Abstract
An improper indoor microclimate has adverse effects on the state of preservation of historical textiles arranged in them, favoring the development of bacteriological microflora. The current study aims to combine traditional and innovative methods for cleaning and preserving a 100-year-old traditional blouse from Bihor, Romania. The material of the blouse was impregnated with 30 and 70 ppm silver nanosuspensions and washed with a substance obtained from boiling natural wood ash (lye). The research goals were to determine the antimicrobial action of lye washing and silver nanoparticles applied to the analyzed textile material and identify the way in which the environmental factors (light) act upon the conservation degree of textile objects impregnated with silver nanoparticles. All these procedures are eco-friendly and do not cause any damage to the constituent material of the fabrics. The use of the hyperspectral imaging technique proved the permeation of both 30 and 70 ppm silver nanosuspensions into the textile, producing changes in the textile’s reflectance spectrum after being treated with them. The results showed anti-bactericidal/fungal properties of both silver nanoparticles and lye. Microbiological analyses revealed that bacterial colonies were reduced to more than 95% in both cases. The antibacterial effect of silver nanoparticles on the textile material of the blouse was maintained throughout the duration of the study, and under normal environmental conditions, the effects would remain active for a long period.
1. Introduction
The spaces in museums where rare, valuable objects are kept face feature conservation problems related to microclimatic conditions. The indoor microclimate is influenced by the architectural features of the building and construction techniques, materials, outdoor climate, etc. Furthermore, the level of biological contamination can determine the biodegradation of artifacts as it is directly influenced by the type of fabrics and furnishing materials, chemical composition, quality of the materials, aging process, internal mechanical stress, and the presence and activity of people (staff and visitors) [1,2]. All these aspects can cause museum exhibits to deteriorate over time with special emphasis on very old items or those made of easily degradable materials, such as textiles, paper or wood [3,4,5,6,7].
The desired preservation of exhibits can be achieved by using both traditional methods and the latest-generation methods. Most of the time, using both as complementary methods is a viable solution because it takes into account both the need to examine and preserve them and the financial aspect; moreover, many museums have limited funds for conservation–restoration interventions, which makes traditional methods useful [8]. Most of the important progress achieved in recent years in conservation and restoration work consists of using modern tools such as X-ray, infrared and Raman spectroscopy, integrated microscopy such as Atomic Force Microscopy (AFM), Scanning Electron microscopy (SEM), or dark-field microscopy, infrared and UV techniques, and 3D and laser scanning techniques [9,10,11], as well as the use of different classical methods, such as photogrammetry [12,13] or the application of different natural solutions [3,14,15,16]. All of these are appropriate proactive and reactive strategies for studying the characteristics of materials that ethnographic textiles are made of, their deterioration and degradation mechanisms, and their behavior in response to the actions of unconventional conservation treatments.
Applying nanotechnology in preserving heritage objects, especially those made of cotton, can constitute a base for the development of alternative techniques in order to complete and boost conventional disinfection, treatment, and conservation methods, providing durability and protection [17,18]. The benefits provided in the conservation of heritage textile objects by applying nanotechnology consist of increasing antibacterial protection and improving antistatic and hydrophobic features [19,20,21,22,23].
The increasing interest in the utilization of metal and mineral nanomaterials is mainly due to two specific properties. The first property is the high specific surface area of nanomaterials, substantially increasing their effectiveness. The second is related to the recent environmental and health concerns in recent decades. Recent developments in nanotechnology have provided the opportunity to utilize materials that are non-toxic to both humans and wildlife, making them quite suitable for a wide variety of indoor and outdoor applications [24,25,26,27].
Recently, special emphasis has been placed on the use of metallic nanoparticles in different sectors of activity. The mode of action of these metallic nanoparticles depends on a number of factors related to both the type of metal and the method of obtaining them (chemical, physical, or biological). The most frequently used nanoparticles are silver (AgNP), gold (AuNP), zinc oxide, titanium oxide (TiO2), silica, and iron oxide (FeO). Gold and silver nanoparticles are frequently used due to their unique physical properties, being less reactive and more stable in air and possessing a large contact surface and a high level of conductivity.
Among these, in the field analyzed in this study, silver nanoparticles have the highest degree of use. This metal is chemically inactive, stable in water, and does not oxidize in air. The optical, thermal, and catalytic properties of silver nanoparticles depend on the size, shape, distance between them, and the environment that surrounds them. Due to their high surface-to-volume ratio, silver nanoparticles show remarkable antimicrobial activity, even at a low concentration. AgNPs have unique properties in terms of toxicity, surface plasmon resonance, and electrical resistance. They also have low costs, low cytotoxicity, and immunological response. It is noted that the smaller the size of the nanoparticles, the more pronounced the antibacterial activity.
In this connection, the bactericidal and fungicidal effects of silver nanoparticles are efficiently increased due to the relatively large specific surface area and their reactivity to proteins and increased action capacity, even when longer periods of time have elapsed since washing them. Silver nanoparticles’ action mechanism is based on their electrostatic affinity towards the bacterial membrane, destabilizing its integrity, reducing cellular metabolism, and inhibiting microorganisms’ growth cells (approximately 99% reduction in bacterial growth within 1 h of contact), even in the case of small quantities of silver nanoparticles. The silver ions’ antimicrobial mechanism is based on membrane protein deterioration, the discrepancy with the electron transport system, and inhibition of respiratory enzymes in order to increase the production of Reactive Oxygen Species (ROSs) [28,29].
For a detailed examination, imaging technology started to generate better and better results, from multispectral information to hyperspectral information and non-invasive microscopy techniques with fast scanning to the nano-metric scale, which targets the topography of a non-conductive sample, providing 2D and 3D images. The applicative perspectives are of high quality and precision regarding the evaluation of heritage objects’ conservation state and monitoring the degradation/deterioration processes on and within the fibers [30], as well as characterizing and mapping the multi-material and polychromatic surfaces [31,32,33,34,35,36].
The Hyperspectral Imaging Technique (HSI), together with other techniques such as AFM or SEM, is useful in characterizing surfaces on a nanometric scale, evaluating them in real-time, and monitoring the efficacy of applied treatments (cleaning, antifungal, antistatic, etc.). It emphasizes the eventual presence of residues (qualitatively and quantitatively—measuring the roughness of the profile surface and section by using specific software). Characterizing the surface on multiple scales makes an important contribution to the restoration of cultural heritage objects, with the conclusions easily extrapolated to a larger scale. Furthermore, the interaction between metallic nanoparticles and different molecules was observed using hyperspectral imaging by evaluating the change in nanoparticles’ plasmonic resonance as a result of surface plasmons’ decoherence when interacting with various molecules or ions [37].
