Abstract
Pellet ore not only has excellent metallurgical and mechanical properties, but is also an important metallurgical raw material used to solve the problem of increasing depletion of global high-grade iron ore resources. Bentonite has long been widely used in pellet ore production, which is not only expensive but also causes serious metallurgical pollution. Organic binders can form stronger adhesion and cohesion with mineral particles inside the green pellets than capillary forces, which greatly improves the pelletizing rate and significantly increases the strength of green and dry pellets, and it becomes an indispensable alternative to bentonite because it volatilizes pyrolytically at high temperatures, leaving almost no inorganic contaminants inside the pellet ore. In order to let more pellet researchers fully understand the research status and pelletizing theory of organic binders, this review systematically summarizes seven common organic binders, and elaborates on their adhesion mechanism and process characteristics, so as to provide references for pellet researchers and readers to further prepare cost-effective pellet binders and improve advanced pelletizing technology.
1. Introduction
Pelletizing binder is an important additive component in the production of iron ore pellets, and it plays a fundamental role in the metal smelting process. It can significantly increase the pelletizing rate, improve the quality of green and dry pellets, and causes almost zero contamination of the pellets [1]. After long-term development, pelletizing binders have gradually formed inorganic binders, organic binders, and composite binders [2]. The inorganic binder is mainly based on bentonite, and the addition of bentonite in China is as high as 2~3 wt.% on average, much higher than the foreign level of 0.5~0.7 wt.% [3]. According to the production experience, for every 1 wt.% of bentonite added, the iron grade of pellet ore will decrease by about 0.6 wt.% [4], and the iron content will be reduced by about 7 kg/ton [5]. Therefore, achieving partial or total replacement of bentonite is an important development direction for metallurgical pellet binders.
After continuous research and testing, organic binders have gradually become one of the most important added components for iron ore pellet production, and there are hundreds of organic binders produced worldwide, which can be divided into natural and artificial binders according to their sources [6]. Natural organic binders include starch, guar gum, and molasses, while artificial binders include carboxymethyl cellulose (CMC), carboxymethyl starch (CMS), humic acids, lignosulfonate, and polyacrylamide. These organic polymers are mainly hydrocarbons. On the one hand, they have good water solubility and high viscosity, and can form strong adhesion with mineral particles, thereby significantly improving the strength of green and dry pellets [7]. On the other hand, they have strong water absorption, which can quickly adjust the moisture content and improve the pelletizing rate [8]. Meanwhile, the organic binder contains no other inorganic components, and there are no inorganic materials remaining after preheating and firing stage at high temperatures, realizing without metallurgical pollution [9]. However, although the organic binder has the above excellent low-temperature properties, it is obvious that there is a serious problem of insufficient strength of preheated and fired pellets after high-temperature heating treatment, which is fundamentally caused by the lack of sufficient liquid slag phase bonding and high porosity of the organic binder [10,11]. Nevertheless, the excellent metallurgical properties and economic benefits possessed by organic binders continue to be the focus of research for many pellet researchers.
In the context of the increasing global depletion of high-quality iron ore, improving production efficiency and reducing resource consumption are important initiatives to sustain the future steel industry [12]. Organic binders have shown obvious advantages in improving pellet ore production efficiency and reducing metallurgical pollution caused by bentonite and decreasing pellet binder costs. However, there is no detailed and systematic theoretical study on the adhesion mechanism between organic pellet binder and iron ore and the process characteristics of pellet ore, especially since there is a lack of review on the solutions and mechanism of the problem of insufficient strength of fired pellets prepared by an organic binder. Through this review, we hope to better demonstrate the opportunities and challenges faced by the current pelletizing theory, technology, and performance of organic pellet binders for the readers and pellet researchers, and to provide references for further preparation of new cost-effective pellet binders and innovative improvement of pellet pelletizing technology.
2. The Binding Mechanism of Organic Binder in the Pellets
The polymer has a high degree of polymerization and several polar functional groups, which results in strong adhesion and adsorption of the organic binder to the iron ore particles. During the formation of green pellets, the presence of water will not only hydroxylate the surface of the mineral particle, but will also promote the dissolution and diffusion of organic polymer molecules, increasing the viscosity and bonding properties of the solution. With the rotation of the pelletizing machine, the colloid adhering to the surface of mineral particles will continuously bond to the surrounding mineral powder, making it roll and compact continuously and gradually tighten internally, thus forming green pellets with certain strength [13].
In the mineral particle–organic binder system, the adhesion of the binder to the mineral particle surface and the cohesion of the binder itself are the main forces inside the pellet [14,15]. The adhesion forces at the interface between the binder and mineral particles are mainly composed of coordination, electrostatic attraction, hydrogen bonding, and van der Waals forces, while coordination and electrostatic attraction are the strongest adsorption forces. The mineral surface is hydroxylated upon contact with water and adsorbs H+ from the solution to form Fe-OH2+, and some of the carboxyl groups in the organic binder molecule (HO-[P]-COOH) ionize to form HO-[P]-COO− [16], which have opposite surface charges and generate electrostatic attraction. Further mutual reactions lead to the formation of HO-P-OC-O-Fe coordination [17]. However, OH− adsorption on the surface of iron ore forms Fe-O−, and the carboxyl group of the HO-[P]-COOH combines with OH− to form HO-[P]-COO−, which have opposite charges and generate electrostatic repulsion [18,19], and the surface interactions in the pellet are shown in Figure 1. Although electrostatic repulsion negatively affects the adsorption on the ore surface, organic polymers can rely on a strong molecular network to form a strong cohesive force to improve the bond strength between mineral particles.
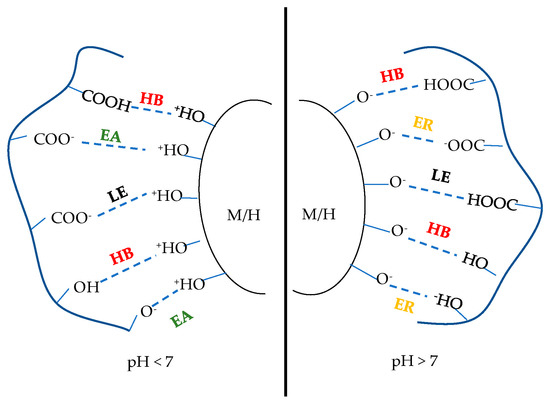
Figure 1.
Surface interactions between organic binders and Magnetite(M)/Hematite(H) minerals HB: hydrogen bind, EA: electrostatic attraction; ER: electrostatic repulsion; LE: ligand exchange.
The cohesion of organic polymers is directly related to the degree of polymerization, chemical structure, and degree of cross-linking and branching [20]. The degree of polymerization is an important factor in increasing the cohesion of a binder. A higher degree of polymerization produces greater chain interactions, resulting in longer branching chains that will entangle together, thus increasing the adhesion and cohesion inside pellets [21]. The viscosity of the organic polymer solution is a macroscopic manifestation of the strength of cohesion, which can control the water transport within the pellet and affect the pelletizing ability of the mineral particles in an effective timeframe [22]. The ideal viscosity enables the water on the pellet and the mineral to coalesce within an effective collision time, while low viscosity leads to faster aggregation of water on the surface, resulting in the uncontrollable and rapid growth of green pellets and the formation of small pellets with a rough surface and low strength [23], while high viscosity tends to slow down the coalescence of water and the growth rate [24]. Therefore, appropriately increasing the viscosity of organic binder is an important way to effectively improve the strength and pelletizing rate of the pellet [25].
However, the organic binder should not only have sufficient bonding force and cohesion, but should also enable its solution to rapidly spread to the solid surface when it contacts the surface of mineral particles, which is called wettability [26]. The key to improving wettability is to reduce the contact angle of the solution on the surface of iron ore, which depends on the binder solution and the nature of the iron ore surface, and can be achieved by introducing solidophilic and hydrophilic functional groups such as carboxyl groups and hydroxyl groups into the molecular structure of organic binders or by increasing the density of hydroxyl groups on the ore surface [27].
Therefore, in the presence of organic binders, adhesion and cohesion are the most important forces that improve the strength of pellets. The adhesion force is closely related to the number of polar functional groups of the organic binder and the adsorption properties of the mineral particles, while the degree of polymerization and cross-linking of the branching chains of the organics have a significant effect on the cohesion force. However, good wettability is an important condition to ensure the adsorption of organic polymers to mineral surfaces. Therefore, the adsorption, cohesion, and wettability of organic binders are important factors for organic polymers to influence the properties of pellets, and the preparation of an ideal organic binder must have a suitable degree of polymerization, as well as the type and number of polar and hydrophilic groups.
