Preparation of ZnFe2O4@TiO2 Novel Core-Shell Photocatalyst by Ultrasonic Method and Its Photocatalytic Degradation Activity
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
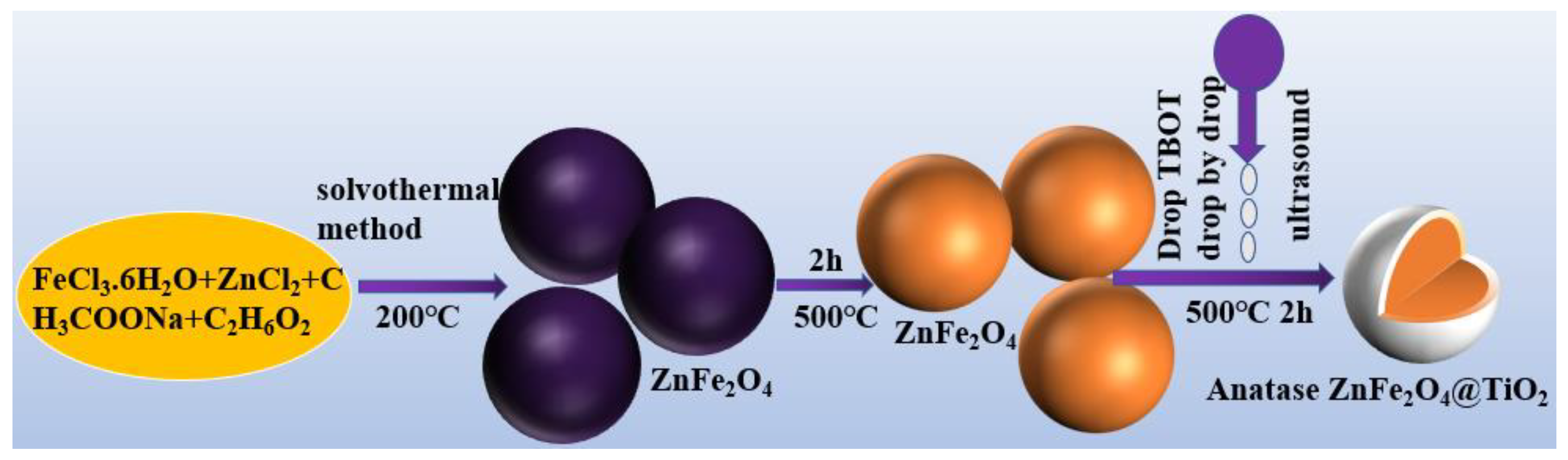
2.2. Preparation of ZnFe2O4@TiO2 Core-Shell Microspheres
2.3. Photocatalytic Performance Evaluation
2.4. Characterization
3. Results and Discussion
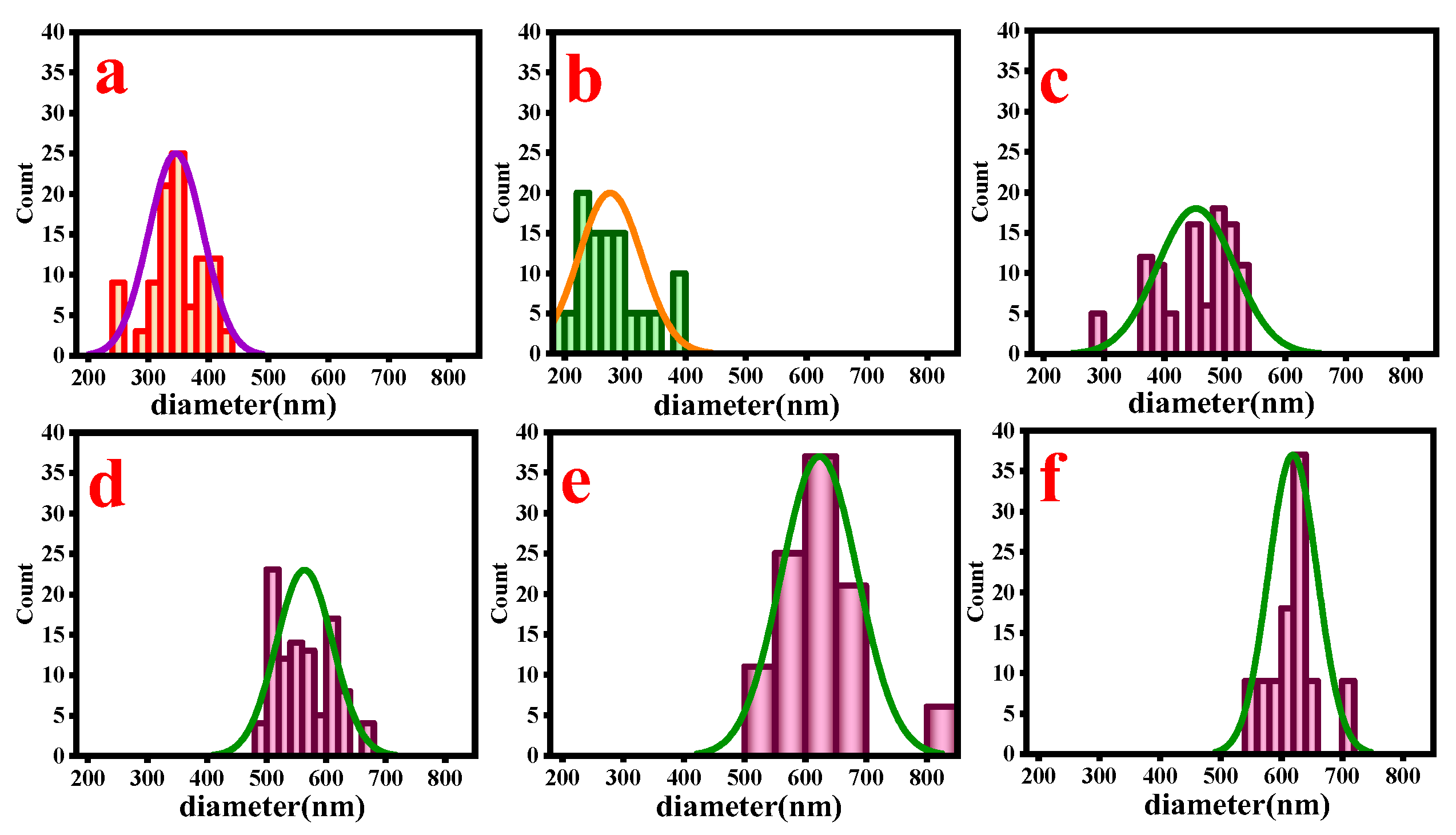
3.1. Morphology Analysis
3.2. EDS Study
3.3. Crystal Analysis
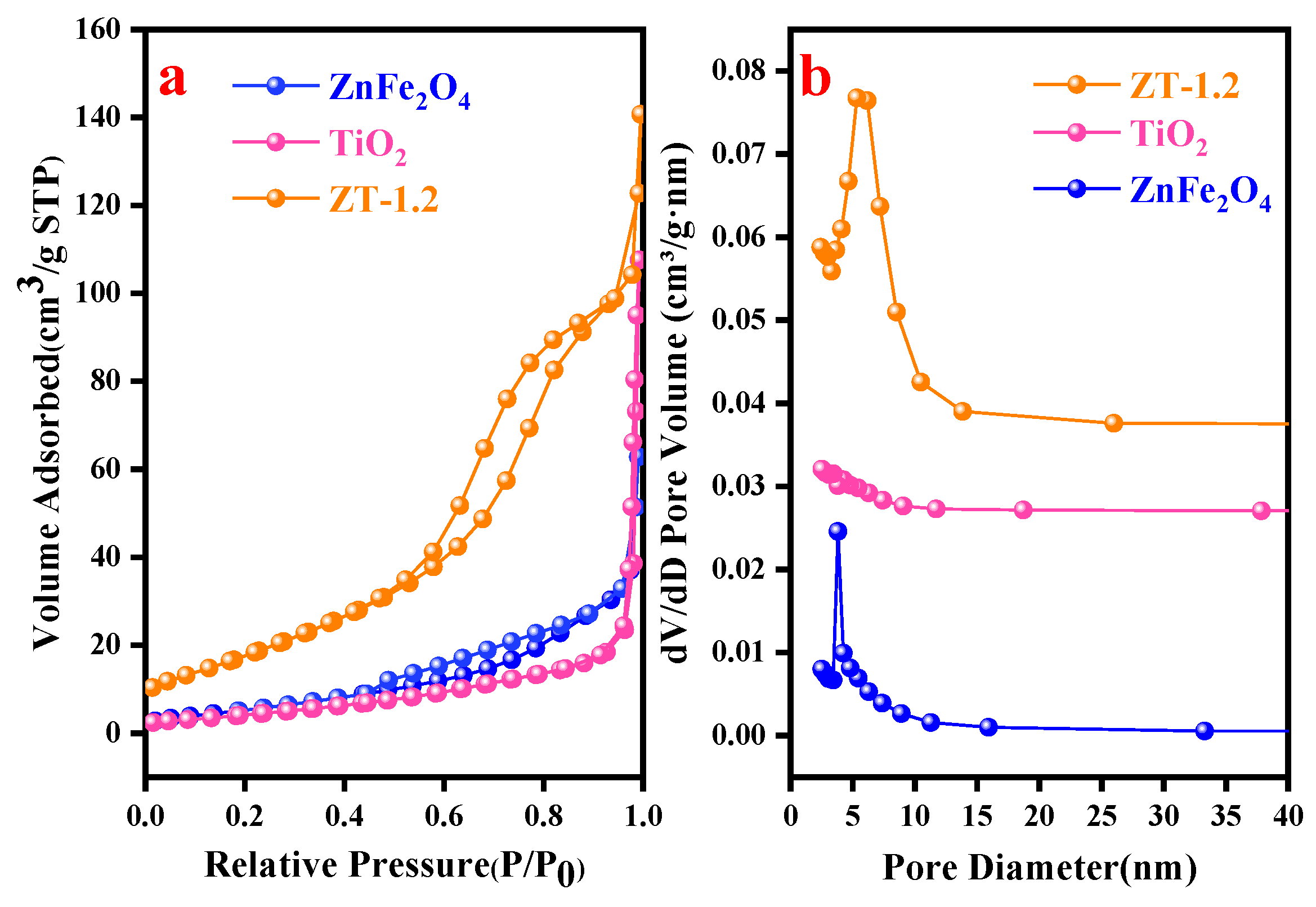
3.4. BET Test
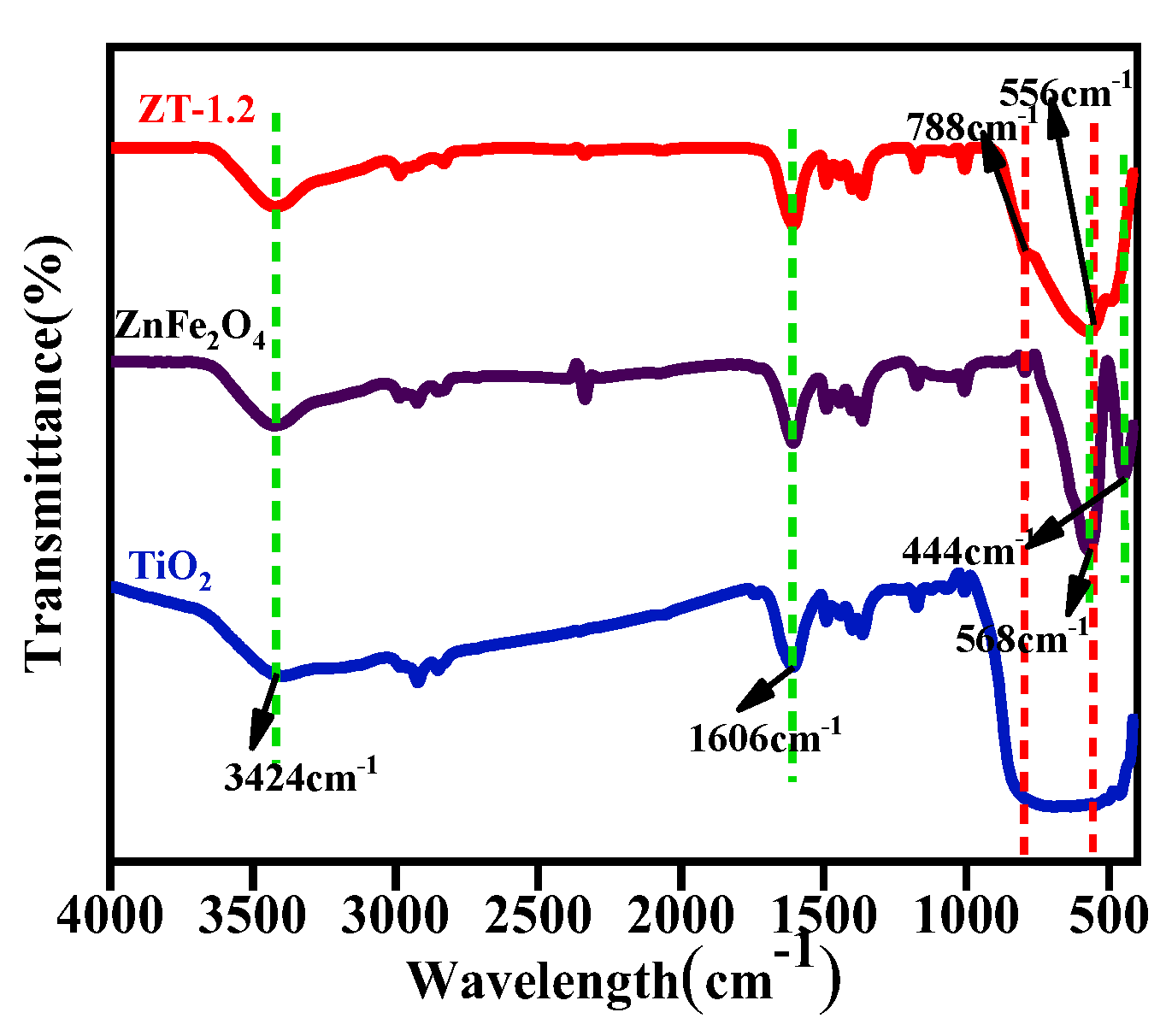
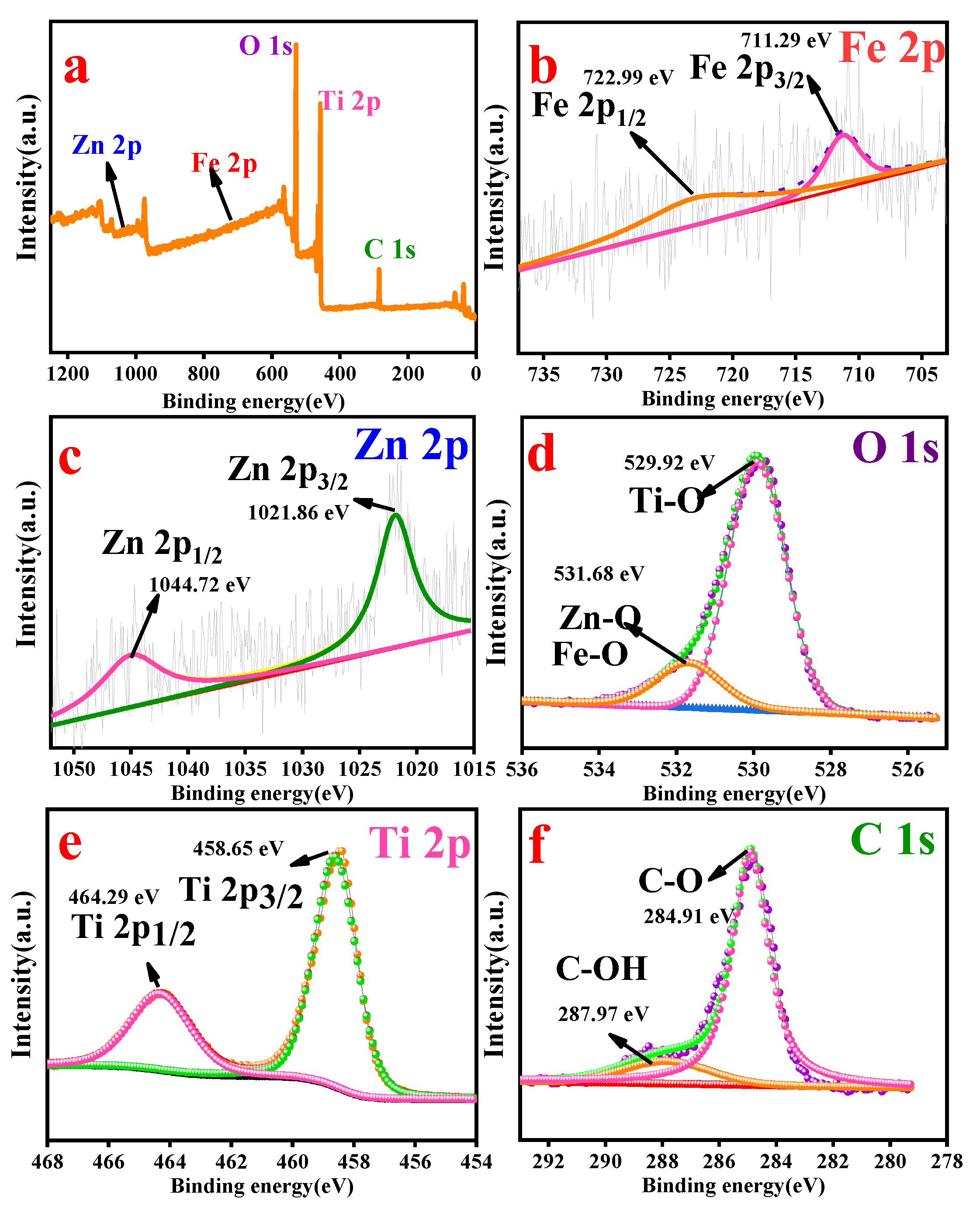
3.5. Chemical State Analysis
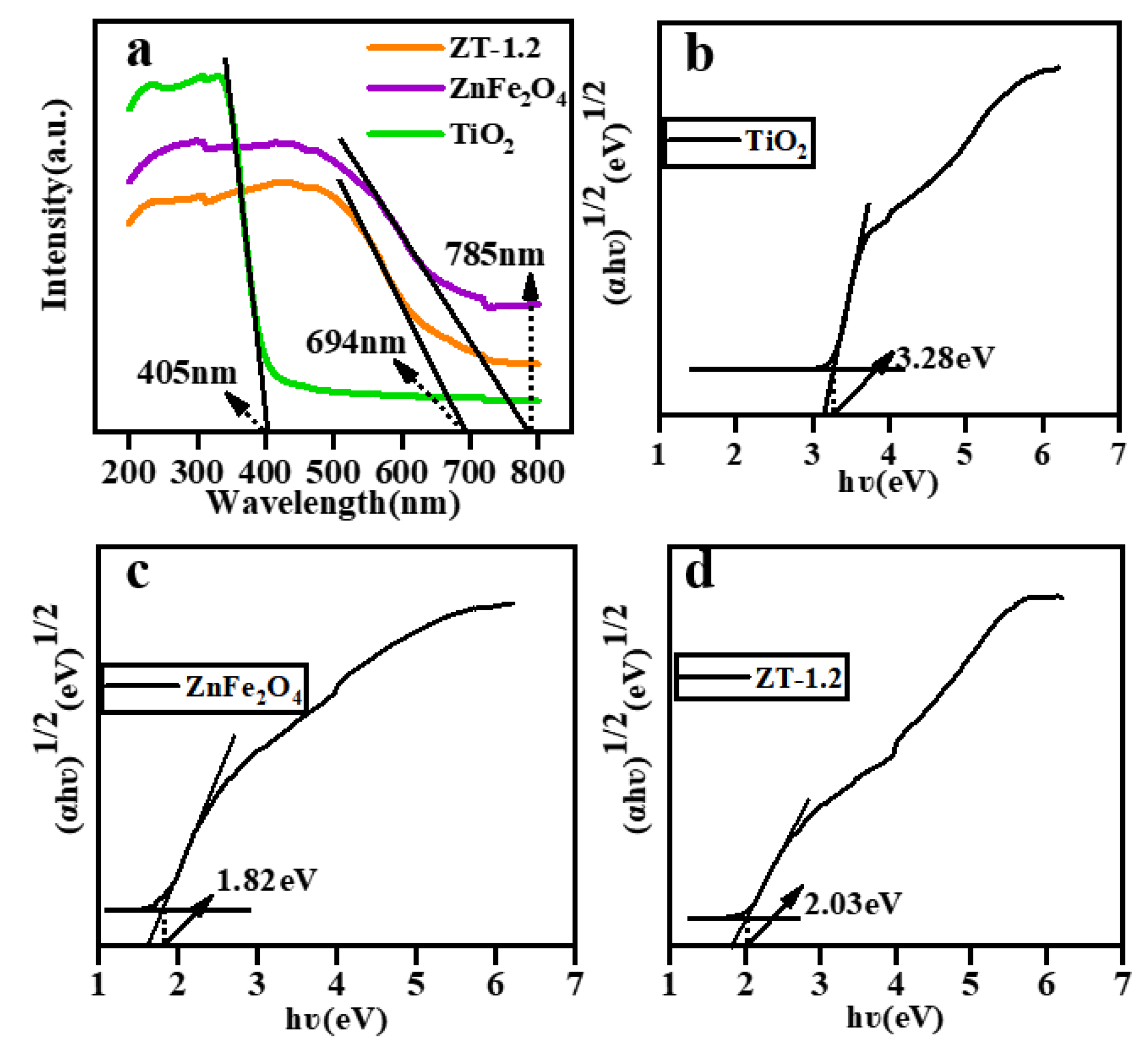
3.6. Optical Performance Analysis
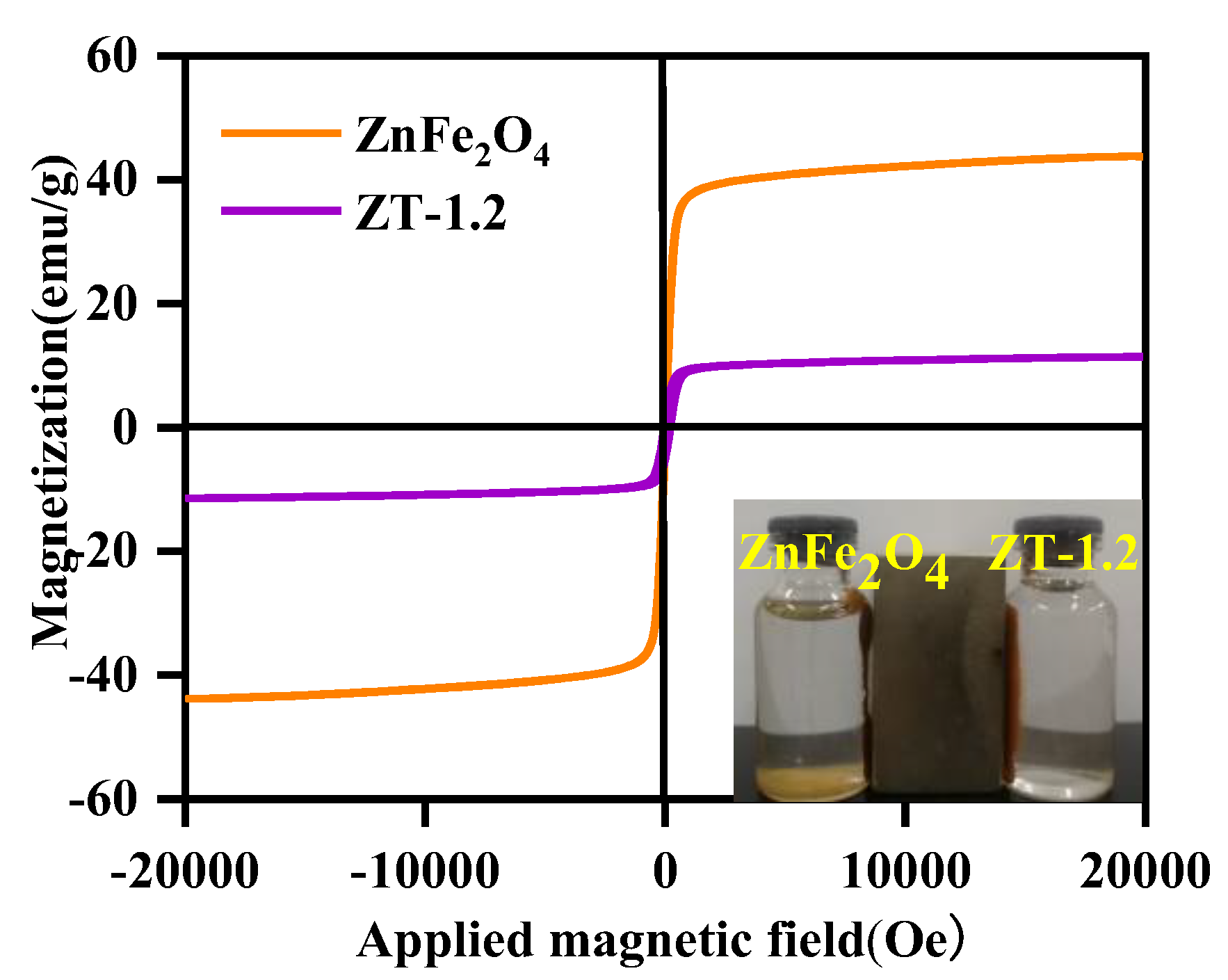
3.7. Magnetic Evaluation
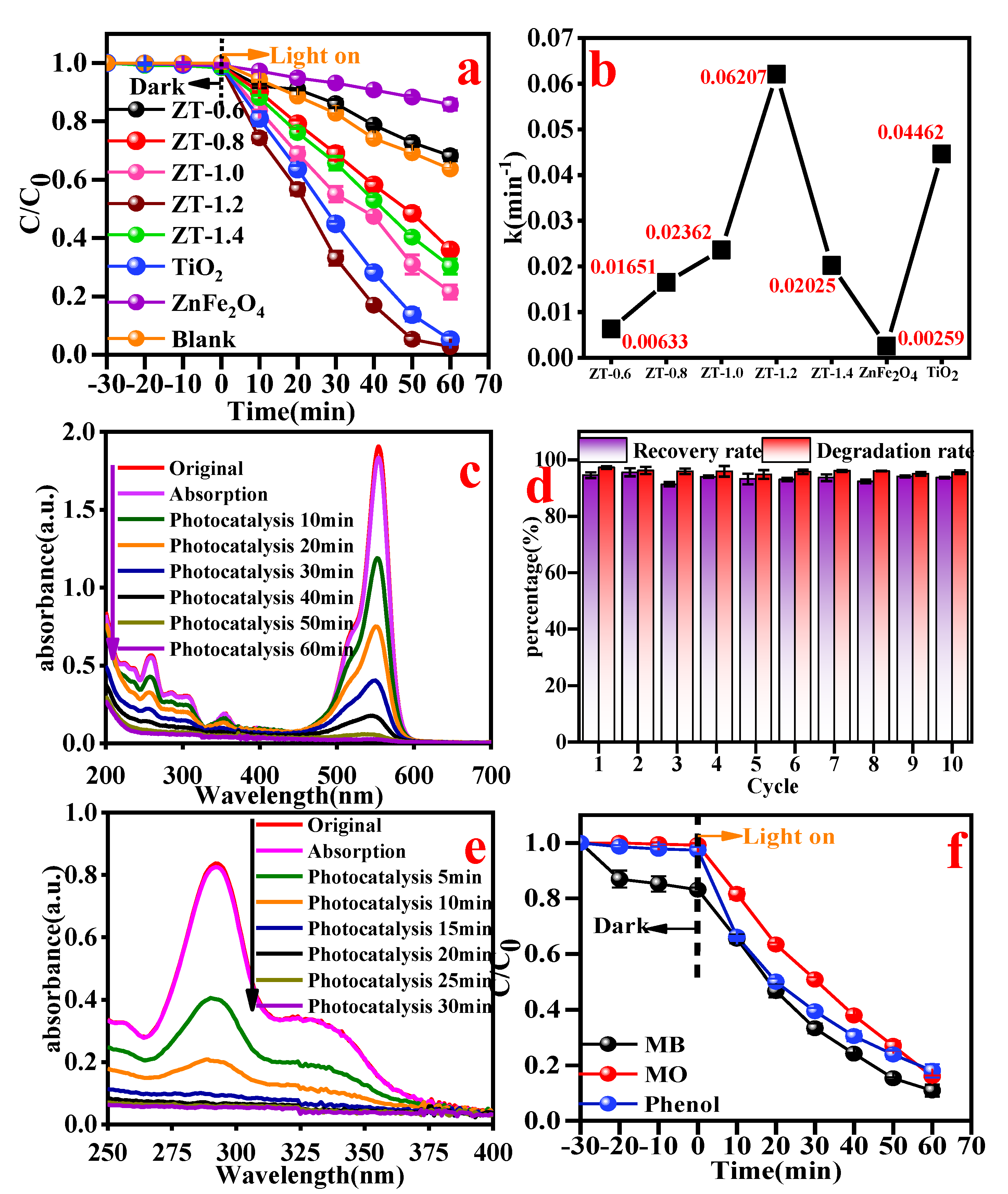
3.8. Photocatalytic Performance
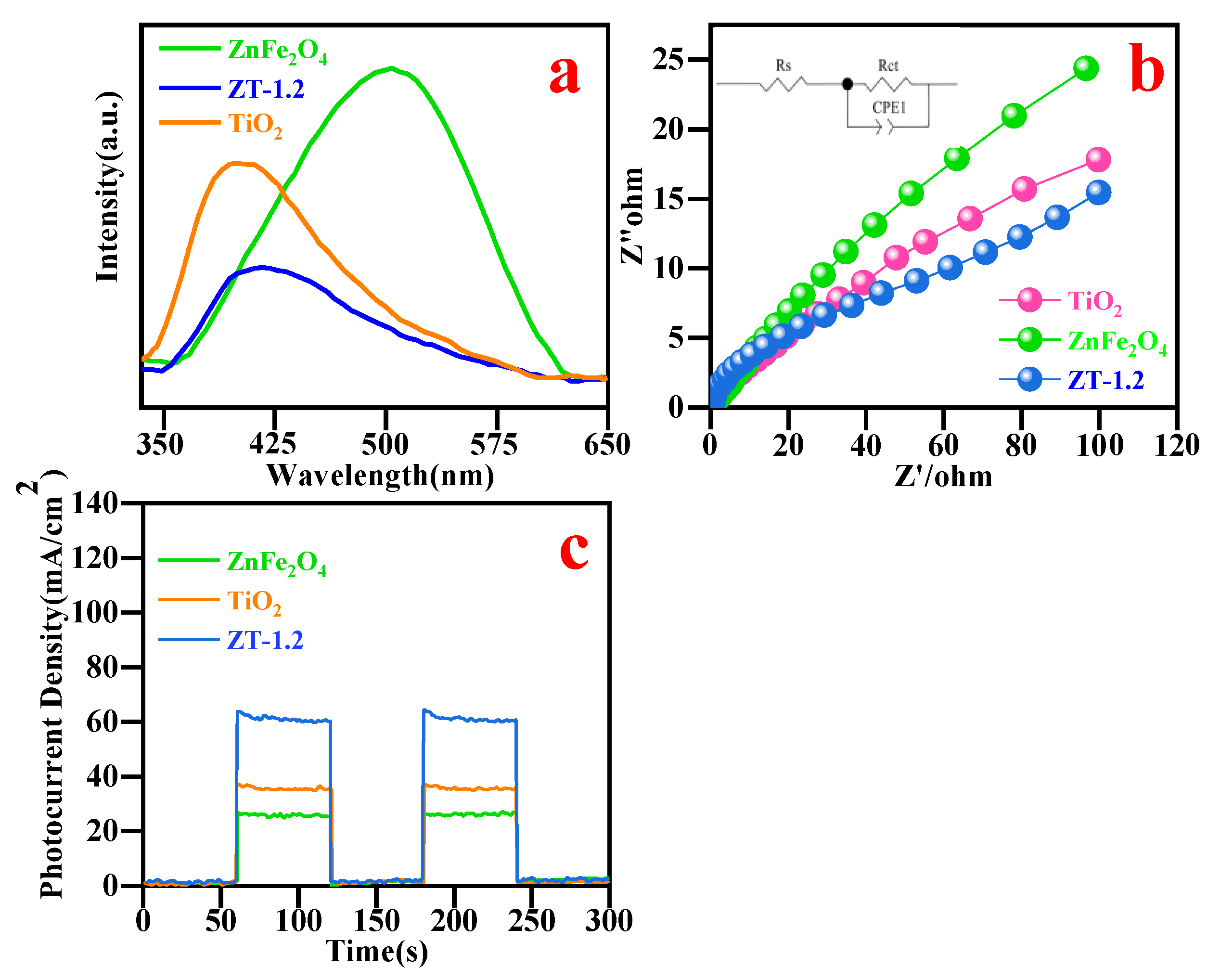
3.9. Photoelectric Properties
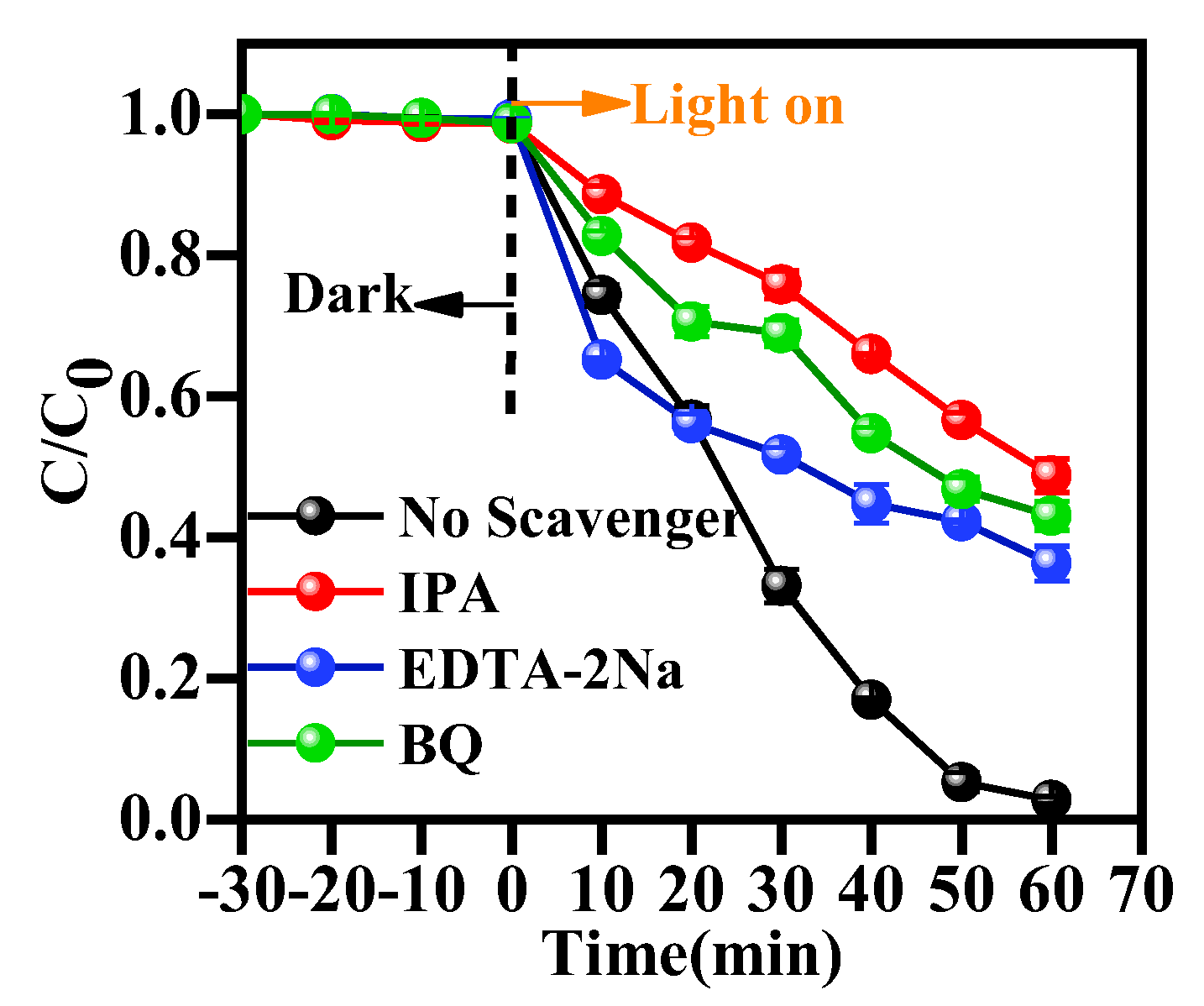
3.10. Photocatalytic Mechanism Study
3.11. Advantages of Ultrasonic Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef]
