Fabrication of a Conductive Additive for the Anticorrosion Enhancement of Zinc-Rich Epoxy Coatings
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Conductive PPy/SCP Particles
2.3. Fabrication of ZRP/PPy/SCP Coating
2.4. Characterization
3. Results and Discussion
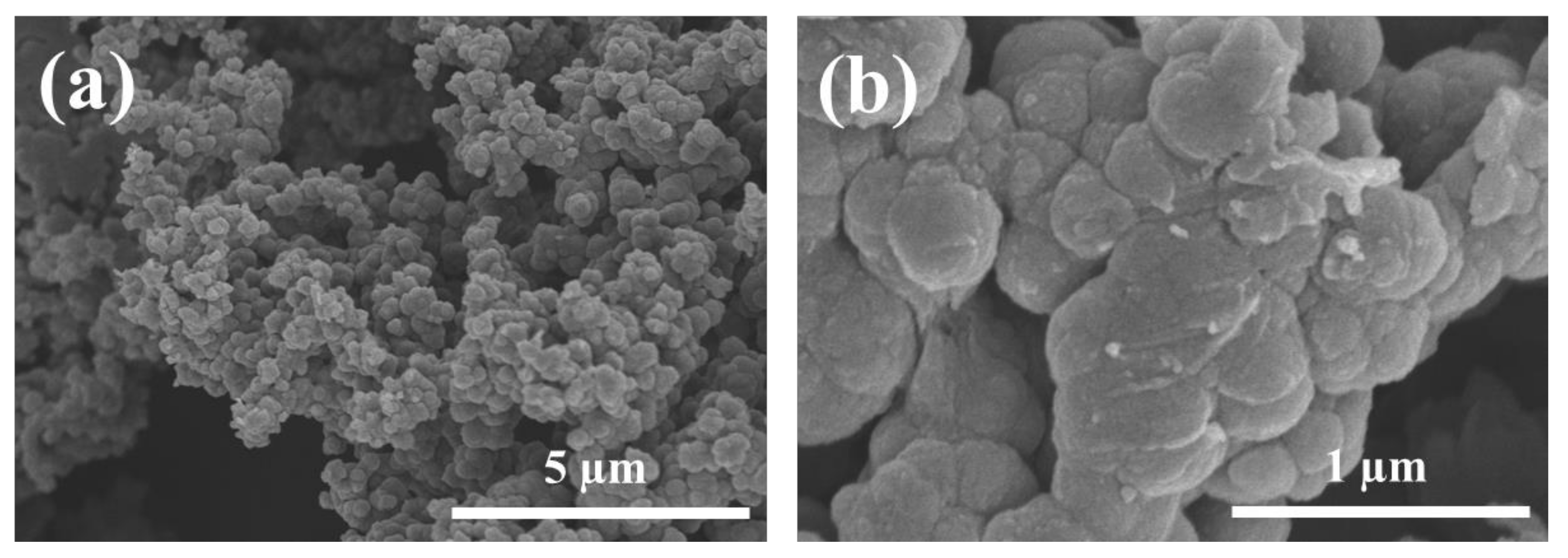
3.1. PPy/SCP Particle Analysis
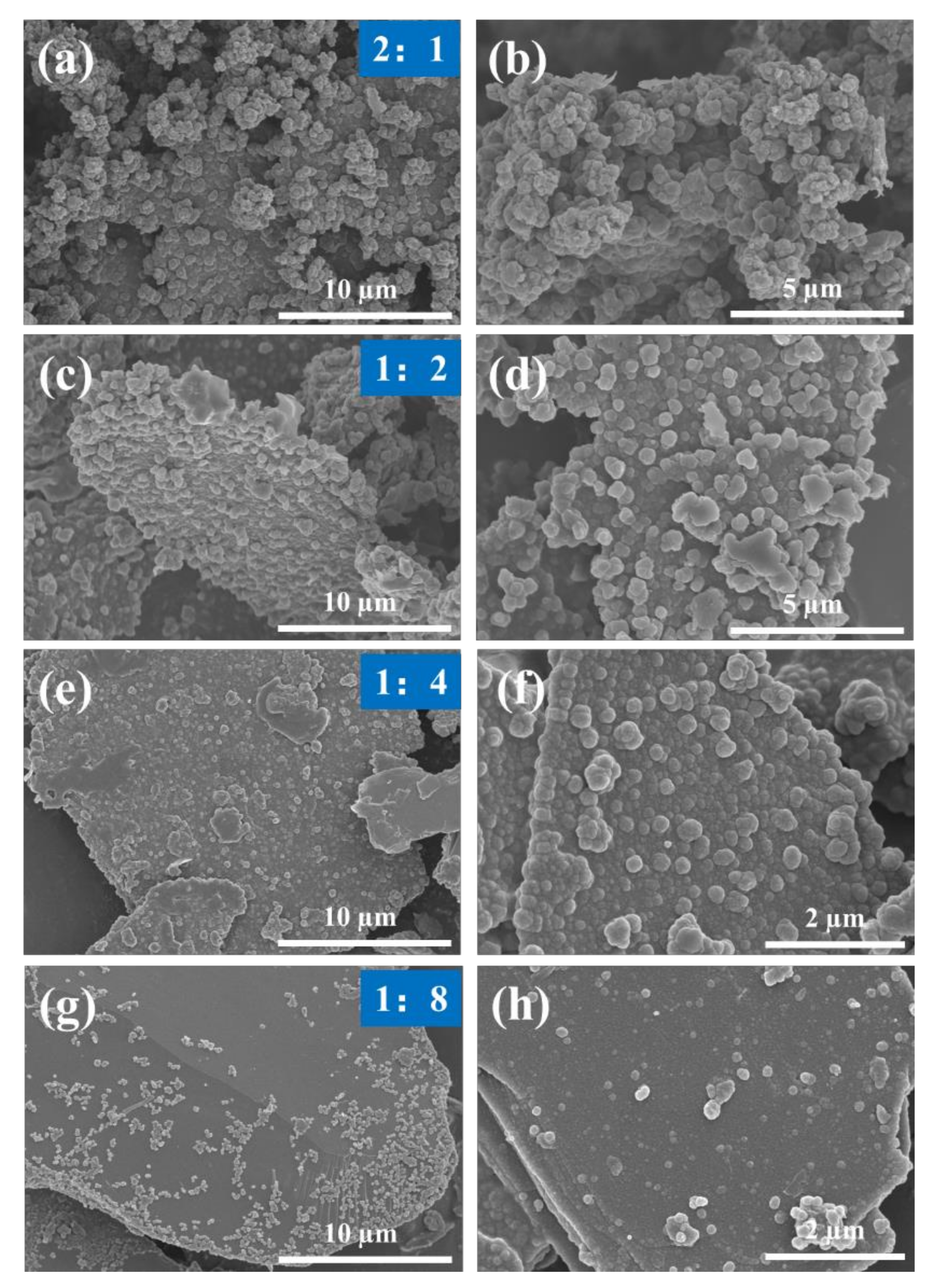
3.2. ZRP/SCP/PPy Coating Analysis
3.2.1. Equal Mass Substitution
3.2.2. Equal Volume Substitution
Surface Morphologies
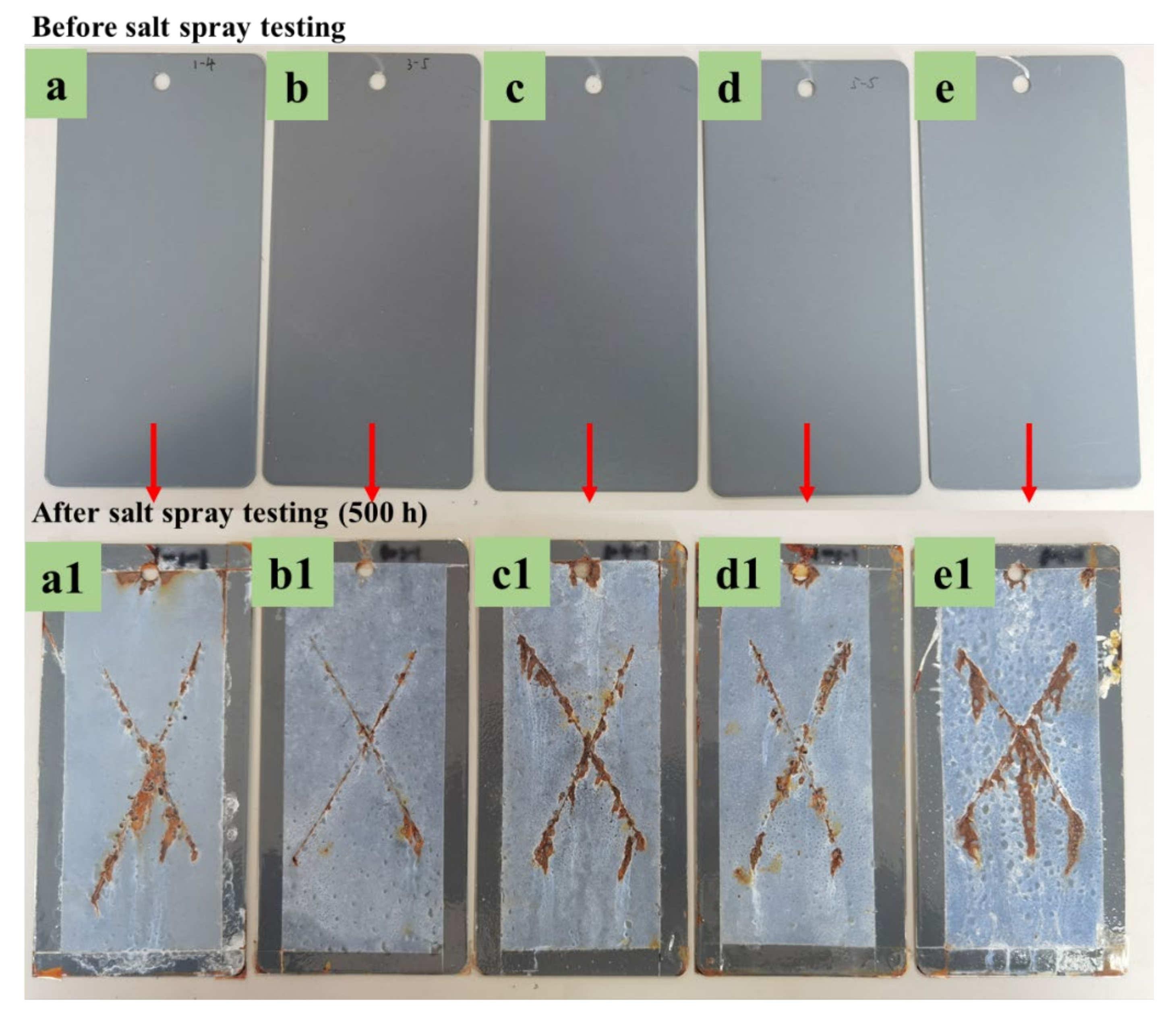
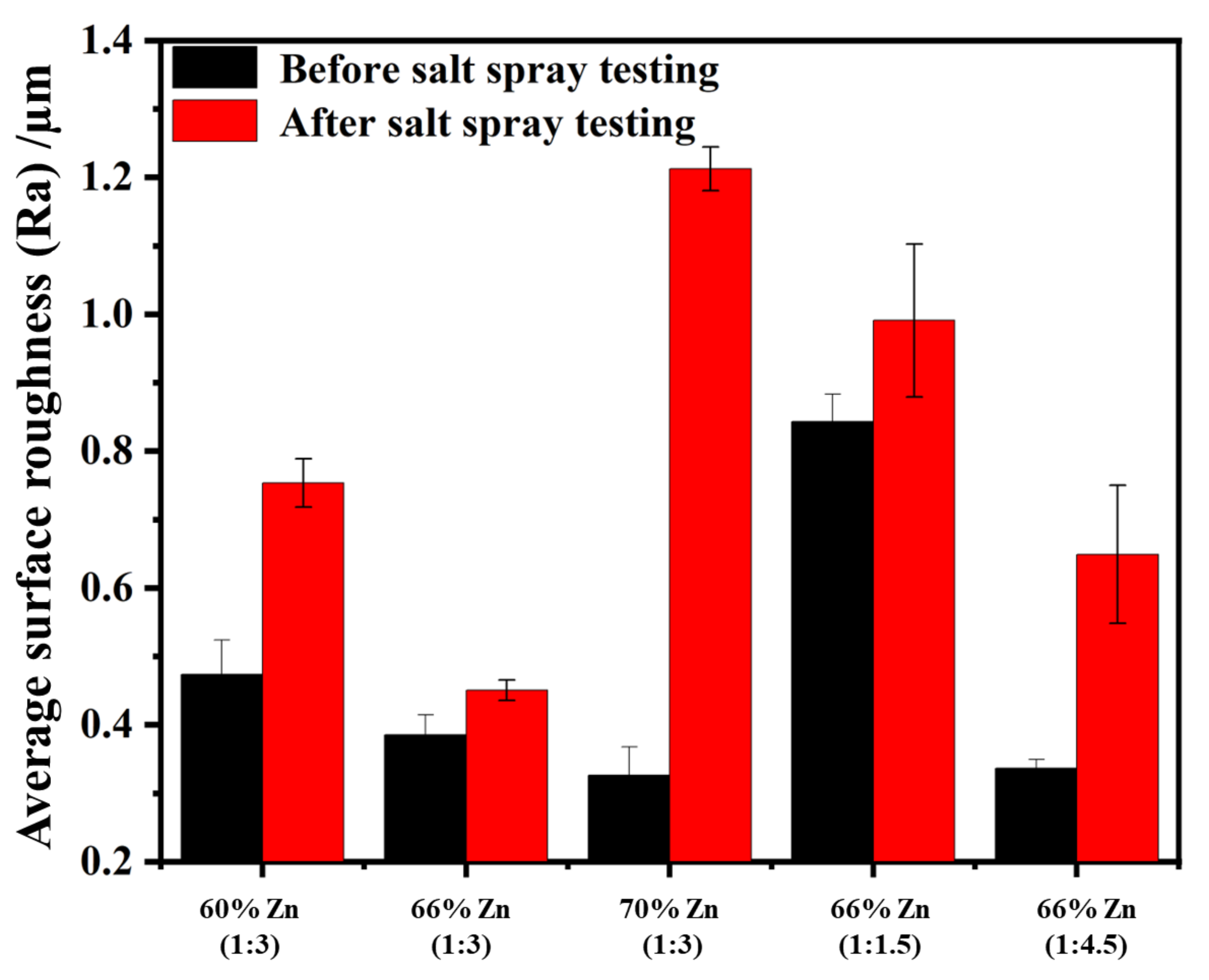
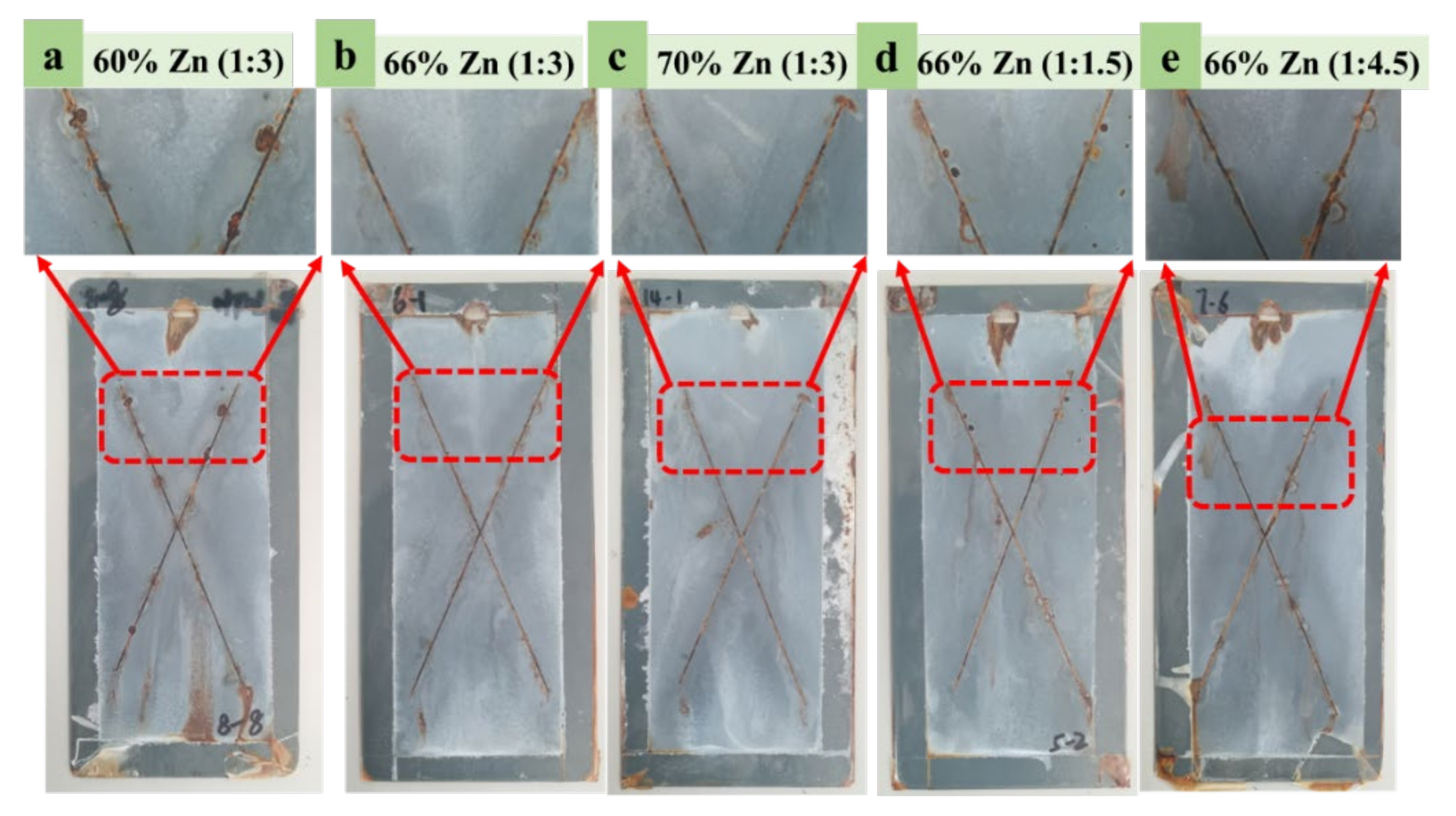
Salt Spray Test
Adhesion Strength
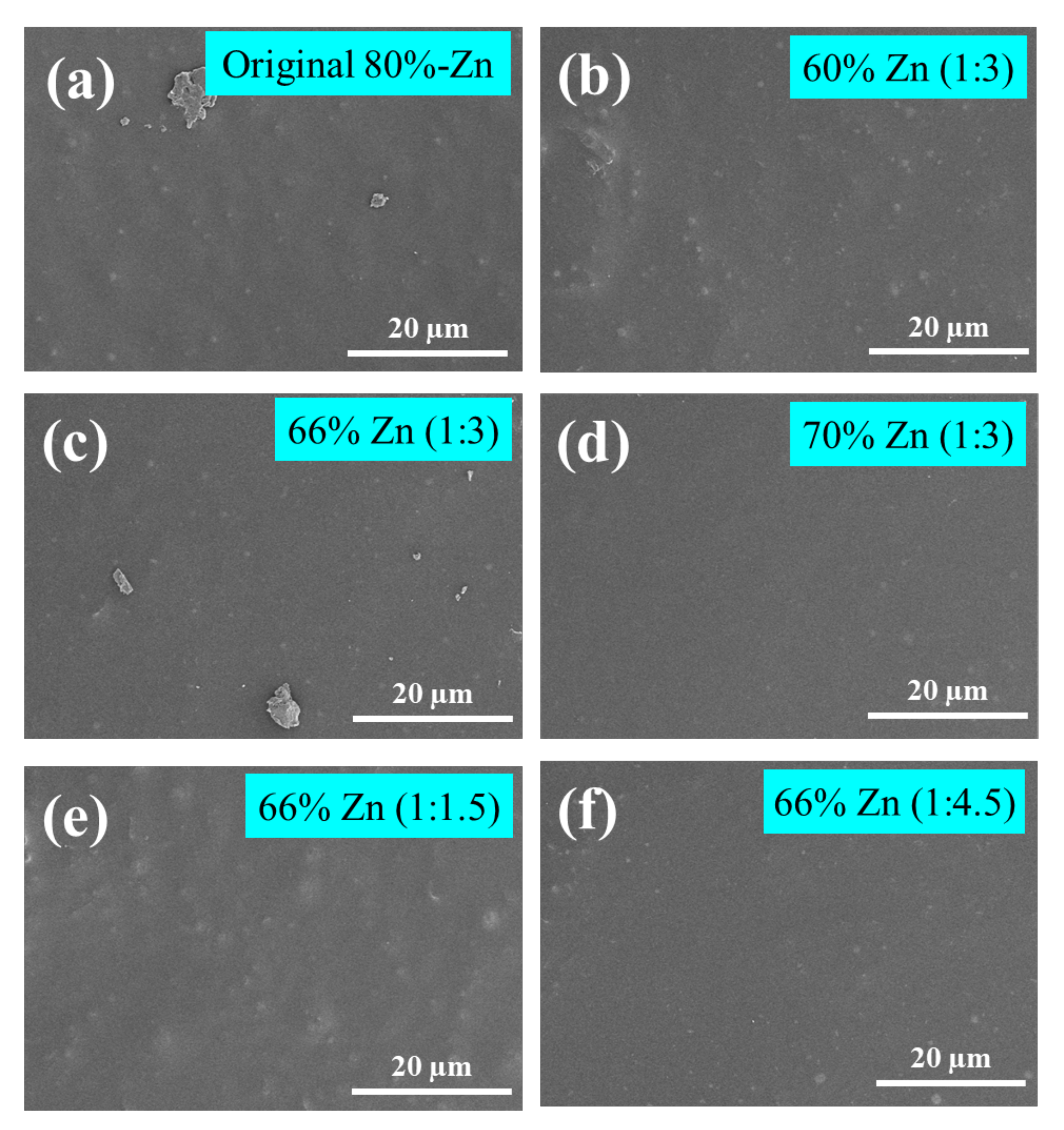
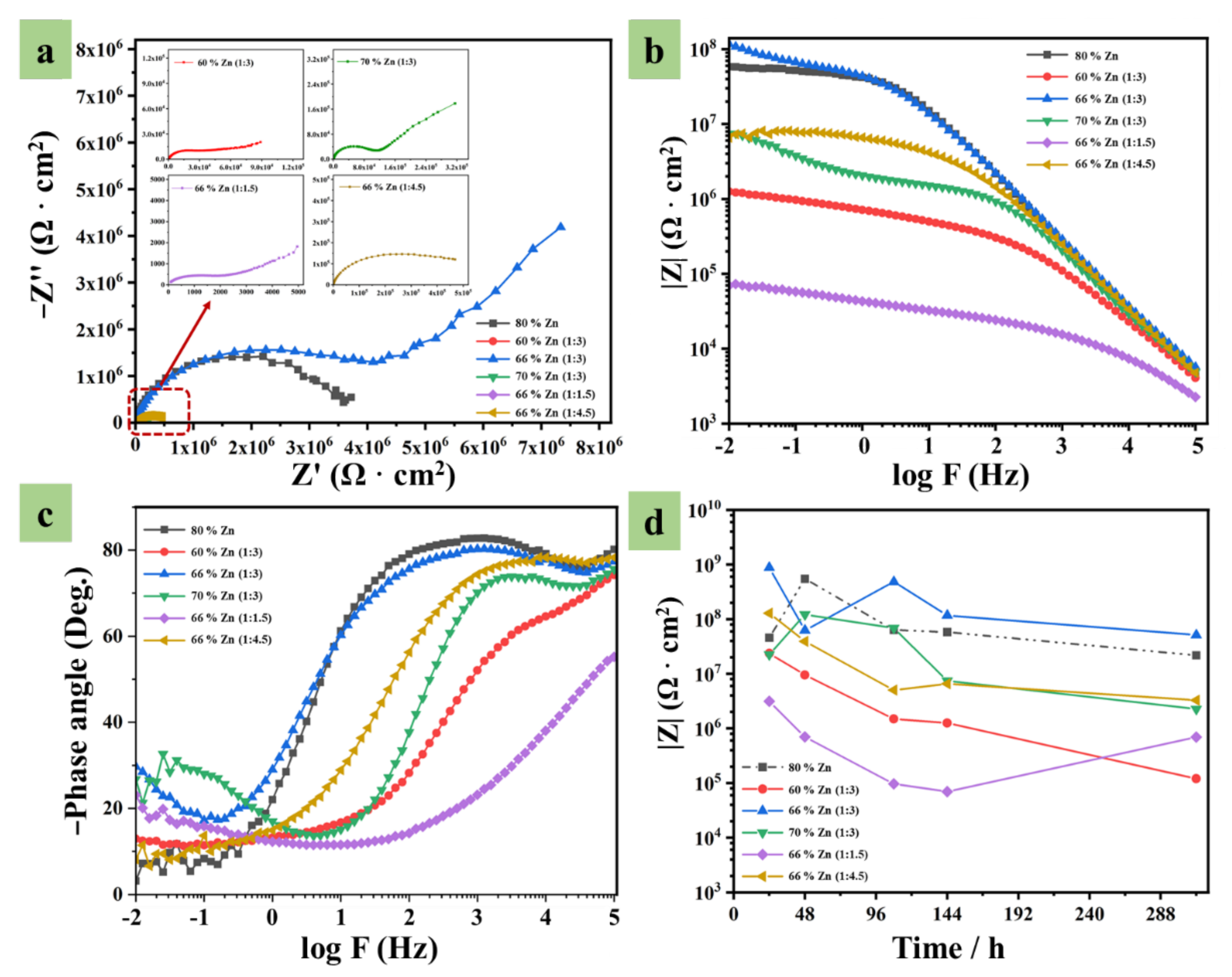
Corrosion Resistance
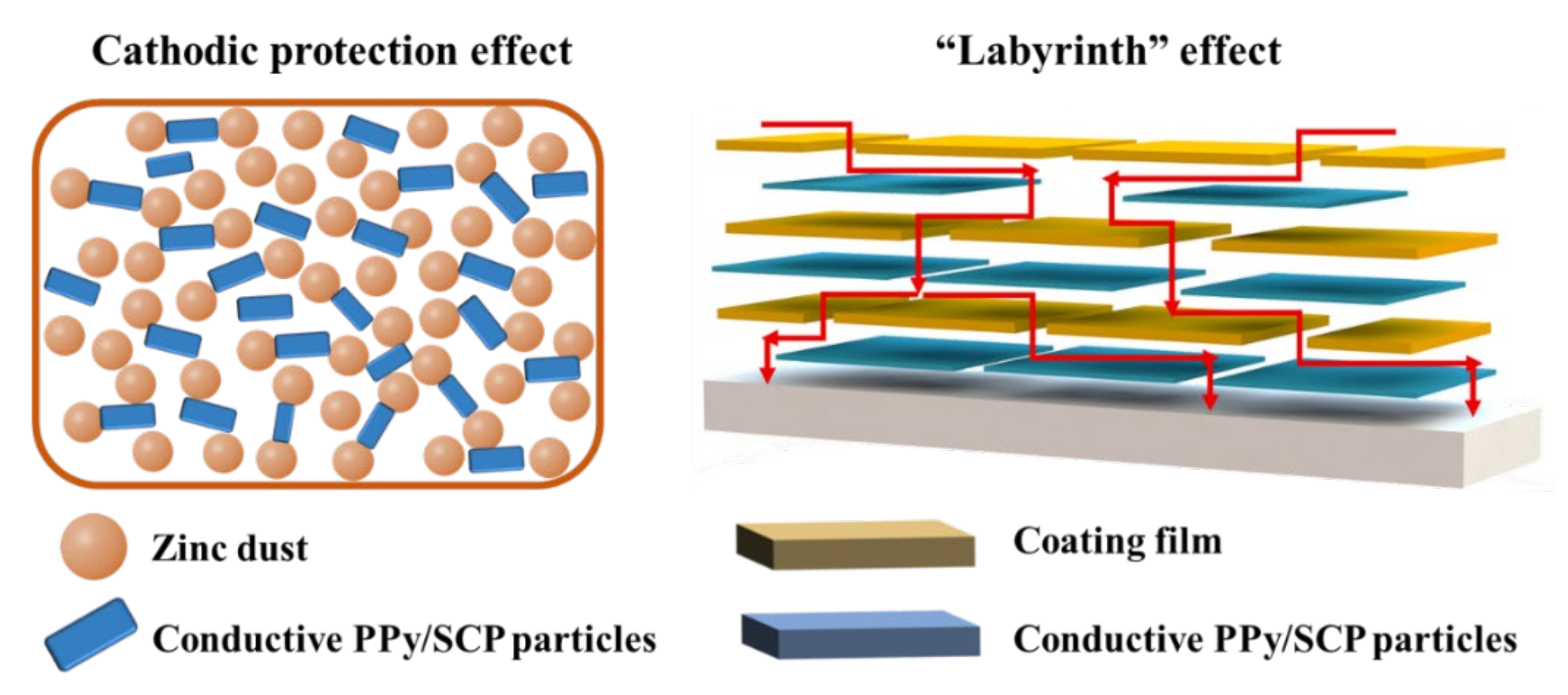
3.3. Anticorrosion Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, Y.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Recent Progresses of Superhydrophobic Coatings in Different Application Fields: An Overview. Coatings 2021, 11, 116. [Google Scholar] [CrossRef]