3. Organic Polymers
3.1. Carboxymethyl Cellulose (CMC)
3.1.1. The Interaction between CMC and Minerals
Carboxymethyl cellulose (CMC) is a water-soluble cellulose derivative produced by partial substitution of the hydroxyl group of cellulose by the carboxymethyl group, which is usually produced by reacting natural cellulose with alkali and chloroacetic acid after alkalinization and etherification [28,29], and the chemical structure is shown in Figure 2 [30]. Under humid conditions, CMC molecules adsorb mineral particles through chemical bonds, electrostatic attraction, van der Waals forces, and hydrogen bonds [31]. Under dry conditions, CMC adsorbs on the surface through the formation of chemical bonds with Fe2+/Fe3+ by the oxygen (-OH, -CH2COOH, or -CH2OCH2-) present in the CMC [32]. Numerous studies have shown [33,34] that the degree of polymerization (Dp) and degree of substitution (DS) of Na-CMC have a significant effect on the green and dry pellets, since the polymers of higher Dp can extend sufficiently into the liquid for maximum thickening effect at higher moisture, while the polymers of higher DS increase the solubility and reduce the interaction effect of the binder with various ions in solution. At the same binder dosage and suitable moisture content, the binders with higher Dp and DS show better bonding forces in terms of green pellet strength, but high viscosity may lead to high-temperature thermal cracking during subsequent processing [35].
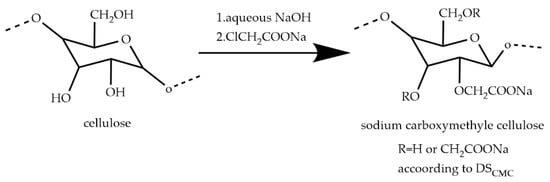
Figure 2.
Reaction scheme of the carboxymethylation of cellulose [30].
3.1.2. The Application Effect of CMC in the Pellets
At present, CMC is one of the most widely used organic substances for pellet binders with many advantages such as high viscosity and water absorption, good hydrophilicity, and strong adsorption with minerals. Peridur, a cellulose derivative produced in the Netherlands in the 1970s, has been widely used in major pellet-producing countries such as Brazil, India, Australia, the United States, and Iran [36]. Since most foreign pellet plants use belt roasters, there is no special requirement for the strength of preheated pellets, but the strength of green and preheated pellets prepared by Peridur makes it difficult to meet the production process requirements of shaft furnaces or rotary kilns. Therefore, this type of organic binder has never been used on a large scale in China [37].
Although the pellet with good low-temperature performance can be obtained by simply adding CMC, the strength of preheated and fired pellets will be significantly reduced after the decomposition by high-temperature combustion. To overcome such defects, many pellet researchers have tried to improve the high temperature by compounding with other inorganic minerals, as shown in Table 1. Haas et al. [38] improved the quality of the pellets by adding Na2CO3 or NaCl. The pellet strength increased with the addition of Na2CO3, reaching a maximum of 0.15 wt.% CMC + 0.06 wt.% Na2CO3. The effect of NaCl addition alone on pellet strength was low, while 0.15 wt.% CMC + 0.03 wt.% Na2CO3 + 0.03 wt.% NaCl resulted in maximum green and dry pellet strength, indicating a synergistic effect of Na2CO3 and NaCl. The addition of Na2CO3 or NaCl can increase the fired pellet strength. The presence of Na+ probably lowers the melting point of the slag phase, increases the number of liquid phase bridges, and improves solid phase consolidation, but too high a Na+ content can cause an increase in the amount of high-temperature molten liquid phase, preventing the Fe2O3 recrystallization process and causing a decrease in pellet strength [39].
Sodium tripolyphosphate (TPP) is an amorphous water-soluble polyphosphate with the chemical formula Na5P3O10 [40], which has dispersing and chelating effects [41]. TPP can isolate harmful ions such as Ca2+ and Mg2+ in water, and can adsorb to the surface of mineral particles, limit the over-adsorption of organic binders on the surface of particles and ensure that organic binders are completely dispersed in the moisture, thus increasing the internal viscosity of the pellet and then joining the mineral particles as the moisture evaporates [42]. In the pelletizing process, De Moraes and Kawatra [43] mixed 0.02 wt.% NaOH or TPP in 0.04 wt.% CMC, respectively, and the compressive strength of the dry pellet increased, but only adding TPP met the requirement of minimum strength. The results indicate that TPP is beneficial for pellet strength, but it introduces phosphorus, which pollutes steel smelting.

Table 1.
The effect of CMC with other additives on the performance of pellets.
Table 1.
The effect of CMC with other additives on the performance of pellets.
| CMC and Other Additives (wt.%) | Green Pellet Drop Number (times/P) | Dry Pellet Compressive Strength (N/P) | Fired Pellet Compressive Strength (N/P) | Ref. | |
|---|---|---|---|---|---|
| CMC1 Dp * = 1825, DS = 0.65 | 0.1 | 4.7 | [33] | ||
| 0.2 | 5.1 | ||||
| 0.3 | 7.8 | ||||
| CMC2 Dp = 560, DS * = 0.51 | 0.1 | 1.3 | |||
| 0.2 | 2.2 | ||||
| 0.3 | 3.1 | ||||
| CMCD Dp = 76, DS = 0.46 | 0.1 | 1.3 | 9.1 | [35] | |
| 0.2 | 2.2 | 9.9 | |||
| CMCS Dp = 552, DS = 0.51 | 0.1 | 3.1 | 11.3 | ||
| 0.2 | 5.1 | 12.9 | |||
| CMCT Dp = 1504, DS = 0.65 | 0.1 | 4.7 | 12.5 | ||
| 0.2 | 7.8 | 13.3 | |||
| 0.15 wt.% CMC | 0 | 4.1 | 56.7 | 2655 | [38] |
| 0.03 wt.% Na2CO3 | 6.6 | 60.75 | 3163.5 | ||
| 0.06 wt.% Na2CO3 | 5.5 | 69.3 | 3136.5 | ||
| 0.03 wt.% NaCl | 5.3 | 49.5 | 3069 | ||
| 0.03 wt.% Na2CO3 + 0.03 wt.% NaCl | 14.7 | 87.75 | 2916 | ||
| 0.04 wt.% CMC | 0 | 2.4 | 15.9 | [43] | |
| 0.02 wt.% NaOH | 2.4 | 19.1 | |||
| 0.02 wt.% TPP | 2.9 | 21.5 | |||
| 0.1 wt.% CMC | 0.25 wt.% Calcined colemanite | 3.0 | 6.7 | 4551.12 | [44] |
| 0.50 wt.% Calcined colemanite | 2.7 | 9.1 | 4557 | ||
| 0.75 wt.% Calcined colemanite | 2.9 | 5.9 | 4968.6 | ||
| 1.00 wt.% Calcined colemanite | 3.0 | 6.1 | 5001.92 | ||
| 0.1 wt.% CMC | 0.50 wt.% boric acid | 3920 | [45] | ||
| 0.75 wt.% boric acid | 4385.5 | ||||
| 1.00 wt.% boric acid | 4434.5 | ||||
*DS: Degree of substitution; *DP: Degree of polymerization.
Calcined colemanite and boric acid are important boron-containing compounds, and their application can significantly improve the strength of preheated and fired pellets [46,47,48]. Calcined colemanite and boric acid contain a large amount of boron oxide (B2O3), which has a low melting temperature and can produce a relatively large amount of liquid phase at a lower temperature, promoting the generation of silicate liquid phase [49,50], and thus reducing the liquid phase viscosity and porosity, enhancing the bonding between mineral particles and facilitating the diffusion of solid phase mass points, promoting Fe2O3 recrystallization and significantly improving the fired pellet strength [51,52]. Sivrikaya and Arol [44] combined 0.1 wt.% CMC with 0~1 wt.% calcined colemanite and the fired pellet strength was significantly improved without a significant negative effect on the green pellet strength. Kumar and Suman [45] compounded 0.1 wt.% CMC with 0.5~1 wt.% boric acid, and the fired pellet strength increased by three to four times at the firing temperature.
3.2. Starch
3.2.1. The Structure and Properties of Starch
Starch is a macromolecular organic substance formed by the enzyme-catalyzed and dehydrated polymerization of glucose, which can be expressed as (C6H10O5)n, where n is the degree of polymerization [53]. Starch contains approximately 20%~30% of amylose and 70%~80% of amylopectin, and their chemical structures are shown in Figure 3 [54]. Amylose and amylopectin are usually encased in an insoluble shell and must be chemically and physically modified to increase their solubility. Then, these substances can be used as pellet binders [55]. Pregelatinized starch is a starch compound with a certain solubility that is formed by boiling and extruding starch granules under high temperature and pressure to make their starch chains unfold [56,57]. Due to many advantages, such as the wide source of starch, low cost, and easy modification, many starch derivatives with excellent properties have been produced and are widely used in food, oilfield chemicals, mineral processing, water treatment additives, medical drugs and other fields [58,59].
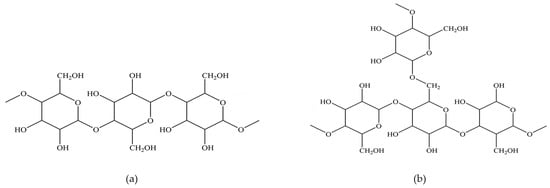
Figure 3.