The current study aims to identify a combined and non-invasive cleaning and conservation methodology of a heritage textile object in a museum (a 100-year-old traditional blouse from Bihor area, Romania). The work methodology aims to combine traditional (washing with lye) and latest-generation (application of silver nanoparticles in concentrations of 30 and 70 ppm) interventions. The analyzed portions were monitored using hyperspectral images, both before and after the application of the treatments, in order to identify the inhibitory effects on the microorganisms present on the fabric, as well as to prevent recontamination of the material.
2. Literature Review
Over the past two decades, metal and mineral nanoparticles and nanostrands have been used to mitigate the susceptibility of biological materials to different bacteria and fungi [38,39,40,41]. Specifically, silver, copper, and zinc nanometals have been clearly reported to significantly improve the biological durability against different fungi [42,43]. As mineral nanomaterials, nanowollastonite and nano-clay also successfully prohibited the growth of different fungi on wood, wood-based composite panels, paper, textiles, and other biomaterials composed of cellulose [41,44,45,46,47].
From the perspective of AgNPs’ antibacterial action, higher sensitivity of Gram-negative bacterial strains compared to Gram-positive strains was noted. Recent studies focused on the effects of AgNPs on the sensitiveness of two Gram-positive bacterial species proved that AgNPs act differently; thus, they are more efficient on Staphylococcus cells and less efficient on Bacillus cereus. Comparisons between AgNPs’ degree of activity on two Gram-negative species indicated higher sensitivity in the case of Pseudomonas aeruginosa than Escherichia coli. There were also correlations made [18,19,20,23,48,49,50,51,52,53,54,55] between the size and shape of AgNPs and the antibacterial and bacterial-static activity, unpleasant smell prevention, decrease in pathogenic germ level, etc.
A comparative analysis of the sensitivity of two Gram-positive bacterial species proved that AgNPs acted efficiently (the inhibition area diameter was between 12.8 mm and 11.4 mm) on Staphylococcus cells in the case of all tested samples (after several washes), while in the case of Bacillus cereus, antimicrobial activity was present but diminished (the inhibition area diameter was between 11.3 mm and 10.3 mm) for one sample and disappeared in the case of treated and washed samples. Regarding Gram-negative bacterial species, Pseudomonas aeruginosa showed higher sensitiveness to AgNPs with its inhibition area diameter between 12.6 mm and 12.3 mm, while in the case of Escherichia coli, it was between 11.3 mm and 10.3 mm, even after 20 washing cycles [56,57].
There are numerous studies (we mention, in this respect, Monteiro et al. [57]) that confirm AgNPs’ antifungicide effect against Candida spp., alone or in combination with classical antifungicides, such as nystatin. The destruction of Cryptosporidium parvum, Bacillus subtilis, and Pseudomonas aeruginosa viability has also been proven [18,58,59,60,61], and that of Fusarium oxysporum and respective nanoparticles in concentrations of 10 and 20 ppm were deposited on cotton fabric in order to analyze their bactericidal effect upon Staphylococcus aureus [62]. The synthesized AgNP-coated fabrics (8–20 nm in size) provided pronounced antibacterial (Aspergillus terreus) activity and excellent laundering durability [63]. The destructive action of AgNPs was also noted in Bacillus subtilis, Klebsiella mobilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonie, Streptococcus pyogenes, and Salmonella typhi [29]. At the same time, they had antifungicide actions against Candida albicans, Penicillium citrium, and Aspergillus niger [57].
Research on AgNP application on biomedical fabrics emphasized their destructive activity in Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive) bacteria, even after 20 washing cycles [55,58], as well as the viability of Cryptosporidium parvum, Bacillus subtilis, and Pseudomonas aeruginosa [18,59,60,61,62]. Kim et al. [62] stated that 10 and 20 ppm concentrations of silver nanoparticles act upon Fusarium oxysporum. Synthesized AgNP-coated fabrics (8–20 nm in size) displayed pronounced antibacterial (Aspergillus terreus) activity and excellent laundering durability [63].
The modern methodologies for studying the structure and physico-chemical properties of stable silver nanoparticles in solutions applied to cotton sample fabrics are UV-VIS spectroscopy, AFM (Atomic Force Microscopy), and TEM (Transmission Electron Microscopy) [55]. The interaction between bacteria Escherichia coli and citrate-reduced silver nanoparticles (AgNPs) (size 25 nm ± 8.5 nm) was studied by the team led by Vishnupriya [64] using Raman Spectroscopy (RS) in conjunction with hyperspectral images obtained through plasmon resonance. The fact that AgNPs can be fixed and well dispersed on the cotton fabric’s surface was demonstrated through a series of analyses: Fourier Transform Infrared Spectroscopy (FTIS), Ultraviolet-Visible spectroscopy (UV), X-ray diffraction, X-ray photoelectron spectroscopy, Scanning Electron Microscopy (SEM), and Energy-Dispersive X-ray Spectroscopy (Table 1) [56].

Table 1.
The working methodology used in the reference bibliography for nanoparticle impregnation and material analysis.
Most of the articles presented in Table 1 classify nanoparticles as non-destructive, versatile, and cheap solutions for cleaning materials, with their efficiency remaining high even after several washes in the case of textiles. Limitations are constituted by the lack of studies comparing the different available techniques, the ability of nanoparticles to remain incorporated without losing their efficiency, and their long-term effects on human health and the integrity of materials.
Some scientific research has also emphasized the fact that the antistatic features of the fabrics can be improved (especially regarding synthetic fibers) by using nanotechnology, e.g., dioxide, zinc oxide whiskers, nanoantimonydoped tin oxide (ATO), silanenanol, etc. [20,23].
3. Materials and Methods
In order to emphasize AgNPs’ effects on 100-year-old homemade cotton fabric textiles and the conservation conditions of the heritage object, the working methodology was divided into three stages, as shown in Figure 1.