- Tan, H.; Wang, D.; Guo, Y. Investigation of graphene effect on the anti-corrosion behaviour of polyurethane coatings in sea-water. Anti-Corros. Methods Mater. 2019, 66, 853–860. [Google Scholar] [CrossRef]
- Wei, J.; Li, B.; Jing, L.; Tian, N.; Zhao, X.; Zhang, J. Efficient protection of Mg alloy enabled by combination of a conventional anti-corrosion coating and a superamphiphobic coating. Chem. Eng. J. 2020, 390, 124562. [Google Scholar] [CrossRef]
- Ge, T.; Zhao, W.; Wu, X.; Lan, X.; Zhang, Y.; Qiang, Y.; He, Y. Incorporation of electroconductive carbon fibers to achieve enhanced anti-corrosion performance of zinc rich coatings. J. Colloid Interface Sci. 2020, 567, 113–125. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L.; Sun, W.; Yang, Z.; Wang, S.; Liu, G. Effect of chemical conversion induced by self-corrosion of zinc powders on enhancing corrosion protection performance of zinc-rich coatings. Corros. Sci. 2022, 194, 109942. [Google Scholar] [CrossRef]
- Marchebois, H.; Joiret, S.; Savall, C.; Bernard, J.; Touzain, S. Characterization of zinc-rich powder coatings by EIS and Raman spectroscopy. Surf. Coat. Technol. 2002, 157, 151–161. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Moghadam, M.H.M.; Shohani, N.; Mandavian, M. Effects of highly crystalline and conductive polyaniline/graphene oxide composites on the corrosion protection performance of a zinc-rich epoxy coating. Chem. Eng. J. 2017, 320, 363–375. [Google Scholar] [CrossRef]
- Shreepathi, S.; Bajaj, P.; Mallik, B.P. Electrochemical impedance spectroscopy investigations of epoxy zinc rich coatings: Role of Zn content on corrosion protection mechanism. Electrochim. Acta 2010, 55, 5129–5134. [Google Scholar] [CrossRef]
- Schaefer, K.; Miszczyk, A. Improvement of electrochemical action of zinc-rich paints by addition of nanoparticulate zinc. Corros. Sci. 2013, 66, 380–391. [Google Scholar] [CrossRef]
- Aminian, O.; Zeinodin, H.; Sadeghniiat-Haghighi, K.; Izadi, N. Respiratory Symptoms and Pulmonary Function Tests among Galvanized Workers Exposed to Zinc Oxide. J. Res. Health Sci. 2015, 15, 159–162. [Google Scholar] [PubMed]
- Raza, M.A.; Rehman, Z.U.; Ghauri, F.A.; Ahmad, A.; Ahmad, R.; Raffi, M. Corrosion study of electrophoretically deposited graphene oxide coatings on copper metal. Thin Solid Film. 2016, 620, 150–159. [Google Scholar] [CrossRef]
- Tan, H.; Wang, D.; Guo, Y. Thermal Growth of Graphene: A Review. Coatings 2018, 8, 40. [Google Scholar] [CrossRef]