The chemical structure of Amylose and Amylopectin. (a) Amylose; (b) Amylopectin [54].
3.2.2. The Application Effect of Starch in the Pellets
After puffing treatment, starch will increase its solubility and water absorption. The dispersibility and water absorption capacity of starch increase with the increase in solubility, the viscosity becomes higher and the adhesive force of the binder increases, which in turn affects the binding effect of starch with mineral particles, and leads to an increase in dry pellet strength [60,61]. However, with the increase in water absorption of soluble starch, more iron oxide particles are completely encapsulated in the starch gel [62]. Once this gel decomposes at high temperatures, more loose particles are produced, resulting in a weaker fired pellet strength, as shown in Table 2.
McDonald and Kawatra [63] mixed starch of different solubilities and bentonite in a certain ratio for pelletizing. Experiments showed that when starch increased, the green and fired pellet compressive strength with the same starch was almost unchanged, but the dry pellet strength increased significantly, and the green pellet drop number with 60% solubility starch was significantly greater than 7.5% solubility starch. The fundamental reason is that high-solubility starch forms a gel film and matrix more easily than insoluble starch in order to encapsulate the mineral particles, which results in higher green and dry pellet strength. During the preheating and firing stage, the starch is decomposed by combustion, and due to the presence of bentonite, the fired strength does not decrease below the minimum value for industrial use, despite the increase in the percentage of starch.
To improve the fired pellet strength, Sivrikaya and Arol [44] used calcined colemanite instead of bentonite, and the fired pellet strength increased with increasing calcined colemanite when maintaining 0.1 wt.% corn starch addition, 0.1 wt.% corn starch + 1.0 wt.% calcined colemanite produced a fired pellet strength of approximately 5000 N/pellet, which is much higher than the minimum strength criterion. Boron-containing compounds such as calcined colemanite and boric acid become one of the most important additions to improve the high-temperature performance of pellets, because they contain low-melting-point B2O3, which forms a strong solid-phase bridging effect.

Table 2.
The effect of starch with other additives on the performance of pellets.
Table 2.
The effect of starch with other additives on the performance of pellets.
| Starch and Other Additives (wt.%) | Green Pellet Drop Number (times/P) | Dry Pellet Compressive Strength (N/P) | Fired Pellet Compressive Strength (N/P) | Ref. | |
|---|---|---|---|---|---|
| Corn starch | 0.5 | 4 | 110.25 | 2320.44 | [64] |
| 1.0 | 5 | 90.98 | 1924.13 | ||
| 1.5 | 5 | 97.02 | 1294.78 | ||
| 2.0 | 6 | 81.21 | 886.9 | ||
| 0.145 wt.% 7.5% solubility starch + 0.435 wt.% Bent. | 7.13 | 61.79 | 3731.86 | [63] | |
| 0.333 wt.% 7.5% solubility starch + 0.333 wt.% Bent. | 5.50 | 70.73 | 3648.09 | ||
| 0.435 wt.% 7.5% solubility starch + 0.145 wt.% Bent. | 7.48 | 84.42 | 3812.13 | ||
| 0.66 wt.% 7.5% solubility starch | 6.92 | 82.91 | 3626.42 | ||
| 0.145 wt.% 60% solubility starch + 0.435 wt.% Bent. | 19.90 | 133.97 | 3542.65 | ||
| 0.333 wt.% 60% solubility starch + 0.333 wt.% Bent. | 19.81 | 187.50 | 3750.04 | ||
| 0.435 wt.% 60% solubility starch + 0.145 wt.% Bent. | 20.19 | 267.13 | 3622.58 | ||
| 0.66 wt.% 60% solubility starch | 19.85 | 305.43 | 3786.61 | ||
| 0.1 wt.% Corn starch | 0.25 wt.% Calcined colemanite | 3.0 | 6.7 | 4551.12 | [44] |
| 0.50 wt.% Calcined colemanite | 2.7 | 9.1 | 4557 | ||
| 0.75 wt.% Calcined colemanite | 2.9 | 5.9 | 4968.6 | ||
| 1.00 wt.% Calcined colemanite | 3.0 | 6.1 | 5001.92 | ||
3.3. Humic Acids (HA)
3.3.1. The Interaction between HA/FA and Minerals
Humic acids (HA) and Fulvi acids (FA) are a complex mixture of a class of large aromatic aliphatic molecules with similar chemical structures obtained by the chemical purification of humic substances. Their main chemical elements are C, H, O, N, and small amounts of S and P. The molecules contain mainly phenolic hydroxyl, carboxyl, alcohol hydroxyl, carbonyl, amine groups and other reactive functional groups [65,66,67]. Related studies have shown that there is no unique form of the molecular structure of humic acid, but the following molecular structure model exists, as shown in Figure 4 [68].
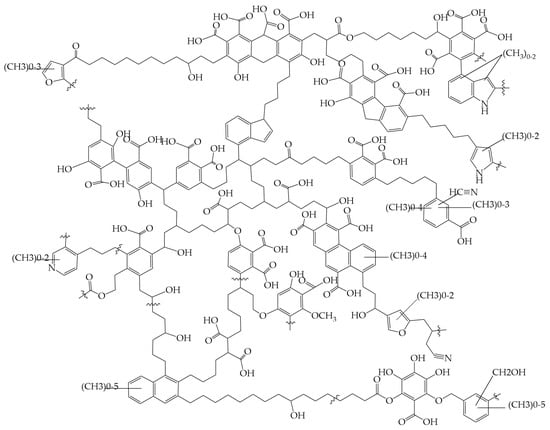
Figure 4.
The humic acids molecular structure model proposed by Schulten [68].
HA and FA molecules rely on a large number of active functional groups to generate adsorption forces such as ligand exchange and electrostatic interaction with Fe2+/Fe3+ on the surface of mineral particles [69], while with the increase in HA content, colloid action is enhanced, which adheres to and wraps around mineral particles, resulting in enhanced bridging between mineral particles and tight agglomeration, thus substantially increasing the green pellet strength [70,71]. Numerous studies have shown [72,73] that the adsorption concentration of HA on magnetite/hematite increases with increasing HA concentration and decreases with increasing pH [74], while metal cations change the stretching and curling of FA molecules, leading to the cohesion of FA together, thus increasing the viscosity of FA solution and promoting bridging between FA and magnetite surfaces, allowing more FA to be adsorbed on the mineral surface [75]. Therefore, the viscosity and adsorption density of humic acid-based pellet binder can be increased by adjusting the pH and concentration of the HA/FA solution or adding metal cations to obtain better green pellet strength [76].
3.3.2. The Application Effect of HA and FA in the Pellets
Compared with other organic binders, humic acid binders are not only inexpensive but also generally have high strength of fired pellets, as shown in Table 3. Modified humic acid binder (MHA) are organic polymers prepared by alkalizing weathered coal and lignite with surface active and colloidal properties, which can reduce the surface tension of water, improve the hydrophilicity of minerals, adsorb on mineral surfaces by chemical bonding, and have good thermal stability [77].

Table 3.
The effect of MHA with other additives on the performance of pellets.
Table 3.
The effect of MHA with other additives on the performance of pellets.
| MHA with Other Additives (wt.%) | Green Pellet Drop Number (times/P) | Dry Pellet Compressive Strength (N/P) | Fired Pellet Compressive Strength (N/P) | Ref. | |
|---|---|---|---|---|---|
| MHA (HA:FA = 7:3) | 0.25 | 8.2 | 57 | 3185.12 | [78] |
| 0.50 | 11.7 | 116 | 3101.43 | ||
| 0.75 | 13.5 | 132 | 2918.16 | ||
| 1.00 | 17.9 | 167 | 2757.51 | ||
| MHA | 0.75 | 3.3 | 2204.61 | [79] | |
| MHA | 0.25 | 5.2 | 2912.39 | [80] | |
| 0.50 | 9.6 | 2813.37 | |||
| 0.75 | 13.7 | 2652.41 | |||
| 1.00 | 18.4 | 2572.64 | |||
| 0.5 wt.% MHA | 4.14 | 131.55 | 3353.67 | [81] | |
| 0.333 wt.% MHA + 0.167 wt.% Ca-Bent.#1 | 5.84 | 149.47 | 3462.63 | ||
| 0.333 wt.% MHA + 0.167 wt.% Ca-Bent.#2 | 9.15 | 160.62 | 3631.51 | ||
The MHA binder prepared via the same process produced significant differences in pellet properties due to the different HA and FA contents in various low-rank coals. To investigate the effect of HA and FA on pellet quality, Han et al. [78] found that the strength of green pellets prepared by Na-HA was higher than that of Na-FA through pelletizing experiments, and the strength of green pellets reached the maximum when the mass ratio of Na-HA to Na-FA was 7:3, and the strength of dry and fired pellets increased with the addition of MHA binder prepared by this mass ratio.