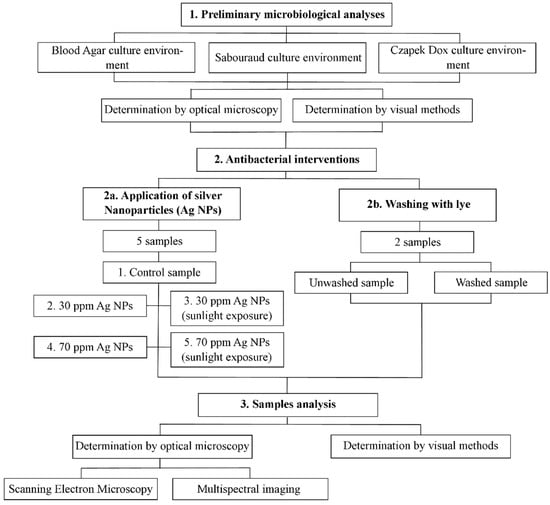
Figure 1.
Schematic diagram of the main methodological stages of the study.
3.1. Preliminary Microbiological Analyses
The first stage was represented by the microbiological analyses undertaken on the material from which the heritage textile piece is made. In this sense, the collection of microbiological samples, their cultivation on Petri dishes, and the identification of their degree of microbiological contamination were considered.
With the help of sterile swabs, microbiological samples were collected from five distinct areas within the object, with these being delimited as the work surfaces. After preservation, each swab was unloaded in three culture environments, as follows: The Blood Agar (bioMérieux-CRAPONNE-France), Sabouraud with Chloramphenicol and Gentamicyn (Mikrobiologie labor-technik, Arad, Romania), and the Czapek Dox environments (Biolab, Budapest, Hungary). The Czapek Dox environment is usually used to cultivate fungi such as Candida, Aspergillus, Penicillium, and Actinomicete and to form Chlamydospori by C. albicans. The Sabouraud Agar Chloramphenicol Gentamicyn environment is another culture environment recommended for the isolation of filamentous fungus species from biological samples. Gentamicyn and chloramphenicol inhibit bacteria development, such as Enterobacteriaceae, Pseudomonas, and Staphylococci. Blood agar is an environment used to cultivate saprophytes and pathogenic bacteria.
The culture media were incubated at a temperature of 28 °C for 10 days in order for the colonies to develop. Starting with the fourth day, the colonies began to be visible, and the isolation and identification of the fungi and bacteria developed on the culture media were carried out. Plates were evaluated daily by observing their evolution in terms of changes in the appearance of the colonies in question, with the assessment undertaken sequentially, following the order of shape, texture, consistency, diameter, color, and outline.
3.2. Antibacterial Interventions
The cleaning and preservation interventions of the textile material constituted a combination of traditional and latest-generation methods. This was performed in order to analyze the antibacterial results offered by two completely different methods in terms of the materials used and the method of application, as well as to draw up a work methodology for the preservation–disinfection of historical textiles that face a high degree of fragility. Therefore, the traditional method consisted of washing the material with lye (an ecological substance obtained from ash) while the innovative method involved the use of AgNPs. The samples used were examined with the help of the Hyperspectral Microscope System produced by CytoViva (Auburn, AL, USA), both before and after applying the treatments. This system allows the analysis and spectral mapping of the samples at the nanometric scale. It also allows non-invasive measurements based on the resulting hyperspectral images within the compatible CytoViva ENVI software.
Washing with Lye
Lye is an ecological substance obtained from boiling wood ash in fresh spring or river water. This is traditionally used in different parts of Romania to wash and disinfect textile materials. When properly prepared it is recognized as having high antifungal effects while also being non-invasive regarding the material from which the fabric is made [55]. Its eco-friendly properties are provided by the fact that it is entirely of natural origin and not chemically treated, existing as a mixture of minerals. In addition to its antifungal properties when washing different types of natural fabrics, it is also used for application on the hair and skin in order to treat various ailments.
For the present study, the substance was prepared from the very fine ash of Fagus Sylvatica, resulting from the natural burning of the wood. Thus, one liter of fresh ash was mixed with 10 L of water, boiled for 30 min, and then left to soak for 48 h. After this period, the entire composition was filtered several times through different sieves with a small diameter to remove all impurities. The surfaces of the analyzed object were soaked in the resulting solution for one hour and rinsed with clean water afterward.
3.3. Application of Silver Nanoparticles (AgNPs)
For the application of silver nanoparticles, the sampling of five material samples from the analyzed object was considered. The sample preparation was performed in aseptic conditions in a bacteriological hood with laminar flow to avoid further sample contamination, which could distort the results (Figure 2).

Figure 2.
Preparation of cotton fabric samples for AgNP impregnation.
The experiment focused on impregnation of the above-mentioned textile with two concentrations of 30 ppm and 70 ppm silver nanosuspensions. Since application in the form of functional coatings plays a protective role for textile materials, we opted for this method in this study. Two types of silver nanoparticle solutions with sizes of 30 nm and 70 nm from Pure Life Group SRL, Bucharest, Romania were used. Because the samples had the same sizes, impregnation with 30 ppm and 70 ppm concentrations of the AgNP solution was performed manually by adding the same volume of solution for each type of nanoparticle (1 mL). To emphasize the influence that silver nanoparticles have on the analyzed fabrics, we used a hyperspectral microscope with improved dark-field technology, which allowed us to obtain morphological and structural information on the molecular level based on spreading the light on the sample components. In order to emphasize the antibacterial property of the silver nanoparticles after a period of time following application and under circumstances of sunlight exposure, we determined the antimicrobial activity of AgNPs-treated samples (both 30 and 70 ppm) exposed to sunlight for 10 days (Samples 4 and 5).
3.4. Samples Analysis
The samples were analyzed both before and after the interventions with the help of electron microscopy and hyperspectral imaging. The microscope allows non-destructive spectral measurements of the samples and the detection of metallic nanoparticles and metallic oxides in textile fabrics. The hyperspectral data were recorded at a spectral resolution of between 400 and 1000 nm of approximately 2 nm. For each pixel from the image, a reflectance spectrum was acquired. Compared to multispectral and ultraspectral imaging, this type of imaging (hyperspectral) is superior due to the improved spectral resolution and the fact that each pixel in the image has a complete spectrum.
The CytoViva microscope allows the observation and spectral characterization of a large variety of nanomaterials while they interact with biological environments or other complex matrices. The basic principle that hyperspectral imaging relies on is the fact that any material, because of its physical structure and chemical composition, interacts with electromagnetic radiation in a unique manner (its spectral signature and spectral print). Among the nanomaterials that can be visualized and characterized from a spectral perspective are noble metals, polymers, lipids, bacteria, and viruses. For each sample, a thread was collected, which was placed on a microscope slide to obtain images and hyperspectral data in the dark field. The sample was focalized using a 40× (N.A.0.60) lens. After the acquisition, the region of interest was selected from each sample, and the average reflectance spectrum was calculated for it.
