Rational Design of Yolk Core-Shell Structure MnO-Co@C Nanospheres for High-Performance Microwave Absorption
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
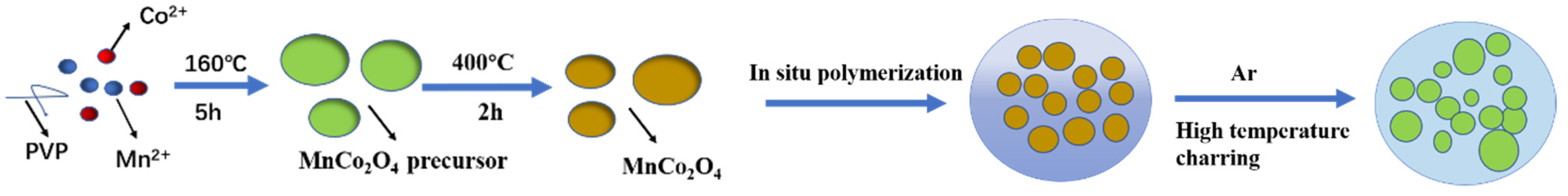
2.2. The Preparation of MnCo2O4 Nanospheres
2.3. The Preparation of Core-Shell MnO-Co@C Nanospheres
2.4. Characterization
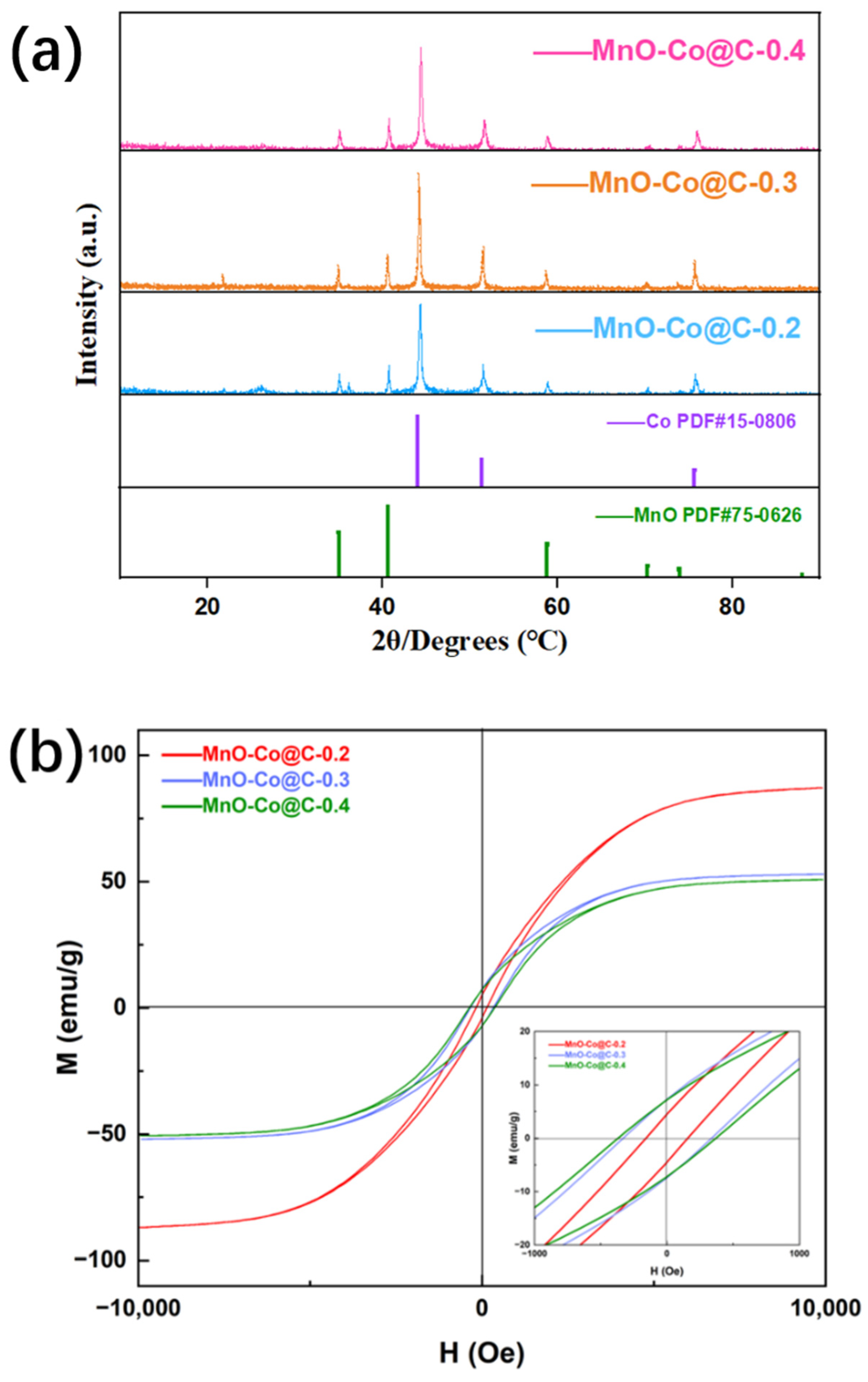
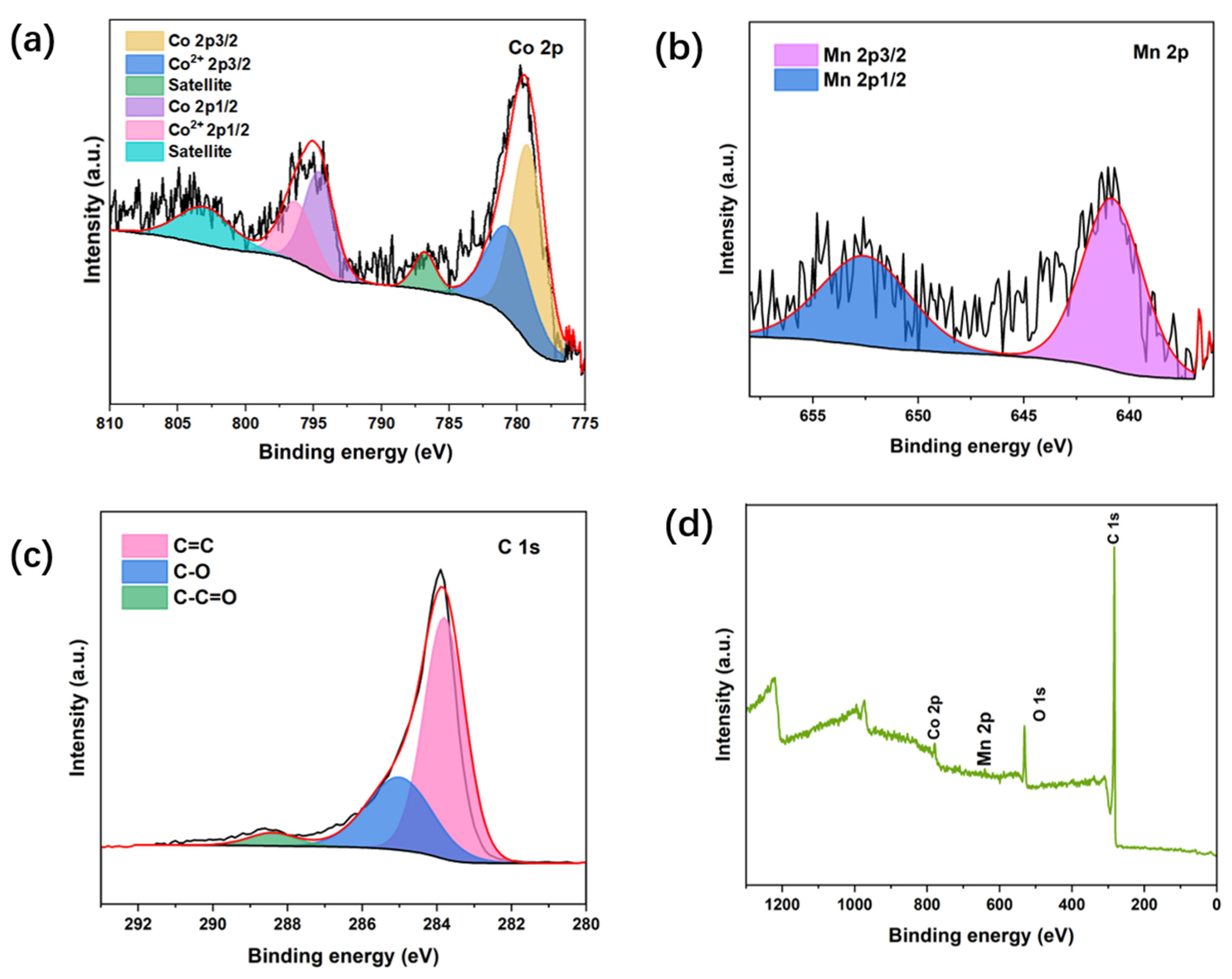
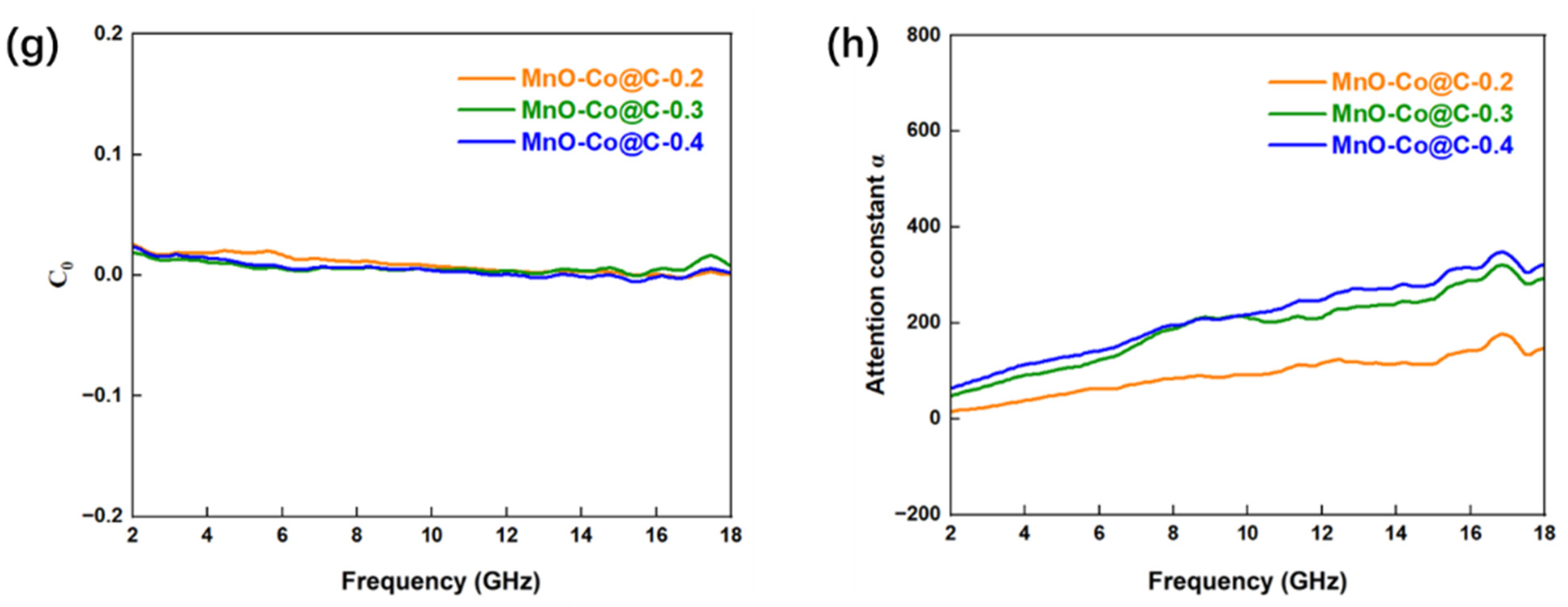
3. Results and Discission
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Micheli, D.; Vricella, A.; Pastore, R.; Marchetti, M. Synthesis and electromagnetic characterization of frequency selective radar absorbing materials using carbon nanopowders. Carbon 2014, 77, 756–774. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Kompotiatis, L.; Kontogeorgakos, A.; Kordas, G. Microwave behavior of ferrites prepared via sol-gel method. J. Magn. Magn. Mater. 2002, 246, 360–365. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Jiao, S.; Ma, K.; Lv, J.; Yang, J. Hierarchical Carbon Fiber@MXene@MoS2 Core-sheath Synergistic Microstructure for Tunable and Efficient Microwave Absorption. Adv. Funct. Mater. 2020, 30, 2002595. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, T.; Cheng, J.; Cao, Q.; Zheng, G.; Liang, S.; Wang, H.; Cao, M.S. Lightweight and High-Performance Microwave Absorber Based on 2D WS2-RGO Heterostructures. Nano-Micro Lett. 2019, 11, 38. [Google Scholar] [CrossRef]