The actual mass ratio of HA to FA in the prepared MHA would be much greater than 7:3 due to the high HA content in weathered coal and lignite, but the pellets prepared from this MHA binder still performed very well, as shown in Table 3. Zhou et al. [79] found that the microstructure of the MHA-fired pellets was denser, and the Fe2O3 grains recrystallized better. Under optimal conditions, the pellet strength of 0.75 wt.% MHA was comparable or better than that of 2 wt.% bentonite pellets, and the total Fe content was 1.06 wt.% higher than that of 2 wt.% bentonite pellets. Han et al. [80] reported that the amount of MHA binder could be reduced to 0.25 wt.% by wet grinding iron ore power with MHA, and that 0.5 wt.% Fe grade of MHA pellets was 0.9 wt.% higher than that of 2.0 wt.% bentonite pellets. Zhou et al. [82] concluded that the green/dry pellet quality of 0.6 wt.% MHA binder was comparable to or even better than that of 0.66 wt.% bentonite pellets.
Due to the excellent performance of MHA, to completely or partially replace bentonite, Zhou and Kawatra [81] mixed humate extracted from lignite with cheap calcium bentonite in proportion to make a composite pellet binder. The pelleting experiment showed that HA adsorbed onto Ca-Mt through complexation and hydrogen bonding, which increased the viscosity inside the pellet, thus significantly improving the green and dry pellet strength [83]. At the same time, the presence of calcium bentonite provided sufficient slag phase for the pellets and improved the fired pellet strength. Therefore, the use of modified humic acid binder not only reduces the amount of bentonite, but also reduces metallurgical cost and pollution.
3.4. Lignosulfonate
3.4.1. The Interaction between Lignosulfonate and Minerals
Lignosulfonates, also known as sulfonated lignin, are the main components of pulp waste streams by the sulfite method [84], and its molecule mainly contain functional groups such as phenolic hydroxyl, alcohol hydroxyl, sulfonic acid groups and carboxyl groups [85], and their molecular structures are shown in Figure 5 [86]. Lignosulfonates are highly hydrophilic, and the unshared electron pairs of oxygen atoms on their functional groups can form coordination bonds with metal ions such as iron and chromium. The lignosulfonate solution easily forms anionic groups with strong electronegativity, and the electrostatic repulsion between anionic groups will keep the plasmas in a highly stable dispersion [87]. Ligninsulfonates have mainly electrostatic attraction, hydrogen bonding, and van der Waals forces at the solid–liquid interface [88], thus increasing the adsorption force with the mineral surface.
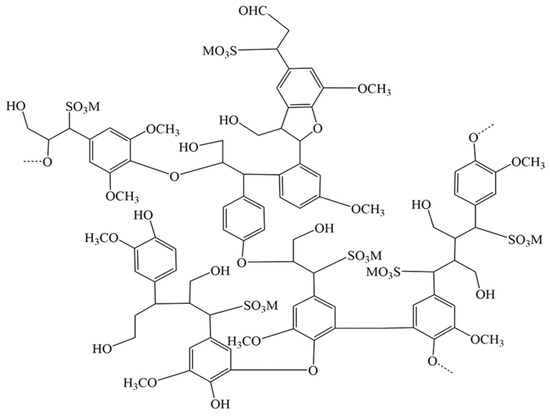
Figure 5.
The structure model for lignosulfonates [86].
3.4.2. The Application Effect of HA and FA in the Pellets
Lignosulfonate also suffers from the problem that the strength of the fired pellets decreases with the increase in the added amount [38]. The reason is that the decomposition of sodium lignosulfonate at high temperature produces gas escaping from the pellets, resulting in too loose internal structure and increased porosity, which is significantly improved by compounding with inorganic substances such as CaCO3, bentonite and copper slag, as shown in Table 4. Ding [89] mixed bentonite or CaCO3 with sodium lignosulfonate in proportions to make pellets, and found that CaCO3 was able to enhance the adsorption and bonding effect of sodium lignosulfonate on minerals, improve the pelletizing properties and increase the strength of green, dry and fired pellets. For the composite binder prepared from sodium lignosulfonate, the use of 0.75 wt.% sodium lignosulfonate was able to reduce the amount of bentonite from 2 wt.% to 0.75 wt.%. The use of 0.5 wt.% sodium lignosulfonate and 0.75 wt.% CaCO3 as a composite binder can completely replace bentonite, and all of its pellet strengths can meet the minimum requirements for production.
Copper slag is a smelting tailing produced during the fire refining of copper using copper sulfide concentrates. For every 1 ton of copper concentrate produced, 2.0~2.2 tons of copper slag are generated, and the annual global emissions of copper slag exceed 20 million tons [90,91]. Copper slag contains oxides such as FeO, Fe3O4, Al2O3, and CaO, which tend to form a certain amount of slag phase at high temperatures, where FeO and Fe3O4 enhance diffusion and recrystallization bonds and contribute to the denseness of pellets. Ammasi and Pal [92] used copper slag to improve the fired pellet strength. At 1250 °C, the fired pellet strength of 3100 N/pellet could be obtained by using 1.0 wt.% copper slag + 0.5 wt.% NLS. Copper slag as a pellet additive can not only improve pellet performance, but can also realize the reuse of industrial wastes, thus saving resources and protecting the environment.

Table 4.
The effect of Lignosulfonate with additives on the performance of pellets.
Table 4.
The effect of Lignosulfonate with additives on the performance of pellets.
| Lignosulfonate with Additives (wt.%) | Green Pellet Drop Number (times/P) | Dry Pellet Compressive Strength (N/P) | Fired Pellet Compressive Strength (N/P) | Ref. | |
|---|---|---|---|---|---|
| ALS * | 0.1 | 3.4 | 26.55 | 3115 | [38] |
| ALS | 0.2 | 3.5 | 42.30 | ||
| CLS * | 0.24 | 3.1 | 35.10 | 2042.55 | |
| CLS | 0.50 | 5.8 | 122.40 | ||
| SLS | 0.5 | 3.16 | 21.79 | 1977.15 | [89] |
| 1.0 | 4.67 | 41.52 | 2217.64 | ||
| 2.0 | 11.92 | 91.82 | 1452.91 | ||
| 0.75 wt.% CaCO3 | 0.5 wt.% SLS | 4.21 | 23.72 | 2609.58 | |
| 0.75 wt.%SLS | 5.22 | 28.58 | 2734.16 | ||
| 1.0 wt.% SLS | 6.10 | 36.04 | 2701.24 | ||
| 0.75 wt.% Bentonite | 0.5 wt.% SLS | 3.88 | 23.37 | 2639.09 | |
| 0.75 wt.%SLS | 4.88 | 28.42 | 2750.52 | ||
| 1.0 wt.% SLS | 5.67 | 34.44 | 2689.74 | ||
| 0.5 wt.% SLS * | 0.5 wt.% Cu-S | 8 | 49 | 3088.10 | [92] |
| 2.0 wt.% Cu-S | 7 | 50.96 | 2994.89 | ||
| 1 wt.% Cu-S * | 0.5 wt.% SLS | 12.4 | 80.26 | 7708.36 | |
| 1.0 wt.% SLS | 19.4 | 93.20 | 8950.73 | ||
*SLS: Sodium Lignosulfonate; *ALS: Ammonium Lignosulfonate; *CLS: Calcium Lignosulfonate; *Cu-S: Copper Slag.
3.5. Guar Gum
3.5.1. The Structure, Properties and Application of Guar Gum
Guar gum is a polysaccharide compound isolated from the guar bean, a seed of the legume family, and is a natural polymeric hydrocolloid, mainly composed of galactose and mannose polymerization, usually with a molecular weight of about 1 to 2 million. Its molecular structure is shown in Figure 6 [93]. Guar gum is easily hygroscopic, and can be soluble in water at different temperatures. It is usually chemically modified in a series of different ways, such as etherification, oxidation, cationization and anionization, to obtain derivatives with more excellent properties, and it is widely used in food, medicine, petroleum and textiles [94,95].
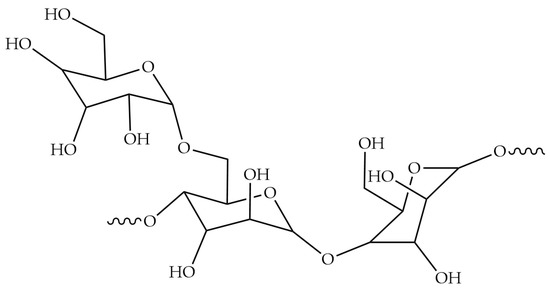
Figure 6.
The structure of Guar gum [93].
3.5.2. The Interaction between Guar Gum and Minerals
Guar gum has a high molecular weight and contains mainly benzene rings and hydroxyl groups. Such binders have high viscosity, good water solubility and good affinity with mineral surfaces [96]. Guar gum tends to form very viscous colloids at lower doses, enhancing the adhesive force between mineral particles, and thus producing higher green pellet strength [97]. Lu et al. [98] used industrial-grade CMC, CMS and Guar gum, respectively, to make pellets with magnetite, and found that CMS and CMC are adsorbed on the magnetite surface by carboxymethyl and hydroxyl groups in a ribbon-like or fibrous structure, effectively adsorbing and filling the voids between the incoming particles to form a continuous smooth film, while Guar gum is adsorbed on the magnetite surface in a bulk structure to form a smaller adsorption layer. The pelletizing results showed that Guar gum produced the highest strength of green pellets, followed by CMC and CMS, while the dry pellet strength did not differ significantly.