4. Results and Discussions
4.1. Preliminary Microbiological Analyses
The identification of fungi and bacteria was performed after evaluating the macroscopical and microscopical characters. On the 10th day, the cultures were examined. On the blood agar culture, the results were negative (no culture developed) (Figure 3a). On the Sabouraud and Czapek Dox cultures, with samples from batches 2 and 3, Penicillium spp. developed with colonies that developed rapidly and were green, blue-green, grey-green (2), and yellow (3) in color. The colony surface was more powdered. The presence of Penicillium spp. was microscopically confirmed (Figure 3b and Figure 4).
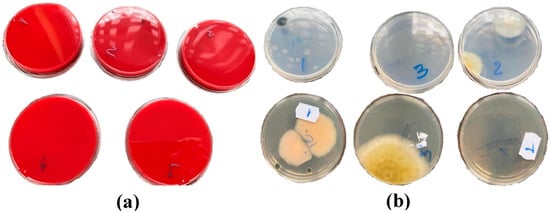
Figure 3.
Plates seeded with primary samples (before interventions) on the 10th day of incubation ((a) dishes with Blood Agar culture environment, samples 1–5; (b) dishes with Sabouraud culture (top) and Czapek Dox (bottom) culture environments, samples 1–3).

Figure 4.
Microscopical aspects sample ((a) Cladosporium spp.; (b) Penicillium spp.).
4.2. Antibacterial Interventions
After the samples were treated with 30 ppm and 70 ppm AgNPs, specimens were taken again with sterile swabs and seeded again in the same culture environments: Blood Agar, Sabouraud with Chloramphenicol and Gentamicyn, and Czapek Dox, with incubation at 28 °C temperature and observation for 7 days (Figure 5).
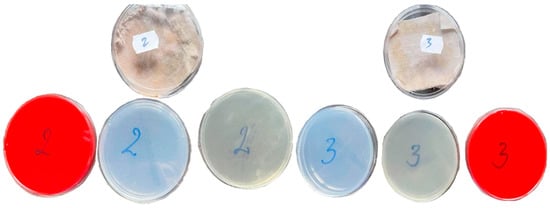
Figure 5.
Culture plates after NNPSAg treatment of samples 2 and 3.
For the best possible accuracy of the results, the samples were tested for microbiological content twice, and then their arithmetic mean was calculated. For the textiles treated with silver nanoparticles, small pieces of fabric were analyzed. For each fabric sample, the mean reflectance spectrum was calculated. For fabrics treated with the 70 ppm nanosuspension, the nanoparticles were incorporated into the fabric since there are noticeable areas where there is a spectrum specific to the silver nanoparticles’ extinction spectrum. The dark-field images with the delimitation of one silver nanoparticle and the specific extinction spectrum are presented in Figure 6. For the fabric treated with 30 ppm nanoparticles, on the spectra from the brighter areas, the incorporation of nanoparticles into the fabric cannot be evaluated using hyperspectral imaging because of the detector’s limitation to under 400 nm. The resonance of silver nanoparticles’ surface plasmons does not have similar shapes to the spectra specific to silver nanoparticles.
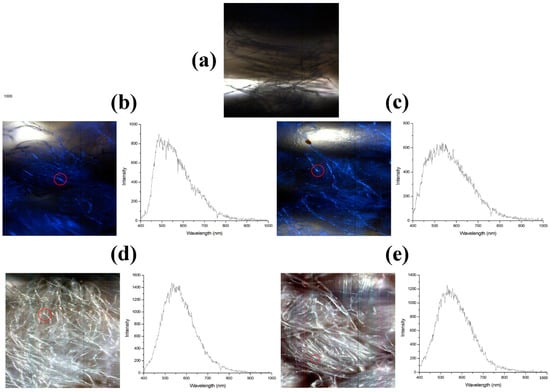
Figure 6.
Dark field images for fabrics treated with silver nanoparticles and the cattering spectra of AgNPs in the fabrics acquired in the marked regions. (a) Control fabric, untreated (b) Fabric treated with AgNPs 30 nm (c) Fabric treated with AgNPs 30 nm and exposed to light (d) Fabric treated with AgNPs 70 nm (e) Fabric treated with AgNPs 70nm and exposed to light.
For each fabric, the spectra specific to the points are indicated, which represent the AgNPs present. For each fabric, the mean spectrum was calculated on an area delimitated around the nanoparticle (Figure 7). We noted a slight shift toward smaller wavelengths in the case of the mean reflectance spectrum of the control fabric compared to that of the fabrics treated with silver nanoparticles.
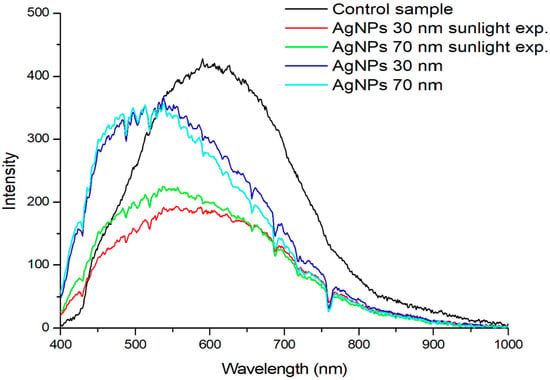
Figure 7.
Mean reflectance spectrum of fabrics.
Figure 7 shows the reflectance spectrum of fabrics treated with AgNPs compared to the control fabric. For each fabric, the average spectrum was calculated on a region delimited around the nanoparticle. A shift towards smaller wavelengths can be noted, irrespective of AgNPs’ dimensions and light exposure. No significant difference was observed between the reflectance spectra of fabrics impregnated with either 30 ppm or 70 ppm nanosuspensions. However, exposure to light led to a significant decrease in the intensity of the reflectance spectra and a slight shift towards larger wavelengths.
Regarding the treatment of the material through traditional method, sample threads from the washed sample and the unwashed sample were measured using the hyperspectral imaging technique. After applying the treatment (Figure 8a), aesthetic changes could be observed in the threads that make up the material, with these being much cleaner than the initial ones (Figure 8b). This can be explained by the reduction in the volume of dust within the fibers and their microbiological load.
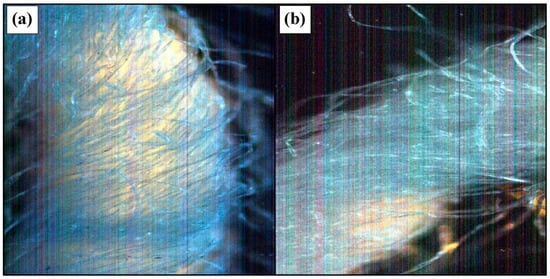
Figure 8.