- Olad, A.; Barati, M.; Shirmohammadi, H. Conductivity and anticorrosion performance of polyaniline/zinc composites: Investigation of zinc particle size and distribution effect. Prog. Org. Coat. 2011, 72, 599–604. [Google Scholar] [CrossRef]
- Lue, Q. Unstirred preparation of soluble electroconductive polypyrrole nanoparticles. Microchim. Acta 2010, 168, 205–213. [Google Scholar] [CrossRef]
- Gergely, A.; Paszti, Z.; Bertoti, I.; Toeroek, T.; Mihaly, J.; Kalman, E. Novel zinc-rich epoxy paint coatings with hydrated alumina and carbon nanotubes supported polypyrrole for corrosion protection of low carbon steel: Part II: Corrosion prevention behavior of the hybrid paint coatings. Mater. Corros. Werkst. Und Korros. 2013, 64, 1091–1103. [Google Scholar] [CrossRef]
- Le, H.N.T.; Garcia, B.; Deslouis, C.; Le Xuan, Q. Corrosion protection and conducting polymers: Polypyrrole films on iron. Electrochim. Acta 2001, 46, 4259–4272. [Google Scholar]
- Arianpouya, N.; Shishesaz, M.; Arianpouya, M.; Nematollahi, M. Evaluation of synergistic effect of nanozinc/nanoclay additives on the corrosion performance of zinc-rich polyurethane nanocomposite coatings using electrochemical properties and salt spray testing. Surf. Coat. Technol. 2013, 216, 199–206. [Google Scholar] [CrossRef]