The US Bureau of Mines conducted pellet tests using different types of guar gums GG211, GG211D, and GG416, where GG211 and GG211D are unmodified nonionic guar gums and GG416 is hydroxypropyl guar gum [32]. Due to the high water absorption and high viscosity of guar gum, a strong cohesive force is produced between the mineral particles, and the presence of hydroxypropyl polar functional groups in GG416 increases the adsorption force between the organic binder and the mineral surface. GG211D has better dispersibility than GG211, but its hydration speed is slower. The pellets made with GG211 can absorb more water in the pelletizing process, and can effectively adjust the surface roughness of the pellets, thereby improving the green and dry pellet compressive strength. However, a large amount of guar gum disappeared during the firing stage, resulting in a great decrease in the fired pellet strength, as shown in Table 5 [38].

Table 5.
The effect of guar gum on the performance of pellets.
3.6. Polyacrylamide
3.6.1. The Interaction between Polyacrylamide and Minerals
Polyacrylamides (PAM) include homopolymers of acrylamide (AM), copolymers of acrylamide (AM) with other ionic monomers and polyacrylamide derivatives [99]. Each PAM molecule usually contains amide groups on its side chains, which are easily attached to other liquids to form a hydrogen bond, and have good water solubility [100]. The molecular weight of polyacrylamide is usually very large, and the molecular structure contains mainly amide groups. The PAM molecule reacts with Fe3+ ions on the surface of hematite through the lone pair of electrons of nitrogen, forming coordination bonds. At the same time, it also forms strong intermolecular hydrogen bonds with the hydrogen atoms of OH− on the hydrated surface of hematite [101]. Hydrogen bonding is the main driving force controlling polymer adsorption, which occurs between the electronegative C=O groups on polyacrylamide and the surface hydroxyl groups of proton-donating oxides, positive MOH2+ and neutral MOH [102,103]. Therefore, coordination and hydrogen bonding are the main adsorption forces between non-ionic polyacrylamide and iron concentrate powder, and there may be more complex interaction types for other types of PAM.
3.6.2. The Application Effect of Polyacrylamide in the Pellets
CMC and PAM are organic compounds that have been used earlier and have good effects on pellet binders. In the 1970s, the Alcotac FE series produced in Switzerland was successfully used in production, and the Alcotac FE series binders are copolymers of acrylamide and acrylic acid monomers, which are widely used in the United States, Chile, Sweden, and Venezuela, and other countries [104]. Floform 1049 V, Cytec Superfloc®A150, and Alcotac® CS are the most commonly used polyacrylamide derivatives on the market today, and their effects on pellet properties are shown in Table 6.

Table 6.
The effect PAM with other additives on the performance of pellets.
Cytec Superfloc®A150LMW and Cytec Superfloc®A150HMW are anionic polyacrylamides produced by the company Kemira. Cytec Superfloc®A150LMW has a low molecular weight, while Cytec Superfloc® A150HMW has a high molecular weight. Sivrikaya and Arol [44] found that Cytec Superfloc®A150LMW had a greater effect on the green pellet strength than Cytec Superfloc® A150HMW at the same addition. Due to the high-temperature volatilization of the organic binder, the fired pellet strength decreased significantly with the addition, but was still higher than 3000 N/pellet. The organic binder Floform 1049 V is a powder based on polyacrylamide, which is made by the company SNF. Chizhikova et al. [105] prepared pellets by mixing Floform 1049 V and bentonite, the green and dry pellet strength increased with increasing Floform 1049 V content, and the pellet strength at Floform 1049 V addition of 0.05 wt.% was close to 0.4 wt.% bentonite pellet. Alcotac® CS is a modified anionic polyacrylamide blend for iron ore palletization. Wang et al. used Alcotac® CS [106] to improve the adhesion of bentonite to mineral particles, and the addition of 0.02 wt.% Alcotac® CS could reduce the addition of bentonite from 1.8 wt.% to 0.6 wt.%. The strength of green and fired pellets still met the basic requirements.
3.7. Molasses
3.7.1. The Properties and Application of Molasses
Molasses is a by-product of the sugar solution produced during the sugar production process, and the brownish viscous liquid remaining after the concentration and precipitation of crystalline sugar and its composition varies depending on the raw materials and processing conditions of sugar production. Its composition is mainly sugar and water, though it contains a small amount of crude protein and minerals [107,108]. Molasses is divided into sugarcane molasses, beet molasses, citrus molasses and corn molasses, according to the source of raw materials, among which beet molasses and sugarcane molasses are produced in larger quantities. In recent years, research on the application of molasses has focused on animal and plant, fermentation, heavy metal adsorption and paper additives [109,110].
3.7.2. The Interaction between Molasses and Minerals
During the green pellet formation process, molasses forms a sticky layer on the surface of iron ore particles under the action of water molecules, which reduces the surface roughness and friction of the particles and increases the contact area between the particles [111], while molasses form a strong cohesive force between the particles and promote the bonding force between mineral particles, thus significantly increasing the green pellet strength [112]. During the drying process, molasses becomes a highly viscous liquid due to the evaporation of water, which greatly improves the dry pellet strength, as shown in Table 7. Kotta et al. [113] concluded that the green and dry pellet strength increased with increasing molasses content, and their strength far exceeded industrial minimum values from the pelletizing experiments.

Table 7.
The effect of molasses with other additives on the performance of pellets.
In the presence of molasses, CaO, and a large amount of CO2, glucose in molasses will catalyze the formation of calcite from lime to form a hardened structure inside the pellet. The reaction process is expressed in Equations (1)–(4) [115].
CaO + H2O→Ca(OH)2
Ca(OH)2 + 2C6H12O6→Ca(C6H12O6)2 + 2H2O
Ca(C6H12O6)2 + CO2 + 2H2O→(glucose)x(CaCO3)y(CaO)z
(glucose)x(CaCO3)y(CaO)z + CO2 + 2H2O→CaCo3 + C6H12O6
The regenerated glucose continues to react with Ca(OH)2, and the whole process is repeated several times, resulting in the formation of large amounts of CaCO3. The strength of the pellets produced using this binder is initially weak, and it takes about 1 h to harden to maximum strength, usually 2~4 times the mass of molasses than lime [116]. Srivastava et al. [114] prepared pellets using 2 wt.% molasses + 2 wt.% CaO and the strength of green and fired pellets was almost close to that of 0.66 wt.% bentonite, while the strength of dry pellets far exceeded that of 0.66 wt.% bentonite. Thus, the reaction of sugar with CaO to form a hardened layer of glycocalcium enhanced the bonding between mineral particles within the pellets, thus increasing the pellet strength.
4. Conclusions
After a long period of development, the pelletizing theory and preparation process of pellet binder has become increasingly mature, and many organic polymers with excellent performance have emerged, which have a great impact on the pelletizing performance and pellet ore strength, as shown in Table 8. Compared with bentonite, organic binders have the advantages of a fast pelletizing rate, high green and dry pellet strength, without metallurgical contamination, and no reduction in the pellet iron grade. Organic binder produces adhesion with the mineral surface through the carboxyl group, and the hydroxyl group can improve the hydrophilicity of organic matter, enhance diffusion, regulate water and viscosity inside the pellet, and improve capillary force, thus effectively controlling the pelletizing rate. At the same time, the organic binder of high molecular weight can produce a strong cohesive force inside the pellet, thus binding the minerals closely together and forming green pellets with smooth surfaces, uniform size, and good denseness. After preheating and firing, the organic binder inside the pellet evaporates by heat and does not leave any residual silicate slag phase, so it will not produce metallurgical pollution and significantly reduce the iron grade. Therefore, the vigorous development of new organic pellet binders is of great significance for the preparation of high-quality pellet ore.

Table 8.
Effect of organic polymers on pellet ore properties.
However, pellet ores prepared with organic binders often suffer from the prominent problem of insufficient strength of preheated and fired pellets. Pellet researchers have added small amounts of inorganic minerals such as bentonite, sodium carbonate, calcium carbonate, calcined colemanite, calcium oxide and other inorganic minerals into the pellet to increase the high-temperature liquid phase, improve solid-phase bridging and reduce the porosity within the pellet, resulting in significant improvements in fired pellet strength. However, this behavior inevitably introduces an inorganic slag phase, which generates metallurgical contamination and increases the cost, where boron-containing additions may have some negative impact on the steel properties. However, compounding organic polymers and inorganic minerals to prepare a composite pellet binder does achieve good overall performance by complementing the strengths and weaknesses, and has also become a commonly used binder in pellet plants today.