Dark-field image of a thread from the cotton fabric ((a) before washing; (b) after lye washing).
We acquired hyperspectral images for the two threads taken from the fabric before and after washing. For comparison, the reflectance spectra for the two samples are shown in Figure 9.
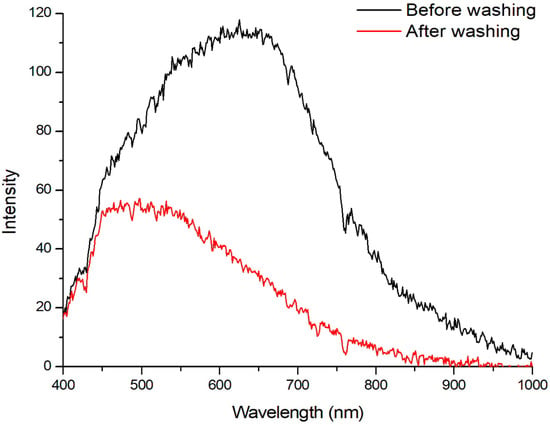
Figure 9.
Mean spectra for the two cotton threads before washing and after lye washing.
We noticed a shift in the thread’s mean spectrum toward smaller wavelengths after the fabric was washed (Figure 9). The reflectance spectrum shift may be the result of eliminating certain components through washing.
Microscopic images of the cotton fabric samples analyzed before and after lye washing were obtained using the CytoViva optical microscope and are presented in Figure 8.
5. Conclusions
Applying non-invasive techniques in the conservation process of heritage objects represents a vital goal for the future to improve the durability of priceless historical heritage. Moreover, it provides a properly protective environment for objects, museum areas, and visitors. Silver nanoparticles applied in 30 and 70 ppm concentrations and washing with lye both had antibacterial/antifungal properties, with microbiological analyses emphasizing the fact that bacterial colonies were diminished by over 95%, a property that was maintained during the time the study was conducted. Using HSI (hyperspectral imaging), analyses of the fabric’s reflectance spectra after treatment with silver nanoparticles provided information regarding the permeation of AgNPs (both 30 and 70 ppm) into the fabric. Moreover, through imaging analysis, we did not notice major changes in the cotton thread as a result of lye washing. As silver is non-toxic and possesses antimicrobial properties, the use of AgNPs is encouraged as coatings on textile fabrics of different types, even on aged fabric.
6. Perspective
The information regarding fibers’ adhesive and elastic properties, in addition to other details such as 2D height and 3D images of the fiber surface, surface roughness, and other parameters of the surface texture, was obtained with the help of AFM (Atomic Force Microscopy) and was used to compare various types of fibers and establish and monitor the damage caused by exposure to different environments and their effects over time on fabric fibers. The main research directions that we have in mind are focused on the synthesis of nanoparticles with properties controlled by the use of reaction/stabilization agents that are as environmentally friendly as possible, as follows:
- -
- In the processes of obtaining silver nanoparticles, the use of certain plant extracts obtained from plants specific to areas from which the cult objects analyzed in this study come as reducer and stabilizing agents to stabilize the silver nanoparticles and follow the dynamics of the rate of increase of silver nanoparticles depending on the operating conditions.
- -
- Expanding the research using gold nanoparticles obtained using the vegetable extracts mentioned in the previous paragraph and comparing their efficiency to those of nanosilver obtained under the same working conditions.
- -
- Synthesis of nanomaterials with controlled properties, using environmentally friendly reagents.
Author Contributions
Conceptualization: A.I., N.H. and D.C.I.; methodology: E.P., M.Z., S.I. and A.C.; software: M.Z., S.I. and C.G.; validation: L.I. and T.C.; formal analysis: A.I., H.R.T. and M.C.; investigation: D.C.I., T.C., M.Z., M.C. and A.I.; resources: L.I., N.H., D.C.I., T.C. and E.P.; data curation: M.C. and C.G.; writing—original draft preparation: D.C.I., M.Z., S.I., E.P. and H.R.T.; writing—review and editing: L.I., N.H. and Ș.B. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the partially contribution of University of Oradea Grants, Competition “Scientific Research of Excellence Related to Priority Areas with Capitalization through Technology Transfer: INO–TRANSFER–UO- Second edition”, Projects No. 232/28.10.2022. Title of the project—Reuse of textile waste in interior decorations.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study may be obtained on request from the corresponding author.
Acknowledgments
The research undertaken was made possible by the equal scientific involvement of all the authors concerned.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pasquariello, G.; Crupi, G.; Strippoli, S.; Maggi, O.; Colaizzi, P.; Balocco, C.; Albertini, R.; Pasquarella, C. Microbial environmental monitoring in museums: Preventive conservation of graphic collections. Conserv. Sci. Cult. Herit. 2016, 14, 275–289. [Google Scholar]
- Franco-Castillo, I.; Hierro, L.; de la Fuente, J.M.; Seral-Ascaso, A.; Mitchell, S.G. Perspectives for Antimicrobial Nanomaterials in Cultural Heritage Conservation. Chem 2021, 7, 629–669. [Google Scholar] [CrossRef]
- Ilieș, D.C.; Hodor, N.; Indrie, L.; Dejeu, P.; Ilieș, A.; Albu, A.; Caciora, T.; Ilieș, M.; Barbu-Tudoran, L.; Grama, V. Investigations of the Surface of Heritage Objects and Green Bioremediation: Case Study of Artefacts from Maramureş, Romania. Appl. Sci. 2021, 11, 6643. [Google Scholar] [CrossRef]
- Ilieș, D.C.; Marcu, F.; Caciora, T.; Indrie, L.; Ilieș, A.; Albu, A.; Costea, M.; Burtă, L.; Baias, Ș.; Ilieș, M.; et al. Investigations of Museum Indoor Microclimate and Air Quality. Case Study from Romania. Atmosphere 2021, 12, 286. [Google Scholar] [CrossRef]