- Chen, Z.; Cai, Y.; Lu, Y.; Cao, Q.; Lv, P.; Zhang, Y.; Liu, W. Preparation and Performance Study of Carboxy-Functionalized Graphene Oxide Composite Polyaniline Modified Water-Based Epoxy Zinc-Rich Coatings. Coatings 2022, 12, 824. [Google Scholar] [CrossRef]
- Li, D.; Ma, L.; Zhang, B.; Chen, S. Large-scale fabrication of a durable and self-healing super-hydrophobic coating with high thermal stability and long-term corrosion resistance. Nanoscale 2021, 13, 7810–7821. [Google Scholar] [CrossRef] [PubMed]
- Ramezanzadeh, B.; Arman, S.Y.; Mehdipour, M. Anticorrosion properties of an epoxy zinc-rich composite coating reinforced with zinc, aluminum, and iron oxide pigments. J. Coat. Technol. Res. 2014, 11, 727–737. [Google Scholar] [CrossRef]
- Gao, H.; Yuan, J.; Wang, X.; Guan, J.; Zhang, L.; Jing, Z.; Mao, Y. Mechanism of surface modification for sericite. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2007, 22, 470–472. [Google Scholar] [CrossRef]
- Sang, W.; Chen, H.; Chen, H.; Fu, W. Reinforced Zinc-Silicate Coatings on Anti-Wear Anticorrosion and Water Resistance by Adding Sericite. Sci. Technol. Rev. 2011, 29, 49–52. [Google Scholar]
- Lv, C.; Wang, H.; Liu, Z.; Wang, C.; Zhang, W.; Li, M.; Zhu, Y. Fabrication of durable fluorine-free polyphenylene sulfide/silicone resin composite superhydrophobic coating enhanced by carbon nanotubes/graphene fillers. Prog. Org. Coat. 2019, 134, 1–10. [Google Scholar] [CrossRef]
- Yang, M.S.; Huang, J.; Noël, J.J.; Chen, J.; Barker, I.; Henderson, J.D.; Zhang, H.; Zhang, H.; Zhu, J. A Mechanistic Study on the Anti-Corrosive Performance of Zinc-Rich Polyester/TGIC Powder Coatings. Processes 2022, 10, 1853. [Google Scholar] [CrossRef]
- Singh, G.; Vasudev, H.; Bansal, A.; Vardhan, S. Influence of heat treatment on the microstructure and corrosion properties of the Inconel-625 clad deposited by microwave heating. Surf. Topogr. Metrol. Prop. 2021, 9, 025019. [Google Scholar] [CrossRef]





| Label | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Zinc dust/wt % | 80 | 60 | 60 | 60 | 50 |
| PPy/SCP particle/wt % | 0 | 4 | 6 | 8 | 12 |
| Ferrotitanium powder/wt % | 0 | 16 | 14 | 12 | 18 |
| EP + other additives/wt % | 20 | 20 | 20 | 20 | 20 |