- Roy, N.; Alex, S.A.; Chandrasekaran, N.; Mukherjee, A.; Kannabiran, K. A comprehensive update on antibiotics as an emerging water pollutant and their removal using nano-structured photocatalysts. J. Environ. Chem. Eng. 2021, 9, 104796. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; Wei, G.; Khan, E.; Hau Ng, Y.; Sik Ok, Y. Recent advances in photodegradation of antibiotic residues in water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef] [PubMed]
- Asim Jilani, A.A.M. MoS2-Cu/CuO@graphene Heterogeneous Photocatalysis for Enhanced Photocatalytic Degradation of MB from Water. Polymers 2022, 14, 3259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhou, Q. Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Carey, J.H.; Lawrence, J.; Tosine, H.M. Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Morawski, A.W. TiO2/graphene-based nanocomposites for water treatment: A brief overview of charge carrier transfer, antimicrobial and photocatalytic performance. Appl. Catal. B Environ. 2019, 253, 179–186. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; He, J.; Lo, I.M.C. Recent developments and challenges in practical application of visible–light–driven TiO2–based heterojunctions for PPCP degradation: A critical review. Water Res. 2020, 170, 115356. [Google Scholar] [CrossRef]
- Ratova, M.; Redfern, J.; Verran, J.; Kelly, P.J. Highly efficient photocatalytic bismuth oxide coatings and their antimicrobial properties under visible light irradiation. Appl. Catal. B Environ. 2018, 239, 223–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Duoerkun, G.; Shi, Z.; Cao, W.; Liu, T.; Liu, J.; Zhang, L.; Li, M.; Chen, Z. Construction of TiO2/Ag3PO4 nanojunctions on carbon fiber cloth for photocatalytically removing various organic pollutants in static or flowing wastewater. J. Colloid Interface Sci. 2020, 571, 213–221. [Google Scholar] [CrossRef]
- Feizpoor, S.; Habibi-Yangjeh, A. Ternary TiO2/Fe3O4/CoWO4 nanocomposites: Novel magnetic visible-light-driven photocatalysts with substantially enhanced activity through p-n heterojunction. J. Colloid Interface Sci. 2018, 524, 325–336. [Google Scholar] [CrossRef]