- Ilies, D.C.; Zlatev, Z.; Ilies, A.; Zharas, B.; Pantea, E.; Hodor, N.; Indrie, L.; Turza, A.; Taghiyari, H.R.; Caciora, T.; et al. Interdisciplinary Research to Advance Digital Imagery and Natural Compounds for Eco-Cleaning and for Preserving Textile Cultural Heritage. Sensors 2022, 22, 4442. [Google Scholar] [CrossRef]
- Wendt, J.A.; Indrie, L.; Dejeu, P.; Albu, A.; Ilieș, D.C.; Costea, M.; Caciora, T.; Ilieș, G.; Hodor, N.; Josan, I.; et al. Natural Sources in Preventive Conservation of Naturally Aged Textiles. Fibres Text. East. Eur. 2021, 29, 80–85. [Google Scholar] [CrossRef]
- Bou-Belda, E.; Indrie, L.; Ilies, D.C.; Hodor, N.; Berdenov, Z.; Herman, G.V.; Caciora, T. Chitosan—A non-invasive approach for the preservation of historical textiles. Ind. Text. 2020, 71, 576–579. [Google Scholar] [CrossRef]
- Marcu, F.; Ilieș, D.C.; Wendt, I.A.; Indrie, L.; Ilieș, A.; Burta, L.; Caciora, T.; Herman, G.V.; Baias, S.; Albu, A. Investigations Regarding the Biodegradation of the Cultural Heritage. Case Study of Traditional Embroidered Peasant Shirt (Maramures, Romania). Rom. Biotechnol. Lett. 2020, 25, 1362–1368. [Google Scholar] [CrossRef]
- Demenchuk, E.; Ilies, D.C.; Wendt, J.A.; Koroleva, Y.; Ilies, A.; Goikhman, A.; Maznitsyna, E.; Caciora, T.; Herman, G.; Bilcec, M. Spectroscopy study of heritage objects for the digitization of cultural heritage. J. Environ. Eng. Landsc. Manag. 2020, 19, 1057–1066. [Google Scholar]
- Lazic, V.; Vadrucci, M.; Fantoni, R.; Chiari, M.; Mazzinghi, A.; Gorghinian, A. Applications of Laser-Induced Breakdown Spectroscopy for Cultural Heritage: A Comparison with X-ray Fluorescence and Particle Induced X-ray Emission Techniques. Spectrochim. Acta Part B At. Spectrosc. 2018, 149, 1–14. [Google Scholar] [CrossRef]
- Xu, K.; Sun, W.; Shao, Y.; Wei, F.; Zhang, X.; Wang, W.; Li, P. Recent Development of PeakForce Tapping Mode Atomic Force Microscopy and Its Applications on Nanoscience. Nanotechnol. Rev. 2018, 7, 605–621. [Google Scholar] [CrossRef]
- Caciora, T.; Herman, G.V.; Baias, S. Computer analysis of a heritage item—The traditional sheepskin waistcoat in Beius area. Rev. Etnogr. Folc. J. Ethnogr. Folk. 2021, 1–2, 195–209. [Google Scholar]
- Albu, A.V.; Caciora, T.; Berdenov, Z.; Ilies, D.C.; Sturzu, B.; Sopota, D.; Herman, G.V.; Ilies, A.; Kecse, G.; Ghergheles, C.G. Digitalization of garment in the context of circular economy. Ind. Text. 2021, 74, 102–107. [Google Scholar] [CrossRef]
- Guiamet, P.S.; Gómez de Saravia, S.G.; Arenas, P.; Pérez, M.L.; De la Paz, J.; Borrego, S.F. Natural products isolated from plants used in biodeterioration control. Pharmacologyonline 2006, 3, 537–544. [Google Scholar]
- Barresi, G.; Carlo, E.; Trapani, M.R.; Parisi, M.G.; Chillè, C.; Mulè, M.F.; Cammarata, M.; Palla, F. Marine organisms as source of bioactive molecules applied in restoration projects. Herit. Sci. 2015, 3, 17–20. [Google Scholar] [CrossRef]
- Borrego, S.; Valdés, O.; Vivar, I.; Lavin, P.; Guiamet, P.; Battistoni, P.; Gómez de Saravia, S.; Borges, P. Essential oils of plants as biocides against microorganisms isolated from Cuban and Argentine Documentary heritage. ISRN Microbiol. 2012, 826786. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, X.; Chen, Y.; Lin, H. Application of Silver Nanoparticles to Cotton Fabric as an Antibacterial Textile Finish. Fibers Polym. 2009, 10, 496–501. [Google Scholar] [CrossRef]
- Pietrzak, K.; Gutarowska, B.; Machnowski, W.; Mikołajczyk, U. Antimicrobial Properties of Silver Nanoparticles Misting on Cotton Fabrics. Text. Res. J. 2016, 86, 812–822. [Google Scholar] [CrossRef]
- Burniston, N.; Bygott, C.; Stratton, J. Nano Technology Meets Titanium Dioxide. Surface Coat. Int. Part A 2004, 87, 179–814. [Google Scholar]
- Kanti Das, T.; Ganguly, S.; Remanan, S.; Das, N.C. Temperature-dependent Study of Catalytic Ag Nanoparticles Entrapped Resin Nanocomposite towards Reduction of 4-nitrophenol. ChemistrySelect 2019, 4, 3665–3671. [Google Scholar] [CrossRef]
- Das, T.K.; Remanan, S.; Ghosh, S.; Das, N.C. An Environment Friendly Free-Standing Cellulose Membrane Derived for Catalytic Reduction of 4-Nitrophenol: A Sustainable Approach. J. Environ. Chem. Eng. 2021, 9, 104596. [Google Scholar] [CrossRef]
- Lei, Q.; Juan, P.H. Application of nanotechnology for high performance textile. J. Text. Appar. Technol. Manag. 2003, 4, 1–8. [Google Scholar]
- Diaz-Visurraga, J.; Gutierrez, C.; von Plessing, C.; Garcia, A. Metalnanostructures as antibacterial agents. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2011; pp. 210–218. [Google Scholar]
- Bashiri Rezaie, A.; Montazer, M.; Mahmoudi Rad, M. Scalable, Eco-Friendly and Simple Strategy for Nano-Functionalization of Textiles Using Immobilized Copper-Based Nanoparticles. Clean Technol. Environ. Policy 2018, 20, 2119–2133. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Menon, S.; Shanmugam, V. Eco-Friendly Synthesis of Zinc Oxide Nanoparticles Using Cinnamomum Tamala Leaf Extract and Its Promising Effect towards the Antibacterial Activity. J. Drug Deliv. Sci. Technol. 2019, 53, 101212. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Maksoud, G.; Abdel-Rahman, M.A.; Salem, S.S.; Hassan, S.E.-D.; El-Sadany, M.A.-H. Eco-Friendly Approach Utilizing Green Synthesized Nanoparticles for Paper Conservation against Microbes Involved in Biodeterioration of Archaeological Manuscript. Int. Biodeterior. Biodegrad. 2019, 142, 160–169. [Google Scholar] [CrossRef]