Pelletizing binder as an important chemical component of metallurgical additives is essential to improve the performance of pellet ore and control smelting costs, but the quality of pellet ore does not only lie in the binder problem. An advanced pelletizing process and metallurgical technology is often the fundamental measure to solve those problems. At present, some pellet researchers are trying to use industrial and agricultural solid waste to partially or fully replace bentonite or high-cost organic binder, to achieve the purpose of reducing the amount of bentonite added and the cost of pellet binder to promote the recycling of waste resources. At the same time, other pellet workers are trying to continuously innovate and improve the pelletizing technology, such as the use of synthetic geopolymers to prepare pellet ores. Continuous efforts to improve the technology are the fundamental way to solve all metallurgical problems.
Author Contributions
Literature review and writing, H.Z., C.M., Z.W., and W.L. Resources and editing, F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities, Grant No. 2-9-2019-141.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Forsmo, S.P.E.; Apelqvist, A.J.; Björkman, B.M.T.; Samskog, P.O. Binding mechanisms in wet iron ore green pellets with a bentonite binder. Powder Technol. 2006, 169, 147–158. [Google Scholar] [CrossRef]
- Wang, R.-R.; Zhang, J.-L.; Liu, Y.-R.; Liu, Z.-J.; Liu, X.-L.; Li, N.-Y. Effects of an inorganic binder on the strength property of cold-bonded pellets. J. Rev. De Metall. Cah. D Inf. Tech. 2017, 114, 604. [Google Scholar] [CrossRef]
- Fan, X.H.; Gan, M.; Jiang, T.; Chen, X.L.; Yuan, L.-S. Decreasing bentonite dosage during iron ore pelletising. J. Ironmak. Steelmak. 2011, 38, 597–601. [Google Scholar]
- Souza, R.P.D.; Mendonca, C.F.D.; Kater, T. Production of acid iron ore pellet for direct reduction, using an organic binder. Min. Eng. 1984, 36, 1437–1441. [Google Scholar]
- Kater, T.; Steeghs, R. Organic Binders for Iron Ore Pelletizing; Mining Symposium: Duluth, MN, USA, 1984; pp. 1–28. [Google Scholar]
- Claremboux, V.; Kawatra, S.K. Iron ore pelletization: Part III. organic binders. Miner. Process. Extr. Metall. Rev. 2022, 1–17. [Google Scholar] [CrossRef]
- Engelleitner, W.H. Binders: How they work and how to select one. Powder Bulk Eng. 2001, 15, 31–37. [Google Scholar]
- Zhu, D.Q.; Pan, J.; Lu, L.; Holmes, R.J. Iron Ore Pelletization. In Iron Ore; Lu, L., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 435–473. [Google Scholar]
- Kawatra, S.K.; Claremboux, V. Iron ore pelletization: Part II. Inorganic binders. Miner. Process. Extr. Metall. Rev. 2022, 43, 813–832. [Google Scholar] [CrossRef]
- Prusti, P.; Barik, K. Effect of additives concentration on pelletization of high grade hematite. Mater. Today: Proc. 2020, 33, 5373–5377. [Google Scholar] [CrossRef]
- Liu, H.; Xie, B.; Qin, Y.L. Effect of bentonite on the pelleting properties of iron concentrate. J. Chem. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Kawatra, S.K.; Claremboux, V. Iron ore pelletization: Part I. fundamentals. Miner. Process. Extr. Metall. Rev. 2021, 1–16. [Google Scholar] [CrossRef]
- Butt, H.J.; Kappl, M. Normal capillary forces. Adv. Colloid Interface Sci. 2009, 146, 48–60. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Pan, J.; Lu, L.M.; Holmes, R.J. Iron Ore Pelletization. In Iron Ore, 2nd ed.; Lu, L., Ed.; Woodhead Publishing: Sawston, UK, 2022; pp. 539–578. [Google Scholar]
- Qiu, G.Z.; Jiang, T.; Fan, X.H.; Zhu, D.Q.; Huang, Z. Effects of binders on balling behaviors of iron ore concentrates. Scand. J. Metall. 2004, 33, 39–46. [Google Scholar] [CrossRef]
- Vermeer, A.W.P.; van Riemsdijk, W.H.; Koopal, L.K. Adsorption of humic acid to mineral particles. 1. specific and electrostatic interactions. Langmuir 1998, 14, 2810–2819. [Google Scholar] [CrossRef]
- Gu, B.; Schmitt, J.; Chen, Z.H.; Liang, L.Y.; McCarthy, J.F. Adsorption and desorption of natural organic matter on iron oxide: Mechanisms and models. J Environ. Sci. Technol. 1994, 28, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Li, P.; Zhou, Y.L.; Han, G.H.; Li, G.H.; Xu, B.; Jiang, T. Adsorption of lignite humic acid onto magnetite particle surface. J. Cent. South Univ. 2012, 19, 1967–1972. [Google Scholar] [CrossRef]
- Yang, K.; Lin, D.; Xing, B. Interactions of humic acid with nanosized inorganic oxides. Langmuir ACS J. Surf. Colloids Surf. A: Physicochem. Eng. Asp. 2009, 25, 3571–3576. [Google Scholar] [CrossRef] [PubMed]
- Casey, L. Organic Binders for Iron Ore Pelletization. Master’s Thesis, Aalto University, Helsinki, Espoo, Finland, 2016. [Google Scholar]
- Qiu, G.Z.; Jiang, T.; Li, H.X.; Wang, D.Z. Functions and molecular structure of organic binders for iron ore pelletization. Colloids Surf. A: Physicochem. Eng. Asp. 2003, 224, 11–22. [Google Scholar] [CrossRef]
- Forsmo, S. Influence of Green Pellet Properties on Pelletizing of Magnetite Iron Ore. Ph.D. Thesis, Luleå Tekniska Universitet, Luleå, Sweden, 2007. [Google Scholar]
- Goetzman, H.; Bleifuss, R.; Engesser, J. Investigation of carboxymethyl cellulose binders for taconite pelletizing. In Proceedings of the SME Annual Meeting, Phoenix, AZ, USA, 25 January 1988; pp. 88–111. [Google Scholar]
- Forsmo, S.P.E.; Samskog, P.O.; Björkman, B.M.T. A study on plasticity and compression strength in wet iron ore green pellets related to real process variations in raw material fineness. Powder Technol. 2008, 181, 321–330. [Google Scholar] [CrossRef]
- Halt, J.A.; Kawatra, S.K. Review of organic binders for iron ore concentrate agglomeration. Min. Metall. Explor. 2014, 31, 73–94. [Google Scholar] [CrossRef]
- Sunde, M. Organic Binder as a Substitute for Bentonite in Ilmenite Pelletization. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2012. [Google Scholar]
- Potapova, E.; Yang, X.; Grahn, M.; Holmgren, A.; Forsmo, S.P.E.; Fredriksson, A.; Hedlund, J. The effect of calcium ions, sodium silicate and surfactant on charge and wettability of magnetite. Colloids Surf. A Physicochem. Eng. Asp. 2011, 386, 79–86. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent developments of carboxymethyl cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.Y.; Zhang, H.Z.; Ma, Y.; Song, D.W.; Shi, X.X.; Zhang, L.Q.; Zhou, Y. Polyglutamic acid binder for high-performance lithium-sulfur batteries. Coatings 2022, 12, 1433. [Google Scholar] [CrossRef]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Hoogendam, C.W.; de Keizer, A.; Cohen Stuart, M.A.; Bijsterbosch, B.H.; Batelaan, J.G.; van der Horst, P.M. Adsorption mechanisms of carboxymethyl cellulose on mineral surfaces. Langmuir 1998, 14, 3825–3839. [Google Scholar] [CrossRef]
- Saha, B.; Patra, A.S.; Mukherjee, A.K.; Paul, I. Interaction and thermal stability of carboxymethyl cellulose on alpha-Fe2O3(001) surface: ReaxFF molecular dynamics simulations study. J. Mol. Graph. Model. 2021, 102, 107787. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-M.; Fan, X.-H.; Chen, X.-L.; Yuan, L.-S.; Huang, X.-X.; Li, X. Interaction mechanism between carboxylmethyl cellulose and iron ore concentrates in iron ore agglomeration. J. Cent. South Univ. 2015, 22, 1241–1246. [Google Scholar] [CrossRef]
- Van Der Merwe, M.; Garbers-Craig, A. Influence of a carboxymethyl cellulose (CMC) binder on the mechanical properties of iron ore pellets. J. South. Afr. Inst. Min. Met. 2017, 117, 337–341. [Google Scholar] [CrossRef]
- Fan, X.-H.; Yang, G.-M.; Chen, X.-L.; He, X.-N.; Huang, X.-X.; Gao, L. Effect of carboxymethyl cellulose on the drying dynamics and thermal cracking performance of iron ore green pellets. Powder Technol. 2014, 267, 11–17. [Google Scholar] [CrossRef]