- Ma, R.-T.; Shang, H.-Y.; Wang, X.; Jiang, D. Dielectric, magnetic and microwave absorbing properties of polyaniline-Co0.7Cr0.1Zn0.2Fe2O4 composites. Rare Met. 2017, 36, 118–122. [Google Scholar] [CrossRef]
- Qiu, Y.; Wen, B.; Yang, H.; Lin, Y.; Cheng, Y.; Jin, L. MOFs derived Co@C@MnO nanorods with enhanced interfacial polarization for boosting the electromagnetic wave absorption. J. Colloid Interface Sci. 2021, 602, 242–250. [Google Scholar] [CrossRef]
- He, G.; Duan, Y.; Pang, H. Microwave Absorption of Crystalline Fe/MnO@C Nanocapsules Embedded in Amorphous Carbon. Nano-Micro Lett. 2020, 12, 57. [Google Scholar] [CrossRef]
- Xu, D.; Qiao, J.; Wu, N.; Liu, W.; Wang, F.; Lv, L.; Pan, J.; Dong, Y.; Liu, J. Facile Synthesis of Three-Dimensional Porous Co/MnO Composites Derived from Bimetal Oxides for Highly Efficient Electromagnetic Wave Absorption. ACS Sustain. Chem. Eng. 2019, 7, 8687–8695. [Google Scholar] [CrossRef]
- Shu, R.; Li, W.; Zhou, X.; Tian, D.; Zhang, G.; Gan, Y.; Shi, J.; He, J. Facile preparation and microwave absorption properties of RGO/MWCNTs/ZnFe2O4 hybrid nanocomposites. J. Alloys Compd. 2018, 743, 163–174. [Google Scholar] [CrossRef]
- Liu, P.-B.; Huang, Y.; Sun, X. Excellent Electromagnetic Absorption Properties of Poly(3,4-ethylenedioxythiophene)-Reduced Graphene Oxide-Co3O4 Composites Prepared by a Hydrothermal Method. ACS Appl. Mater. Interfaces 2013, 5, 12355–12360. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, J.; Du, Y.; Bai, J.; Fan, H.; Zhang, H.; Peng, Y.; Li, F. One-pot synthesis of CoFe2O4/graphene oxide hybrids and their conversion into FeCo/graphene hybrids for lightweight and highly efficient microwave absorber. J. Mater. Chem. A 2015, 3, 5535–5546. [Google Scholar] [CrossRef]