- Kagithoju, S.; Godishala, V.; Nanna, R.S. Eco-Friendly and Green Synthesis of Silver Nanoparticles Using Leaf Extract of Strychnos Potatorum Linn.F. and Their Bactericidal Activities. 3 Biotech 2015, 5, 709–714. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Stuart, B.H. Analytical Techniques in Materials Conservation; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Piñar, G.; Sterflinger, K. Natural Sciences at the Service of Art and Cultural Heritage: An Interdisciplinary Area in Development and Important Challenges. Microb. Biotechnol. 2021, 14, 806–809. [Google Scholar] [CrossRef]
- Chen, C.-C.; Wactlar, H.D.; Wang, J.Z.; Kiernan, K. Digital Imagery for Significant Cultural and Historical Materials. Int. J. Digit. Libr. 2005, 5, 275–286. [Google Scholar] [CrossRef]
- Stork, D. Computer Image Analysis of Paintings and Drawings: An Introduction to the Literature. In Proceedings of the Image Processing for Artist Identification Workshop, Amsterdam, The Netherlands, 20 June 2008. [Google Scholar]
- Ivanova, K.; Dobreva, M.; Stanchev, P.; Totkov, G. Access to Digital Cultural Heritage: Innovative Applications of Automated Metadata Generation; Plovdiv University Publishing House “Paisii Hilendarski”: Plovdiv, Bulgaria, 2012; p. 249. [Google Scholar]
- Bicchieri, M.; Biocca, P.; Colaizzi, P.; Pinzari, F. Microscopic Observations of Paper and Parchment: The Archaeology of Small Objects. Herit. Sci. 2019, 7, 47. [Google Scholar] [CrossRef]
- Fistos, T.; Fierascu, I.; Fierascu, R.C. Recent Developments in the Application of Inorganic Nanomaterials and Nanosystems for the Protection of Cultural Heritage Organic Artifacts. Nanomaterials 2022, 12, 207. [Google Scholar] [CrossRef]
- Logofătu, P.C.; Vasile, N.T. Image resampling by interpolation guided by sensor geometry. Rom. Rep. Phys. 2021, 73, 402. [Google Scholar]
- Karimi, A.; Taghiyari, H.R.; Fattahi, A.; Karimi, S.; Ebrahimi, G.; Tarmian, A. Effects of Wollastonite Nanofibers on Biological Durability of Poplar Wood (Populus Nigra) against Trametes Versicolor. BioResources 2013, 8, 4134–4141. [Google Scholar] [CrossRef]
- Lykidis, C.; Mantanis, G.; Adamopoulos, S.; Kalafata, K.; Arabatzis, I. Effects of Nano-Sized Zinc Oxide and Zinc Borate Impregnation on Brown Rot Resistance of Black Pine (Pinus nigra L.) Wood. Wood Mat. Sci. Eng. 2013, 8, 242–244. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Norton, J.; Tajvidi, M. Effects of Nano-Materials on Different Properties of Wood-Composite Materials. In Bio-Based Wood Adhesives: Preparation, Characterization, and Testing; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 310–339. [Google Scholar]
- Taghiyari, H.R.; Bari, E.; Sistani, A.; Najafian, M.; Ghanbary, M.A.T.; Ohno, K.M. Biological Resistance of Nanoclay-Treated Plastic Composites with Different Bamboo Contents to Three Types of Fungi. J. Thermoplast. Compos. Mater. 2020, 33, 1048–1060. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Moradi-Malek, B.; Ghorbani Kookandeh, M.; Farajpour Bibalan, O. Effects of Silver and Copper Nanoparticles in Particleboard to Control Trametes Versicolor Fungus. Int. Biodeterior. Biodegrad. 2014, 94, 69–72. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Kalantari, A.; Ghorbani, M.; Bavaneghi, F.; Akhtari, M. Effects of Fungal Exposure on Air and Liquid Permeability of Nanosilver- and Nanozincoxide-Impregnated Paulownia Wood. Int. Biodeterior. Biodegrad. 2015, 105, 51–57. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Bari, E.; Schmidt, O.; Ghanbary, M.A.T.; Karimi, A.; Tahir, P.M.D. Effects of Nanowollastonite on Biological Resistance of Particleboard Made from Wood Chips and Chicken Feather against Antrodia Vaillantii. Int. Biodeterior. Biodegrad. 2014, 90, 93–98. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Bari, E.; Schmidt, O. Effects of Nanowollastonite on Biological Resistance of Medium-Density Fiberboard against Antrodia Vaillantii. Eur. J. Wood Wood Product. 2014, 72, 399–406. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Majidinajafabadi, R.; Vahidzadeh, R. Wollastonite to Hinder Growth of Aspergillus Niger Fungus on Cotton Textile. An. Acad. Bras. Cienc. 2018, 90, 2797–2804. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Kalantari, A.; Kalantari, A.; Avramidis, S. Effect of Wollastonite Nanofibers and Exposure to Aspergillus Niger Fungus on Air Flow Rate in Paper. Measurement 2019, 136, 307–313. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, S.; Pan, N. Studying the Mechanisms of Titanium Dioxide as Ultraviolet-Blocking Additive for Films and Fabrics by an Improved Scheme. J. Appl. Polym. Sci. 2004, 92, 3201–3210. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Liu, H.; Lee, Y.-Y.; Norsten, T.B.; Chong, K. In Situ Formation of Anti-Bacterial Silver Nanoparticles on Cotton Textiles. J. Ind. Text. 2014, 44, 198–210. [Google Scholar] [CrossRef]
- Simončič, B.; Klemenčič, D. Preparation and Performance of Silver as an Antimicrobial Agent for Textiles: A Review. Text. Res. J. 2016, 86, 210–223. [Google Scholar] [CrossRef]
- Gokarneshan, N.; Velumani, K. Application of Nano Silver Particles on Textile Materials for Improvement of Antibacterial Finishes. Glob. J. Nanomed. 2017, 2, 42–45. [Google Scholar]
- Radetić, M. Functionalization of Textile Materials with Silver Nanoparticles. J. Mater. Sci. 2013, 48, 95–107. [Google Scholar] [CrossRef]