- Ayyappan, S.; Paneerselvam, G.; Antony, M.P.; Philip, J. Structural stability of ZnFe2O4 nanoparticles under different annealing conditions. Mater. Chem. Phys. 2011, 128, 400–404. [Google Scholar] [CrossRef]
- Janani, B.; Syed, A.; Sruthi, L.; Sivaranjani, P.R.; Elgorban, A.M.; Bahkali, A.H.; Zaghloul, N.S.S.; Badawy, M.M.; Das, A.; Khan, S.S. Visible light driven photocatalytic activity and efficient antibacterial activity of ZnFe2O4 decorated CdO nanohybrid heterostructures synthesized by ultrasonic-assisted method. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127307. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, B.; Li, X.; Wang, X.; Huang, K.; Chen, Z. MOF-derived magnetically recoverable Z-scheme ZnFe2O4/Fe2O3 perforated nanotube for efficient photocatalytic ciprofloxacin removal. Chem. Eng. J. 2022, 430, 132728. [Google Scholar] [CrossRef]
- Choudhary, S.; Bisht, A.; Mohapatra, S. Microwave-assisted synthesis of α-Fe2O3/ZnFe2O4/ZnO ternary hybrid nanostructures for photocatalytic applications. Ceram. Int. 2021, 47, 3833–3841. [Google Scholar] [CrossRef]