- Liao, Q.; He, M.; Zhou, Y.; Nie, S.; Wang, Y.; Hu, S.; Yang, H.; Li, H.; Tong, Y. Highly Cuboid-Shaped Heterobimetallic Metal-Organic Frameworks Derived from Porous Co/ZnO/C Microrods with Improved Electromagnetic Wave Absorption Capabilities. ACS Appl. Mater. Interfaces 2018, 10, 29136–29144. [Google Scholar] [CrossRef]
- Yu, M.; Liang, C.; Liu, M.; Liu, X.; Yuan, K.; Cao, H.; Che, R. Yolk–shell Fe3O4@ZrO2 prepared by a tunable polymer surfactant assisted sol–gel method for high temperature stable microwave absorption. J. Mater. Chem. C 2014, 2, 7275–7283. [Google Scholar] [CrossRef]
- Tian, C.; Du, Y.; Xu, P.; Qiang, R.; Wang, Y.; Ding, D.; Xue, J.; Ma, J.; Zhao, H.; Han, X. Constructing Uniform Core-Shell PPy@PANI Composites with Tunable Shell Thickness toward Enhancement in Microwave Absorption. ACS Appl. Mater. Interfaces 2015, 7, 20090–20099. [Google Scholar] [CrossRef]
- Liu, X.G.; Li, B.; Geng, D.Y.; Cui, W.B.; Yang, F.; Xie, Z.G.; Kang, D.J.; Zhang, Z.D. (Fe, Ni)/C nanocapsules for electromagnetic-wave-absorber in the whole Ku-band. Carbon 2009, 47, 470–474. [Google Scholar] [CrossRef]
- Liu, X.; Cui, X.; Chen, Y.; Zhang, X.-J.; Yu, R.; Wang, G.-S.; Ma, H. Modulation of electromagnetic wave absorption by carbon shell thickness in carbon encapsulated magnetite nanospindles-poly (vinylidene fluoride) composites. Carbon 2015, 95, 870–878. [Google Scholar] [CrossRef]