| Replacing ratio of PPy/SCP | 0 | 1:5 | 1:3 | 1:2.5 | 1:2.5 |
| Label | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Zinc dust/wt % | 80 | (60.0) | 66.9 | 66.5 | 66.4 | 70 |
| PPy/SCP particle/wt % | 0 | 6.6 | 8.9 | 4.5 | 3.0 | 3.3 |
| Ferrotitanium/wt % | 0 | 10.2 | 1.9 | 6.8 | 8.5 | 5.1 |
| EP + other additives/wt % | 20 | 23.2 | 22.3 | 22.2 | 22.1 | 21.6 |
| Replacing ratio of PPy/SCP | 0 | 1:3 | 1:1.5 | 1:3 | 1:4.5 | 1:3 |
| Sample | 60% Zn (1:3) | 66% Zn (1:3) | 70% Zn (1:3) | 66% Zn (1:1.5) | 66% Zn (1:4.5) |
|---|---|---|---|---|---|
| Pull-off strength /Mpa | 8.24 | 8.38 | 7.30 | 6.44 | 8.27 |
| Sample | E Corr (mV) | I Corr (A/cm2) | CR (mpy) | βa (mV) | βc (mV) | Rp (Ω·cm2) | PE (%) |
|---|---|---|---|---|---|---|---|
| Bare steel | −811 | 2.4 × 10−1 | 7.8 × 10−1 | 76 | 196 | 99.2 | - |
| 80% Zn | −953 | 3.2 × 10−4 | 1.1 × 10−5 | 106 | 176 | 9.0 × 104 | 99.87 |
| 60% Zn (1:3) | −1035 | 9.4 × 10−3 | 3.1 × 10−4 | 195 | 87 | 2.8 × 103 | 96.08 |
| 66% Zn (1:3) | −1006 | 1.9 × 10−4 | 6.1 × 10−6 | 108 | 142 | 1.4 × 105 | 99.92 |