- Huang, Y.; Xing, W.; Fan, J.; Dai, J.; Liu, Q.; Hu, F.; Xu, G. Preparation and microwave absorption properties of the hollow ZnFe2O4@C composites with core-shell structure. J. Magn. Magn. Mater. 2020, 502, 166543. [Google Scholar] [CrossRef]
- Wang, D.; Yang, J.; Li, X.; Zhai, H.; Lang, J.; Song, H. Preparation of magnetic Fe3O4@SiO2@mTiO2–Au spheres with well-designed microstructure and superior photocatalytic activity. J. Mater. Sci. 2016, 51, 9602–9612. [Google Scholar] [CrossRef]
- Hou, L.; Li, W.; Wu, Z.; Wei, Q.; Yang, H.; Jiang, Y.; Wang, T.; Wang, Y.; He, Q. Embedding ZnCdS@ZnIn2S4 into thiazole-modified g-C3N4 by electrostatic self-assembly to build dual Z-scheme heterojunction with spatially separated active centers for photocatalytic H2 evolution and ofloxacin degradation. Sep. Purif. Technol. 2022, 290, 120858. [Google Scholar] [CrossRef]
- Xu, Q.; Feng, J.; Li, L.; Xiao, Q.; Wang, J. Hollow ZnFe2O4/TiO2 composites: High-performance and recyclable visible-light photocatalyst. J. Alloys Compd. 