRef]
- Moreno Morales, G.E.; Araiza Garcia, M.E.; Cruz Cruz, S.; Rebollo Plata, B.; Portillo Moreno, O.; Gutiérrez Pérez, R. CdCO3 nanocrystalline thin film grown by chemical bath and its transition to porous CdO by thermal annealing treatment. Optik 2018, 171, 347–355. [Google Scholar] [CrossRef]
- Sahin, B.; Bayansal, F.; Çakmak, H.M.; Kahraman, S.; Çetinkara, H.A. Effect of heat treatment on the properties of Cd(OH)2 and CdO films grown by chemical bath deposition. Philos. Mag. Lett. 2013, 93, 101–108. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredia-Cancino, J.A.; Mendoza-Peña, K.J.; Higuera-Valenzuela, H.J.; Soto B., M.A.; Ochoa-Landín, R.; Castillo, S.J. Synthesis Optimization of Cadmium Carbonate Films as Potential Precursor to Produce CdSe, CdTe, and CdO Films. Coatings 2022, 12, 1691. https://doi.org/10.3390/coatings12111691
Heredia-Cancino JA, Mendoza-Peña KJ, Higuera-Valenzuela HJ, Soto B. MA, Ochoa-Landín R, Castillo SJ. Synthesis Optimization of Cadmium Carbonate Films as Potential Precursor to Produce CdSe, CdTe, and CdO Films. Coatings. 2022; 12(11):1691. https://doi.org/10.3390/coatings12111691
Chicago/Turabian StyleHeredia-Cancino, J. A., K. J. Mendoza-Peña, H. J. Higuera-Valenzuela, M. Anahí Soto B., R. Ochoa-Landín, and S. J. Castillo. 2022. "Synthesis Optimization of Cadmium Carbonate Films as Potential Precursor to Produce CdSe, CdTe, and CdO Films" Coatings 12, no. 11: 1691. https://doi.org/10.3390/coatings12111691
APA StyleHeredia-Cancino, J. A., Mendoza-Peña, K. J., Higuera-Valenzuela, H. J., Soto B., M. A., Ochoa-Landín, R., & Castillo, S. J. (2022). Synthesis Optimization of Cadmium Carbonate Films as Potential Precursor to Produce CdSe, CdTe, and CdO Films. Coatings, 12(11), 1691. https://doi.org/10.3390/coatings12111691