- Perkas, N.; Perelshtein, I.; Gedanken, A. Coating Textiles with Antibacterial Nanoparticles Using the Sonochemical Technique, Journal of Machine Construction and Maintenance. J. Mach. Constr. Maint. 2018, 4, 15–26. [Google Scholar]
- Khe, Y.; Sv, M.; Aa, S.; Jz, J.; Fm, T.; Ssh, R.; Renat, L. Antibacterial Effect of Cotton Fabric Treated with Silver Nanoparticles of Different Sizes and Shapes. Int. J. Nanomater. Nanotechnol. Nanomed. 2019, 5, 016–023. [Google Scholar] [CrossRef]
- Sivasanmugam, S.Y. Silver Nanoparticle Impregnated Biomedical Fiber. Int. J. RF Technol. Res. Appl. 2015, 3, 194–197. [Google Scholar]
- Wu, Y.; Yang, Y.; Zhang, Z.; Wang, Z.; Zhao, Y.; Sun, L. Fabrication of Cotton Fabrics with Durable Antibacterial Activities Finishing by Ag Nanoparticles. Text. Res. J. 2019, 89, 867–880. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Silva, S.; Negri, M.; de Camargo, E.R.; Oliveira, R.; Barbosa, D.B.; Henriques, M. Silver Colloidal Nanoparticles: Antifungal Effect against Adhered Cells and Biofilms of Candida Albicans and Candida Glabrata. Biofouling 2011, 27, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Cameron, P.; Gaiser, B.K.; Bhandari, B.; Bartley, P.M.; Katzer, F.; Bridle, H. Silver Nanoparticles Decrease the Viability of Cryptosporidium Parvum Oocysts. Appl. Environ. Microbiol. 2016, 82, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Marcato, P.D.; De Souza, G.I.H.; Alves, O.L.; Esposito, E. Antibacterial Effect of Silver Nanoparticles Produced by Fungal Process on Textile Fabrics and Their Effluent Treatment. J. Biomed. Nanotechnol. 2007, 3, 203–208. [Google Scholar] [CrossRef]
- Rojas-Lema, S.P.; Galeas-Hurtado, S.G.; Guerrero-Barragán, V.H. Improvement of silver nanoparticle impregnation on cotton fabrics using a binder. Rev. Fac. Ing. 2017, 26, 109–119. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Di Girolamo, M.; Sarret, G.; Bureau, S.; Fernandez-Martinez, A.; Lelong, C.; Eymard Vernain, E. In Situ Formation of Silver Nanoparticles (Ag-NPs) onto Textile Fibers. ACS Omega 2021, 6, 1316–1327. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, H.-S.; Ryu, D.-S.; Choi, S.-J.; Lee, D.-S. Antibacterial Activity of Silver-Nanoparticles Against Staphylococcus Aureus and Escherichia Coli. Microbiol. Biotechnol. Lett. 2011, 39, 77–85. [Google Scholar]
- Balakumaran, R.R.; Jagadeeswari, S.; Kalaichelvan, P.T. In Vitro Biological Properties and Characterization of Nanosilver Coated Cotton Fabrics—An Application for Antimicrobial Textile Finishing. Int. Biodeterior. Biodegrad. 2016, 107, 48–55. [Google Scholar] [CrossRef]
- Vishnupriya, S.; Chaudhari, K.; Jagannathan, R.; Pradeep, T. Single-Cell Investigations of Silver Nanoparticle-Bacteria Interactions. Part. Part. Syst. Charact. 2013, 30, 1056–1062. [Google Scholar] [CrossRef]
- Roth, G.A.; Sosa Peña, M.d.P.; Neu-Baker, N.M.; Tahiliani, S.; Brenner, S.A. Identification of Metal Oxide Nanoparticles in Histological Samples by Enhanced Darkfield Microscopy and Hyperspectral Mapping. J. Vis. Exp. 2015, 106, e53317. [Google Scholar] [CrossRef]
- Neu-Baker, N.M.; Eastlake, A.C.; Brenner, S.A. Sample Preparation Method for Visualization of Nanoparticulate Captured on Mixed Cellulose Ester Filter Media by Enhanced Darkfield Microscopy and Hyperspectral Imaging. Microsc. Res. Techn. 2019, 82, 878–883. [Google Scholar] [CrossRef]
- Shi, R.; Chen, X.; Huo, J.; Guo, S.; Smith, Z.J.; Chu, K. Epi-Illumination Dark-Field Microscopy Enables Direct Visualization of Unlabeled Small Organisms with High Spatial and Temporal Resolution. J. Biophotonics 2022, 15, e202100185. [Google Scholar] [CrossRef] [PubMed]
- Badireddy, A.R.; Wiesner, M.R.; Liu, J. Detection, Characterization, and Abundance of Engineered Nanoparticles in Complex Waters by Hyperspectral Imagery with Enhanced Darkfield Microscopy. Environ. Sci. Technol. 2012, 46, 10081–10088. [Google Scholar] [CrossRef] [PubMed]
- Hebeish, A.; El-Naggar, M.E.; Fouda, M.M.G.; Ramadan, M.A.; Al-Deyab, S.S.; El-Rafie, M.H. Highly Effective Antibacterial Textiles Containing Green Synthesized Silver Nanoparticles. Carbohydr. Polym. 2011, 86, 936–940. [Google Scholar] [CrossRef]
- Saad, E.R.; Hafez, N.M. Effect of Coating with Silver Nanoparticles (AgNPs) on Cotton Fabric Functional Properties. Int. Des. J. 2014, 4, 33–39. [Google Scholar]
- Memon, H.; Wang, H.; Yasin, S.; Halepoto, A. Influence of Incorporating Silver Nanoparticles in Protease Treatment on Fiber Friction, Antistatic, and Antibacterial Properties of Wool Fibers. J. Chem. Chem. Eng. 2018, 4845687. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and Effect of Silver Nanoparticles on the Antibacterial Activity of Different Antibiotics against Staphylococcus Aureus and Escherichia Coli. Nanomedicine 2007, 3, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.I.; Shvalya, V.; Cvelbar, U.; Silva, R.; Marques-Oliveira, R.; Remião, F.; Felgueiras, H.P.; Padrão, J.; Zille, A. Stabilization of Silver Nanoparticles on Polyester Fabric Using Organo-Matrices for Controlled Antimicrobial Performance. Polymers 2022, 14, 1138. [Google Scholar] [CrossRef]
- Selvakumar, R.; Suriyaraj, S.P.; Jayavignesh, V.; Swaminathan, K. Silver nanoparticle impregnated bio-based activated carbon with enhanced antimicrobial activity. Int. J. Nanosci. 2013, 12, 1350024. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).