2015, 641, 110–118. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Su, M.; Xie, Y.; Chen, D. Dual light-driven p-ZnFe2O4/n-TiO2 catalyst: Benzene-breaking reaction for malachite green. Environ. Res. 2022, 207, 112081. [Google Scholar] [CrossRef]
- Lu, Y.; Feng, Y.; Wang, F.; Zou, X.; Chen, Z.F.; Chen, P.; Liu, H.; Su, Y.; Zhang, Q.; Liu, G. Facile hydrothermal synthesis of carbon dots (CDs) doped ZnFe2O4/TiO2 hybrid materials with high photocatalytic activity. J. Photochem. Photobiol. A Chem. 2018, 353, 10–18. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Li, H.; Jiang, H.; Tian, G. Achieving cadmium selenide-decorated zinc ferrite@titanium dioxide hollow core/shell nanospheres with improved light trapping and charge generation for photocatalytic hydrogen generation. J. Colloid Interface Sci. 2020, 575, 158–167. [Google Scholar] [CrossRef]
- Zeng, D.; Wang, J.; Xie, Y.; Ling, Y.; Zhao, J.; Ye, H.; Chen, T. TiO2@ZnFe2O4 heterojunctions for effecicent photocatalytic degradation of persistent pollutants and hydrogen evolution. Mater. Chem. Phys. 2022, 277, 125462. [Google Scholar] [CrossRef]
- Fu, N.; Ren, X.C.; Wan, J.X. Preparation of ag-coated SiO2@TiO2 core-shell nanocomposites and their photocatalytic applications towards phenol and methylene blue degradation. J. Nanomater. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Huang, Y.; Jia, Y.; Chen, L.; Cui, H. Synergistic effect of adsorptive photocatalytic oxidation and degradation mechanism of cyanides and Cu/Zn complexes over TiO2/ZSM-5 in real wastewater. J. Hazard. Mater. 2021, 416, 125802. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y.; Tian, C.; Zhang, H.; Li, X.; Liu, W.; Li, W.; Kuang, S.; Tong, H. Boosted photocatalytic removal of Cr(VI) using MoS2 modified g-C3N4/ZnFe2O4 magnetic heterojunction composites. Process Saf. Environ. Prot. 2022, 162, 72–82. [Google Scholar] [CrossRef]
- GB11911-89; Ministry of Environmental Protection of the People’s Republic of China. China Environmental Monitoring Station. Water Quality Determination of Fe and Mn Flame Atomic Absorption Spectrophotometry. China Standard Press: Beijing, China, 1990.
