Mechanism of Chemical Bath Deposition of CdS Thin Films: Influence of Sulphur Precursor Concentration on Microstructural and Optoelectronic Characterizations
Abstract
1. Introduction
2. Methodology
2.1. Experimental
2.2. Simulation
2.3. Characterization
3. Results and Discussion
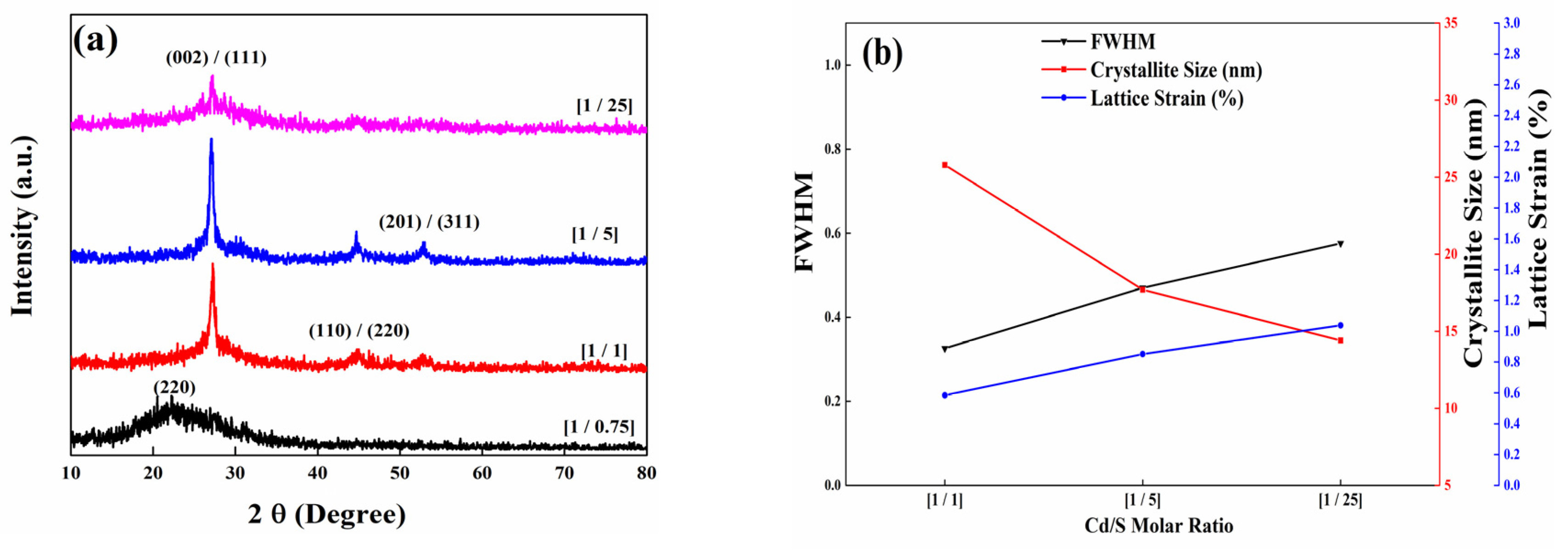
3.1. XRD Studies
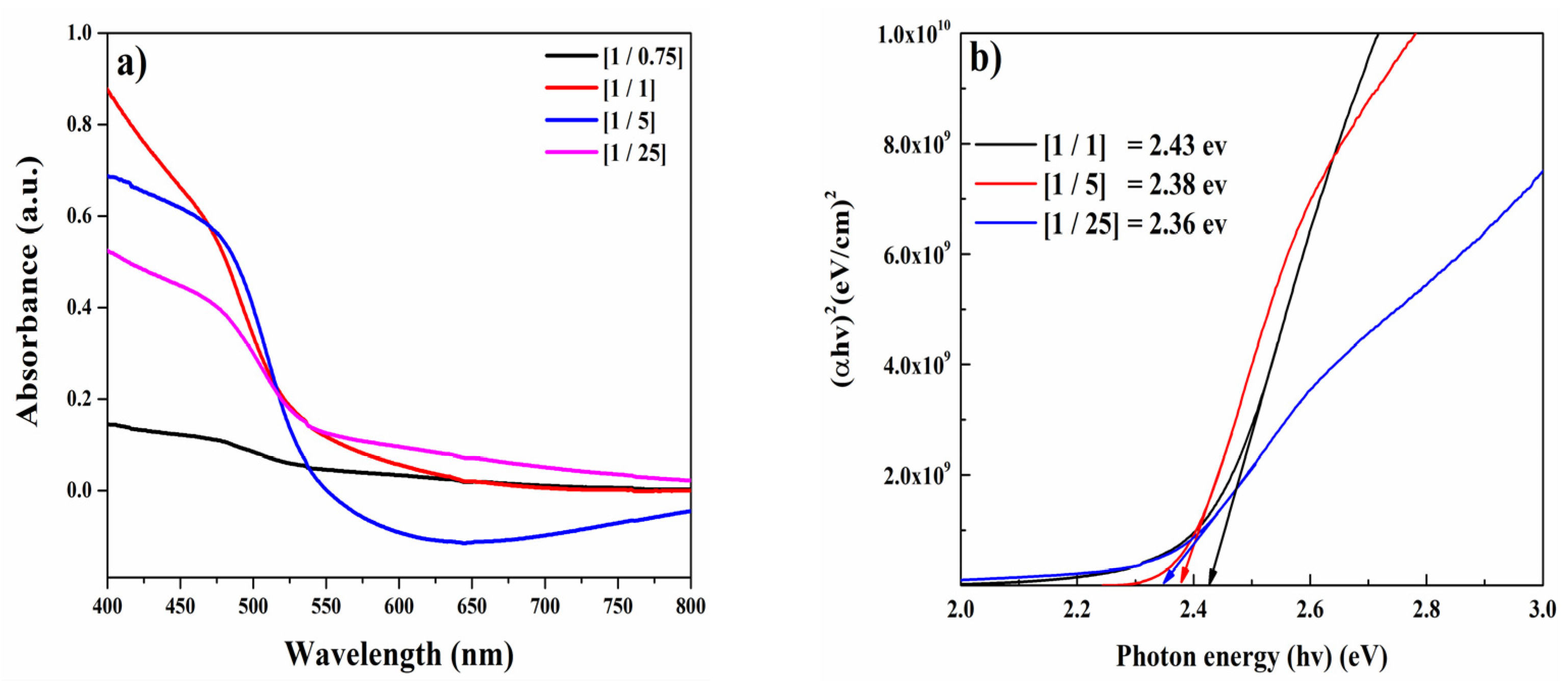