- De Moraes, S.L.; Lima, J.R.B.D.; Neto, J.B.F.; Fredericci, C.; Saccoccio, E.M. Binding mechanism in green iron ore pellets with an organic binder. Miner. Process. Extr. Metall. Rev. 2020, 41, 247–254. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zhou, F.S.; Evelina, L.M.A.; Liu, J.L.; Zhou, Y. A review on the industrial solid waste application in pelletizing additives: Composition, mechanism and process characteristics. J. Hazard. Mater. 2022, 423, 127056. [Google Scholar] [CrossRef]
- Haas, L.A.; Aldinger, J.A.; Zahl, R.K. Effectiveness of Organic Binders for Iron Ore Pelletization; US Department of the Interior, Bureau of Mines: Pittsburgh, PA, USA, 1989. [Google Scholar]
- Song, W.; Luo, G.P.; Sun, C.C.; Zhang, J.; Zhu, J.G. Effect of K and Na on reduction swelling performance of oxidized roasted briquettes. High Temp. Mater. Process. 2021, 40, 241–252. [Google Scholar] [CrossRef]
- Makara, A.; Smol, M.; Kulczycka, J.; Kowalski, Z. Technological, environmental and economic assessment of sodium tripolyphosphate production-a case study. J. Clean. Prod. 2016, 133, 243–251. [Google Scholar] [CrossRef]
- Wang, X.; Jia, W.H.; Yang, C.R.; He, R.; Jiao, F.; Qin, W.Q.; Cui, Y.F.; Zhang, Z.Q.; Li, W.; Song, H. Innovative application of sodium tripolyphosphate for the flotation separation of scheelite from calcite. Miner. Eng. 2021, 170, 106981. [Google Scholar] [CrossRef]
- Cassola, M.S.; Chaves, A.P. Effect of the addition of organic binders on the behavior of iron ore pellets. KONA Powder Part. J. 1998, 16, 136–142. [Google Scholar] [CrossRef]
- De Moraes, S.L.; Kawatra, S.K. Laboratory study of an organic binder for pelletization of a magnetite concentrate. Min. Metall. Explor. 2010, 27, 148–153. [Google Scholar] [CrossRef]
- Sivrikaya, O.; Arol, A.I. Pelletization of magnetite ore with colemanite added organic binders. Powder Technol. 2011, 210, 23–28. [Google Scholar] [CrossRef]
- Kumar, S.; Suman, S.K. Compressive strength of fired pellets using organic binder: Response surface approach for analyzing the performance. Trans. Indian Inst. Met. 2018, 71, 1629–1634. [Google Scholar] [CrossRef]
- Sivrikaya, O.; Arol, A. Use of Organic Binders and Borates in Pelletizing of Iron Oxides. In Proceedings of the IV International Boron Symposium, Eskişehir, Turkey, 15–17 October 2009. [Google Scholar]
- Sivrikaya, O.; Arol, A.I. Use of boron compounds as binders in iron ore pelletization. Open Miner. Process. J. 2010, 3, 25–35. [Google Scholar] [CrossRef]
- Sivrikaya, O.; Arol, A. The bonding/strengthening mechanism of colemanite added organic binders in iron ore pelletization. Int. J. Miner. Process. 2012, s110–s111, 90–100. [Google Scholar] [CrossRef]
- Guo, H.W.; Bai, J.L.; Zhang, J.L.; Li, H.G. Mechanism of strength improvement of magnetite pellet by adding boron-bearing iron concentrate. J. Iron Steel Res. Int. 2014, 21, 9–15. [Google Scholar] [CrossRef]
- Zhuchkov, V.; Zayakin, O.; Akberdin, A. Prospects for using boron in metallurgy. report Izvestiya. Ferr. Metall. 2021, 64, 471–476. [Google Scholar] [CrossRef]
- Dolitskaya, O.A. Effect of mineral additives on the strengthening of pellets of skarn-type ores. Russ. Metall. 2001, 2001, 230–232. [Google Scholar]
- Malysheva, T.; Chesnokova, G.; Akberdin, A.; Dolitskaya, O. Effect of boron on the quality of iron ore pellets. Russ. Metall. Met. 1996, 1, 1–4. [Google Scholar]
- BeMiller, J.N.; Huber, K.C. Starch. Ullmann’s Encycl. Ind. Chem. 2011, 32, 113–141. [Google Scholar] [CrossRef]
- Whistler, R.L.; Daniel, J.R. Starch. Kirk-Othmer Encycl. Chem. Technol. 2000, 1–18. [Google Scholar] [CrossRef]
- Swinkels, J.J.M. Composition and properties of commercial native starches. Starch-Stärke 1985, 37, 1–5. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Jackson, D.S. Starch Gelatinization. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2008; Volume 55, pp. 221–268. [Google Scholar]
- Ai, Y.F.; Jane, J.L. Gelatinization and rheological properties of starch. Starch 2015, 67, 213–224. [Google Scholar] [CrossRef]
- Song, X.Y. Effect of storage conditions on the physicochemical characteristics of bilayer edible films based on iron Yam-pea starch blend and corn zein. Coatings 2022, 12, 1524. [Google Scholar] [CrossRef]
- Li, N.N.; Zhou, Z.G.; Wu, F.Q.; Lu, Y.Y.; Jiang, D.Y.; Zhong, L.; Xie, F.W. Development of pH-indicative and antimicrobial films based on polyvinyl alcohol/starch incorporated with ethyl lauroyl arginate and mulberry anthocyanin for active packaging. Coatings 2022, 12, 1392. [Google Scholar] [CrossRef]
- Halt, J.A. Controlling Properties of Agglomerates for Chemical Processes. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2017. [Google Scholar]
- Halt, J.A.; Kawatra, S.K. Can modified starch be used as a binder for iron ore pellets? Miner. Process. Extr. Metall. Rev. 2017, 38, 73–82. [Google Scholar] [CrossRef]
- McDonald, J. Advances in Alternative Binders for Iron Ore Pellets. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2017. [Google Scholar]
- McDonald, J.E.D.; Kawatra, S.K. Agglomeration of hematite concentrate by starches. Miner. Process. Extr. Metall. Rev. 2017, 38, 1–6. [Google Scholar] [CrossRef]
- Mishra, B.; Dishwar, R.K.; Omar, R.J.; Mahobia, G.S. Hardening behaviour of pellets prepared from a novel combination of rare multimetallic magnetite ore and binder. Trans. Indian Inst. Met. 2021, 74, 2049–2055. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.Q.; Li, H.; Yu, J.D.; Xie, W.N.; Wei, H. The molecular structure of inner mongolia lignite utilizing XRD, solid state 13C NMR, HRTEM and XPS techniques. Fuel 2017, 203, 764–773. [Google Scholar] [CrossRef]
- Xiang, J.H.; Zeng, F.G.; Li, B.; Zhang, L.; Li, M.F.; Liang, H.Z. Construction of macromolecular structural model of anthracite from Chengzhuang coal mine and its molecular simulation. J. Fuel Chem. Technol. 2013, 41, 391–400. [Google Scholar] [CrossRef]
- Peuravuori, J.; Žbánková, P.; Pihlaja, K. Aspects of structural features in lignite and lignite humic acids. Fuel Process. Technol. 2006, 87, 829–839. [Google Scholar] [CrossRef]
- Schnitzer, M. Humic substances: Chemistry and reactions. In Developments in Soil Science; Schnitzer, M., Khan, S.U., Eds.; Elsevier: Amsterdam, The Netherlands, 1978; Volume 8, pp. 1–64. [Google Scholar]
- Han, G.H.; Huang, Y.; Li, G.H.; Zhang, Y.B.; Jiang, T. Detailed adsorption studies of active humic acid fraction of a new binder on iron ore particles. Miner. Process. Extr. Metall. Rev. 2013, 35, 1–14. [Google Scholar] [CrossRef]
- Han, G.H.; Su, S.P.; Cao, Y.J.; Huang, Y.F.; Song, X.Y. Research on the interaction of humic acid with iron minerals. In Characterization of Minerals, Metals, and Materials 2018; Springer: Cham, Switzerland, 2018; pp. 653–660. [Google Scholar]
- Jiang, T.; Han, G.H.; Zhang, Y.B.; Li, G.H.; Huang, Y.F. A further study on the interaction between one of organic active fractions of the MHA binder and iron ore surface. Int. J. Miner. Process. 2011, 100, 172–178. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zhou, F.S.; Bao, X.C.; Zhou, S.H.; Wei, Z.J.; Long, W.J.; Zhou, Y. A review on the humic substances in pelletizing binders: Preparation, interaction mechanism, and process characteristics. ISIJ Int. 2022, advpub. [Google Scholar] [CrossRef]
- Han, G.H. Relationship between Structure and Performance of Humic Substances Based Binder for Iron Ore Pellets. Ph.D. Thesis, Central South University, Changsha, China, 2013. [Google Scholar]