- Ahmadpour, N.; Sayadi, M.H.; Sobhani, S.; Hajiani, M. Photocatalytic degradation of model pharmaceutical pollutant by novel magnetic TiO2@ZnFe2O4/Pd nanocomposite with enhanced photocatalytic activity and stability under solar light irradiation. J. Environ. Manag. 2020, 271, 110964. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Sayadi, M.H.; Sobhani, S.; Hajiani, M. A potential natural solar light active photocatalyst using magnetic ZnFe2O4@TiO2/Cu nanocomposite as a high performance and recyclable platform for degradation of naproxen from aqueous solution. J. Clean. Prod. 2020, 268, 122023. [Google Scholar] [CrossRef]
- Chen, J.; Gao, J.; Liu, X.; Wang, P.; Yu, X.; Zhao, F.; Sun, Y.; Feng, W.; Wang, Q. Controllable Phase Transformation and Enhanced Photocatalytic Performance of Nano-TiO2 by Using Oxalic Acid. Nanomaterials 2022, 12, 3019. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.V.A. The Growth of Extended Melem Units on g-C3N4 by Hydrothermal Treatment and Its Effect on Photocatalytic Activity of g-C3N4 for Photodegradation of Tetracycline Hydrochloride under Visible Light Irradiation. Nanomaterials 2022, 12, 2945. [Google Scholar] [CrossRef] [PubMed]
- Aihemaiti, X.; Wang, X.; Wang, Z.; Bai, Y.; Qi, K.; Ma, Y.; Tao, K.; Simayi, M.; Kuerban, N. Effective prevention of charge trapping in red phosphorus with nanosized CdS modification for superior photocatalysis. J. Environ. Chem. Eng. 2021, 9, 106479. [Google Scholar] [CrossRef]
- Kobkeatthawin, T.; Chaveanghong, S.; Trakulmututa, J.; Amornsakchai, T.; Kajitvichyanukul, P.; Smith, S.M. Photocatalytic Activity of TiO2/g-C3N4 Nanocomposites for Removal of Monochlorophenols from Water. Nanomaterials 2022, 12, 2852. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lei, J.; Wang, Y.; Tang, Q.; Kang, C. Fabrication of a magnetic ternary ZnFe2O4/TiO2/RGO Z-scheme system with efficient photocatalytic activity and easy recyclability. RSC Adv. 2020, 10, 17293–17301. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, C.; Li, M.; Lin, X.; Guo, F.; Shi, J. Fabrication of ternary Ag3PO4/Co3(PO4)2/g-C3N4 heterostructure with following Type II and Z-Scheme dual pathways for enhanced visible-light photocatalytic activity. J. Hazard. Mater. 2020, 389, 121907. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Meng, L.; Ning, X.; Yin, W.; Hou, J.; Xu, Q.; Yi, J.; Wang, S.; Wang, X. Switching charge transfer of g-C3N4/BiVO4 heterojunction from type II to Z-scheme via interfacial vacancy engineering for improved photocatalysis. Int. J. Hydrogen Energy 2022, 47, 8749–8760. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Luo, J.; Feng, Z.; Huang, J. Hydrothermal synthesized novel nanoporous g-C3N4/MnTiO3 heterojunction with direct Z-scheme mechanism. Electrochim. Acta 2017, 258, 998–1007. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; Zeng, X.; Lo, I.M.C. Development of g-C3N4/TiO2/Fe3O4@SiO2 heterojunction via sol-gel route: A magnetically recyclable direct contact Z-scheme nanophotocatalyst for enhanced photocatalytic removal of ibuprofen from real sewage effluent under visible light. Chem. Eng. J. 2018, 353, 645–656. [Google Scholar] [CrossRef]
- Ding, M.; Miao, X.; Cao, L.; Zhang, C.; Ping, Y. Core-shell nanostructured SiO2@a-TiO2@Ag composite with high capacity and safety for Li-ion battery anode. Mater. Lett. 2022, 308, 131276. [Google Scholar] [CrossRef]
- Ahmed Alshehri, N.; Assaifan, A.K.; Albalawi, A.A.; Alghamdi, E.H.; Niu, Y.; Pleydell-Pearce, C.; Pavloudis, T.; Kioseoglou, J.; Alsawat, M.; Maffeis, T.T.G. Fabrication and characterisation of ZnO@TiO2 core/shell nanowires using a versatile kinetics-controlled coating growth method. Appl. Surf. Sci. 2022, 594, 153463. [Google Scholar] [CrossRef]
- Eskandari, P.; Zand, Z.; Kazemi, F.; Ramdar, M. Enhanced catalytic activity of one-dimensional CdS @TiO2 core-shell nanocomposites for selective organic transformations under visible LED irradiation. J. Photochem. Photobiol. A Chem. 2021, 418, 113404. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, L.; Xu, C.; Zhang, W.; Chen, M.; Xu, Q.; Diao, G. A novel method for facile preparation of recoverable Fe3O4@TiO2 core-shell nanospheres and their advanced photocatalytic application. Chem. Phys. Lett. 2020, 761, 138073. [Google Scholar] [CrossRef]
- Fu, H.; Sun, S.; Yang, X.; Li, W.; An, X.; Zhang, H.; Dong, Y.; Jiang, X.; Yu, A. A facile coating method to construct uniform porous α-Fe2O3@TiO2 core-shell nanostructures with enhanced solar light photocatalytic activity. Powder Technol. 2018, 328, 389–396. [Google Scholar] [CrossRef]
- Chi, Y.; Yuan, Q.; Li, Y.; Zhao, L.; Li, N.; Li, X.; Yan, W. Magnetically separable Fe3O4@SiO2@TiO2-Ag microspheres with well-designed nanostructure and enhanced photocatalytic activity. J. Hazard. Mater. 2013, 262, 404–411. [Google Scholar] [CrossRef]






| Photocatalysts | Preparation Method | Ambient Temperature | Reaction Time | Citations |
|---|---|---|---|---|
| ZnFe2O4@TiO2/CdSe | Mechanical agitation | 45 °C | 24 h | [24] |
| SiO2@a-TiO2@Ag | Mechanical agitation | 80 °C | 2 h | [41] |
| ZnO@TiO2 | Mechanical agitation | 35 °C | 6 h | [42] |
| CdS@TiO2 | Mechanical agitation | Room temperature | 4 h | [43] |
| Fe3O4@TiO2 | Mechanical agitation | Room temperature | 3 h | [44] |
| α-Fe2O3@TiO2 | Mechanical agitation | Room temperature | 9 h | [45] |
| Fe3O4@SiO2@TiO2-Ag | Mechanical agitation | 85 °C | 1.5 h | [46] |
| ZnFe2O4@TiO2 | Ultrasonic method | Room temperature | 1.5 h | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Ding, S.; Wang, B.; Ren, X. Preparation of ZnFe2O4@TiO2 Novel Core-Shell Photocatalyst by Ultrasonic Method and Its Photocatalytic Degradation Activity. Coatings 2022, 12, 1407. https://doi.org/10.3390/coatings12101407
Chen R, Ding S, Wang B, Ren X. Preparation of ZnFe2O4@TiO2 Novel Core-Shell Photocatalyst by Ultrasonic Method and Its Photocatalytic Degradation Activity. Coatings. 2022; 12(10):1407. https://doi.org/10.3390/coatings12101407
Chicago/Turabian StyleChen, Renhua, Suying Ding, Bo Wang, and Xuechang Ren. 2022. "Preparation of ZnFe2O4@TiO2 Novel Core-Shell Photocatalyst by Ultrasonic Method and Its Photocatalytic Degradation Activity" Coatings 12, no. 10: 1407. https://doi.org/10.3390/coatings12101407
APA StyleChen, R., Ding, S., Wang, B., & Ren, X. (2022). Preparation of ZnFe2O4@TiO2 Novel Core-Shell Photocatalyst by Ultrasonic Method and Its Photocatalytic Degradation Activity. Coatings, 12(10), 1407. https://doi.org/10.3390/coatings12101407