- Zhou, S.; Luo, X.; Chen, L.; Xu, C.; Yan, D. MnCo2O4 nanospheres for improved lithium storage performance. Ceram. Int. 2018, 44, 17858–17863. [Google Scholar] [CrossRef]
- Qiao, Z.-A.; Guo, B.; Binder, A.J.; Chen, J.; Veith, G.M.; Dai, S. Controlled Synthesis of Mesoporous Carbon Nanostructures via a “Silica-Assisted” Strategy. Nano Lett. 2013, 13, 207–212. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Y.; Han, G.; Yang, W.; Yu, Y. Three-dimensional (Fe3O4/ZnO)@C Double-core@shell porous nanocomposites with enhanced broadband microwave absorption. Carbon 2020, 162, 356–364. [Google Scholar] [CrossRef]
- Ding, D.; Wang, Y.; Li, X.; Qiang, R.; Xu, P.; Chu, W.; Han, X.; Du, Y. Rational design of core-shell Co@C microspheres for high-performance microwave absorption. Carbon 2017, 111, 722–732. [Google Scholar] [CrossRef]
- Saini, P.; Choudhary, V.; Vijayan, N.; Kotnala, R.K. Improved Electromagnetic Interference Shielding Response of Poly(aniline)-Coated Fabrics Containing Dielectric and Magnetic Nanoparticles. J. Phys. Chem. C 2012, 116, 13403–13412. [Google Scholar] [CrossRef]
- Wu, Y.; Shu, R.; Zhang, J.; Wan, Z.; Shi, J.; Liu, Y.; Zhao, G.; Zheng, M. Oxygen vacancies regulated microwave absorption properties of reduced graphene oxide/multi-walled carbon nanotubes/cerium oxide ternary nanocomposite. J. Alloys Compd. 2020, 819, 152944. [Google Scholar] [CrossRef]