- Zhou, Y.L.; Zhang, Y.B.; Li, P.; Li, G.H.; Jiang, T. Comparative study on the adsorption interactions of humic acid onto natural magnetite, hematite and quartz: Effect of initial HA concentration. Powder Technol. 2014, 251, 1–8. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Zhang, Y.B.; Li, G.H.; Jiang, T. Effects of metal cations on the fulvic acid (FA) adsorption onto natural iron oxide in iron ore pelletizing process. Powder Technol. 2016, 302, 90–99. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Kawatra, S.K. Humic substance-based binder in iron ore pelletization: A review. Miner. Process. Extr. Metall. Rev. 2017, 38, 321–337. [Google Scholar] [CrossRef]
- Qiu, G.Z.; Jiang, T.; Fa, K.Q.; Zhu, D.Q.; Wang, D.Z. Interfacial characterizations of iron ore concentrates affected by binders. Powder Technol. 2004, 139, 1–6. [Google Scholar] [CrossRef]
- Han, G.H.; Huang, Y.F.; Li, G.H.; Zhang, Y.B.; Zhou, Y.L.; Jiang, T. Optimizing the mass ratio of two organic active fractions in modified humic acid (MHA) binders for iron ore pelletizing. ISIJ Int. 2012, 52, 378–384. [Google Scholar] [CrossRef][Green Version]
- Zhou, Y.L.; Zhang, Y.B.; Liu, B.B.; Li, G.H.; Jiang, T. Effect of modified humic acid binder on pelletisation of specularite concentrates. J. Cent. South Univ. 2015, 22, 1247–1255. [Google Scholar] [CrossRef]
- Han, G.H.; Zhang, Y.B.; Jiang, T.; Huang, Y.F.; Li, G.H. Effects of binders on oxidized pellets preparation from vanadium/titanium-bearing magnetite. In 2nd International Symposium on High-Temperature Metallurgical Processing, Hoboken, NJ, USA; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2011; pp. 279–287. [Google Scholar]
- Zhou, Y.L.; Kawatra, S.K. Pelletization using humic substance-based binder. Miner. Process. Extr. Metall. Rev. 2017, 38, 83–91. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Wattanaphan, P.; Kawatra, S.K. Application of modified humic acid (MHA) binder in the pelletizing of fluxed hematite concentrate. Miner. Process. Extr. Metall. Rev. 2017, 38, 126–131. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Lu, M.M.; Su, Z.J.; Wang, J.; Tu, Y.K.; Chen, X.J.; Cao, C.T.; Gu, F.Q.; Liu, S.; Jiang, T. Interfacial reaction between humic acid and Ca-montmorillonite: Application in the preparation of a novel pellet binder. Appl. Clay Sci. 2019, 180, 105177. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- White, J. Top Value-Added Chemicals from Biomass Volume II-Results of Screening for Potential Candidates from Biorefinery Lignin; Biomass Fuels; U.S. Department of Energy Office of Scientific and Technical Information: Richland, WA, USA, 2007; pp. 1–79. [Google Scholar]
- Ruwoldt, J. A critical review of the physicochemical properties of lignosulfonates: Chemical structure and behavior in aqueous solution, at surfaces and interfaces. Surfaces 2020, 3, 42. [Google Scholar] [CrossRef]
- Nanthakumar, B.; Arinaitwe, E.; Pawlik, M. Adsorption of sodium lignosulfonates on hematite. Adsorption 2010, 16, 447–455. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Ding, B. Study on the Binding Mechanisms Andexperiment of Sodium Lignosulfonate in Pellets. Master’s Thesis, Wuhan University of Science and Technology, Wuhan, China, 2018. [Google Scholar]
- Gorai, B.; Jana, R.K. Premchand, Characteristics and utilisation of copper slag-a review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. The potential for copper slag waste as a resource for a circular economy: A review-part II. Miner. Eng. 2021, 172, 107150. [Google Scholar] [CrossRef]
- Ammasi, A.; Pal, J. Replacement of bentonite in hematite ore pelletisation using a combination of sodium lignosulphonate and copper smelting slag. Ironmak. Steelmak. 2016, 43, 203–213. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Wang, S.B.; Wang, C.A.; Zhao, F.; Lei, S.; Yi, H.Y.; Guo, J.C. Influence of nanomaterial morphology of guar-gum fracturing fluid, physical and mechanical properties. Carbohydr. Polym. 2020, 234, 115915. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.D.; Pawlik, M. Adsorption of guar gum onto quartz from dilute mixed electrolyte solutions. J. Colloid Interface Sci. 2006, 298, 609–614. [Google Scholar] [CrossRef]
- Mhlanga, S.S.; O’Connor, C.T.; McFadzean, B. A study of the relative adsorption of guar onto pure minerals. Miner. Eng. 2012, 36–38, 172–178. [Google Scholar] [CrossRef]
- Lu, S.S.; Yuan, Z.T.; Zhang, C. Binding mechanisms of polysaccharides adsorbing onto magnetite concentrate surface. Powder Technol. 2018, 340, 17–25. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M.J.N.C.W. Polyacrylamide degradation and its implications in environmental systems. Clean Water 2018, 1, 1–9. [Google Scholar] [CrossRef]
- Kulicke, W.M.; Kniewske, R.; Klein, J. Preparation, characterization, solution properties and rheological behaviour of polyacrylamide. Prog. Polym. Sci. 1982, 8, 373–468. [Google Scholar] [CrossRef]
- Samsonova, N.S.; Il’chenko, L.G.; Gol’dman, M.M.; Ni, L.P. IR spectroscopic study of the adsorption of polyacrylamide on hematite. J. Appl. Spectrosc. 1975, 23, 963–966. [Google Scholar] [CrossRef]
- Lee, L.T.; Somasundaran, P. Adsorption of polyacrylamide on oxide minerals. Langmuir 1989, 5, 854–860. [Google Scholar] [CrossRef]
- Deng, Y.; Dixon, J.B.; White, G.N.; Loeppert, R.H.; Juo, A.S.R. Bonding between polyacrylamide and smectite. Colloids Surf. A: Physicochem. Eng. Asp. 2006, 281, 82–91. [Google Scholar] [CrossRef]
- Xiang, A.P. Study on Bentonite-Based Composite Binder for Iron Ore Pellets. Master’s Thesis, Anhui University of Technology, Ma’anshan, China, 2020. [Google Scholar]
- Chizhikova, V.M.; Vainshtein, R.M.; Zorin, S.N.; Zainetdinov, T.I.; Zinyagin, G.A.; Shevchenko, A.A. Production of iron ore pellets with an organic binder. Metallurgist 2003, 47, 141–146. [Google Scholar] [CrossRef]
- Wang, C.; Xu, C.Y.; Liu, Z.J.; Wang, Y.Z.; Wang, R.R.; Ma, L.M. Effect of organic binders on the activation and properties of indurated magnetite pellets. Int. J. Miner. Metall. Mater. 2021, 28, 1145–1152. [Google Scholar] [CrossRef]
- Palmonari, A.; Cavallini, D.; Sniffen, C.J.; Fernandes, L.; Holder, P.; Fagioli, L.; Fusaro, I.; Biagi, G.; Formigoni, A.; Mammi, L. Short communication: Characterization of molasses chemical composition. J. Dairy Sci. 2020, 103, 6244–6249. [Google Scholar] [CrossRef]
- Olbrich, H. The molasses. J Biotechnol. -Kempe GmbH 2006, 128, 1–131. [Google Scholar]
- Fahmy, T.Y. Introducing molasses as a new additive in papermaking. Tappi J. 2007, 8, 23–25. [Google Scholar]
- Kolemen, S.; Baran Acarali, N.; Tugrul, N.; Moroydor Derun, E.; Piskin, S. The zinc adsorption study by using orhaneli fly ash, bentonite, and molasses in wastewater. Water Air Soil Pollut. 2012, 224, 1367. [Google Scholar] [CrossRef]
- Zambrano, A.P.; Takano, C.; Mourão, M.B.; Tagusagawa, S.Y. Influence of the Binder on the Mechanical Properties of the Chromite Self-Reducing Pellets. Int. J. Bus. Humanit. Technol. 2013, 3, 99–108. [Google Scholar]
- Ripke, S.J. Advances in Iron Ore Pelletization by Understanding Bonding and Strengthening Mechanisms. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2002. [Google Scholar]
- Kotta, A.B.; Patra, A.; Kumar, M.; Karak, S.K. Effect of molasses binder on the physical and mechanical properties of iron ore pellets. Int. J. Miner. Metall. Mater. 2019, 26, 41–51. [Google Scholar]
- Srivastava, U.; Kawatra, S.K.; Eisele, T.C. Study of organic and inorganic binders on strength of iron oxide pellets. Metall. Mater. Trans. B 2013, 44, 1000–1009. [Google Scholar] [CrossRef]
- Sah, R.; Dutta, S.K. Effects of binder on the properties of iron ore-coal composite pellets. Miner. Process. Extr. Metall. Rev. 2010, 31, 73–85. [Google Scholar] [CrossRef]
- Eisele, T.C.; Kawatra, S.K. A review of binders in iron ore pelletization. Miner. Process. Extr. Metall. Rev. 2003, 24, 1–90. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).