| 70% Zn (1:3) | −1001 | 5.5 × 10−4 | 1.8 × 10−5 | 62 | 108 | 3.1 × 104 | 99.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Jin, X.; Xie, J.; Lv, X.; Guo, T.; Zhang, L.; Zhu, J.; Shao, Y.; Zhang, H.; Zhang, H.; et al. Fabrication of a Conductive Additive for the Anticorrosion Enhancement of Zinc-Rich Epoxy Coatings. Coatings 2022, 12, 1406. https://doi.org/10.3390/coatings12101406
Bai Y, Jin X, Xie J, Lv X, Guo T, Zhang L, Zhu J, Shao Y, Zhang H, Zhang H, et al. Fabrication of a Conductive Additive for the Anticorrosion Enhancement of Zinc-Rich Epoxy Coatings. Coatings. 2022; 12(10):1406. https://doi.org/10.3390/coatings12101406
Chicago/Turabian StyleBai, Yuxing, Xuliang Jin, Junqing Xie, Xiao Lv, Tingting Guo, Li Zhang, Jesse Zhu, Yuanyuan Shao, Haiping Zhang, Hui Zhang, and et al. 2022. "Fabrication of a Conductive Additive for the Anticorrosion Enhancement of Zinc-Rich Epoxy Coatings" Coatings 12, no. 10: 1406. https://doi.org/10.3390/coatings12101406
APA StyleBai, Y., Jin, X., Xie, J., Lv, X., Guo, T., Zhang, L., Zhu, J., Shao, Y., Zhang, H., Zhang, H., Yuan, B., Yin, A., Nie, J., Cao, F., & Xu, Z. (2022). Fabrication of a Conductive Additive for the Anticorrosion Enhancement of Zinc-Rich Epoxy Coatings. Coatings, 12(10), 1406. https://doi.org/10.3390/coatings12101406