- Wang, J.; Jia, Z.; Liu, X.; Dou, J.; Xu, B.; Wang, B.; Wu, G. Construction of 1D Heterostructure NiCo@C/ZnO Nanorod with Enhanced Microwave Absorption. Nano-Micro Lett. 2021, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Barman, B.K.; Mallick, S.; Raj, C.R. Three-Dimensional Nitrogen-Doped Graphitic Carbon-Encapsulated MnO-Co Heterostructure: A Bifunctional Energy Storage Material for Zn-Ion and Zn–Air Batteries. ACS Appl. Energy Mater. 2020, 3, 10108–10118. [Google Scholar] [CrossRef]
- Kuchi, R.; Sharma, M.; Lee, S.W.; Kim, D.; Jung, N.; Jeong, J.-R. Rational design of carbon shell-encapsulated cobalt nanospheres to enhance microwave absorption performance. Prog. Nat. Sci. Mater. Int. 2019, 29, 88–93. [Google Scholar] [CrossRef]
- Ren, F.; Yu, H.; Wang, L.; Saleem, M.; Tian, Z.; Ren, P. Current progress on the modification of carbon nanotubes and their application in electromagnetic wave absorption. RSC Adv. 2014, 4, 14419–14431. [Google Scholar] [CrossRef]
- Panwar, R.; Puthucheri, S.; Singh, D.; Agarwala, V.; Lee, J.-R. Microwave absorption properties of FSS-impacted composites as a broadband microwave absorber. Adv. Compos. Mater. 2017, 26, 99–113. [Google Scholar] [CrossRef]
- Wen, B.; Cao, M.-S.; Hou, Z.-L.; Song, W.-L.; Zhang, L.; Lu, M.-M.; Jin, H.-B.; Fang, X.-Y.; Wang, W.-Z.; Yuan, J. Temperature dependent microwave attenuation behavior for carbon-nanotube/silica composites. Carbon 2013, 65, 124–139. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.D.; Hui, S.; Xiao, T.D.; Ge, S.; Hines, W.A.; Budnick, J.I.; Taylor, G.W. Microwave magnetic properties of Co50/(SiO2)50 nanoparticles. Appl. Phys. Lett. 2002, 80, 4404–4406. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Haldolaarachchige, N.; Young, D.P.; Guo, Z. Electromagnetic Field Shielding Polyurethane Nanocomposites Reinforced with Core-Shell Fe-Silica Nanoparticles. J. Phys. Chem. C 2011, 115, 15304–15310. [Google Scholar] [CrossRef]
- Jian, X.; Wu, B.; Wei, Y.; Dou, S.X.; Wang, X.; He, W.; Mahmood, N. Facile Synthesis of Fe3O4/GCs Composites and Their Enhanced Microwave Absorption Properties. ACS Appl. Mater. Interfaces 2016, 8, 6101–6109. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Z.; Wu, J.; Sun, S.; Zhang, M.; Sun, X. Rational Design of Yolk Core-Shell Structure MnO-Co@C Nanospheres for High-Performance Microwave Absorption. Coatings 2022, 12, 1405. https://doi.org/10.3390/coatings12101405
Xin Z, Wu J, Sun S, Zhang M, Sun X. Rational Design of Yolk Core-Shell Structure MnO-Co@C Nanospheres for High-Performance Microwave Absorption. Coatings. 2022; 12(10):1405. https://doi.org/10.3390/coatings12101405
Chicago/Turabian StyleXin, Zhen, Junjie Wu, Shuchen Sun, Mu Zhang, and Xudong Sun. 2022. "Rational Design of Yolk Core-Shell Structure MnO-Co@C Nanospheres for High-Performance Microwave Absorption" Coatings 12, no. 10: 1405. https://doi.org/10.3390/coatings12101405
APA StyleXin, Z., Wu, J., Sun, S., Zhang, M., & Sun, X. (2022). Rational Design of Yolk Core-Shell Structure MnO-Co@C Nanospheres for High-Performance Microwave Absorption. Coatings, 12(10), 1405. https://doi.org/10.3390/coatings12101405