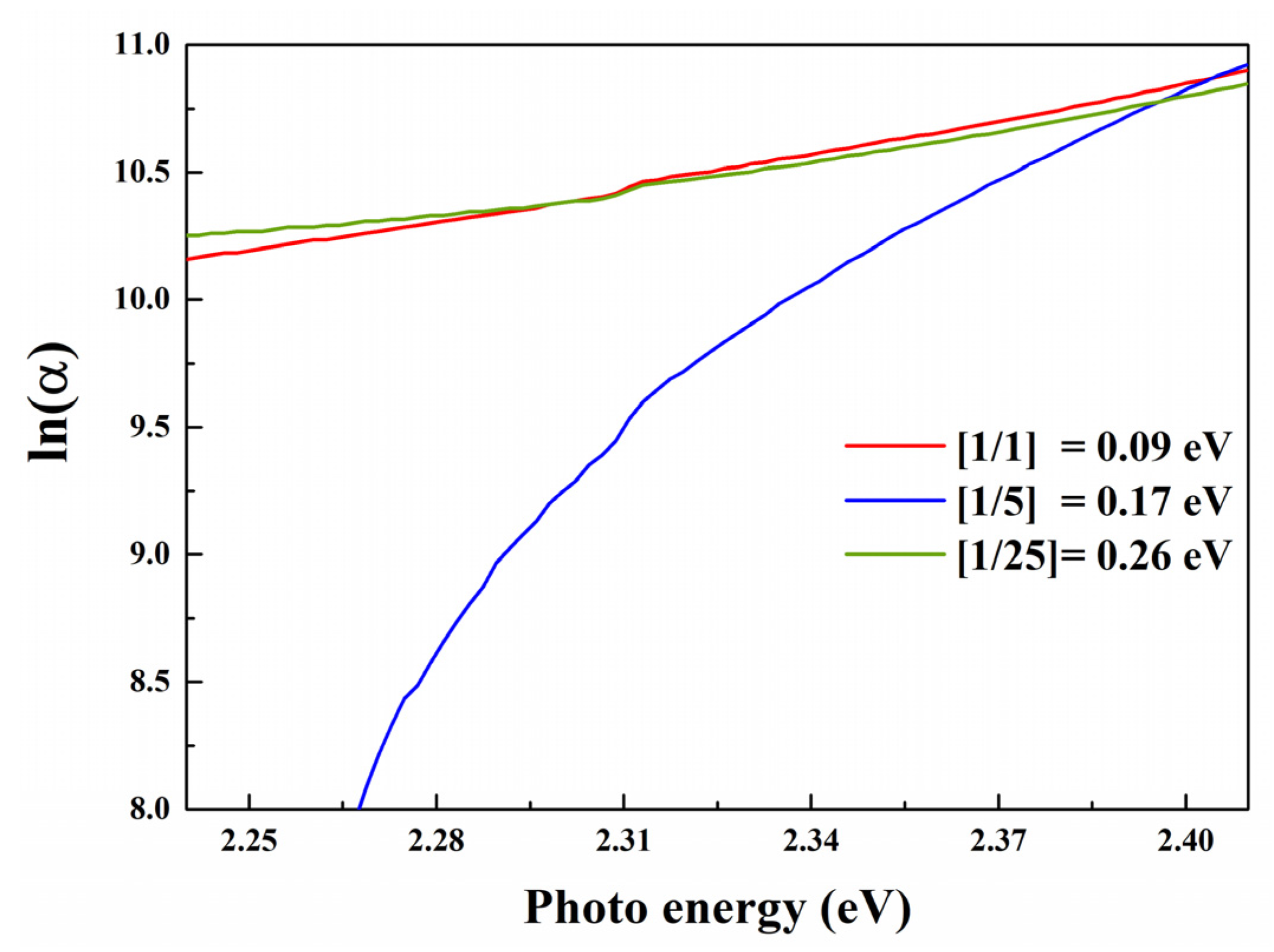
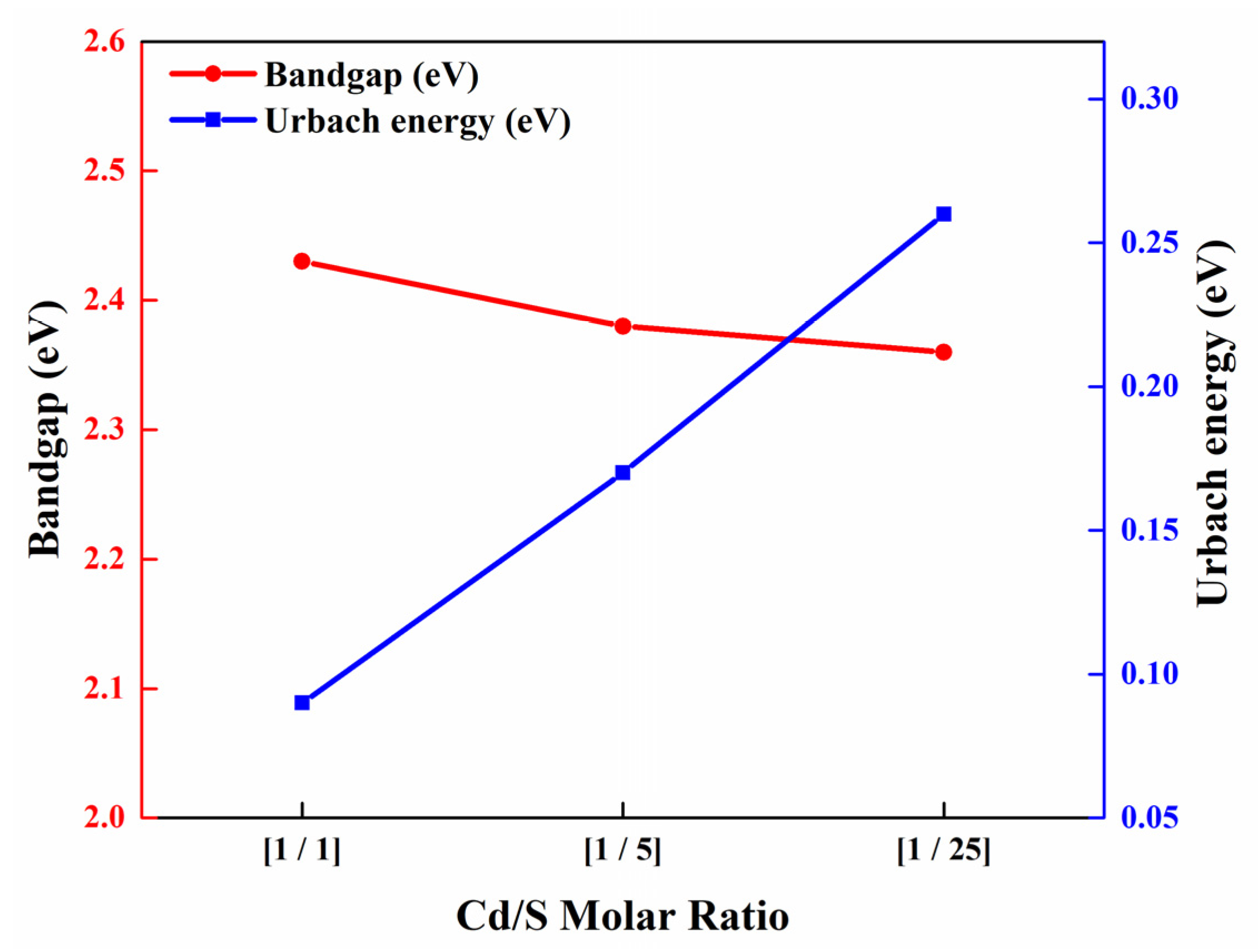
3.2. Optical Properties
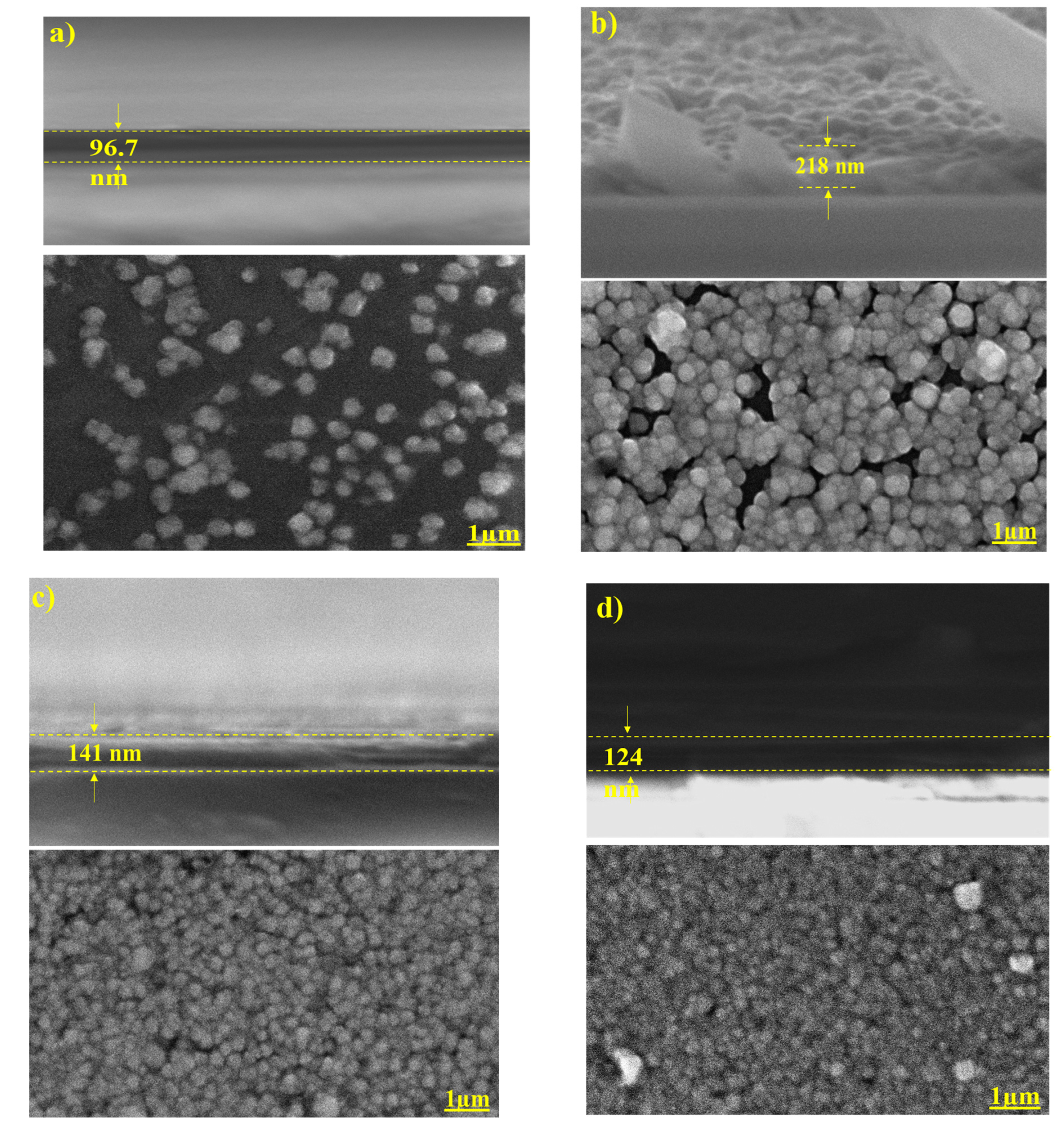
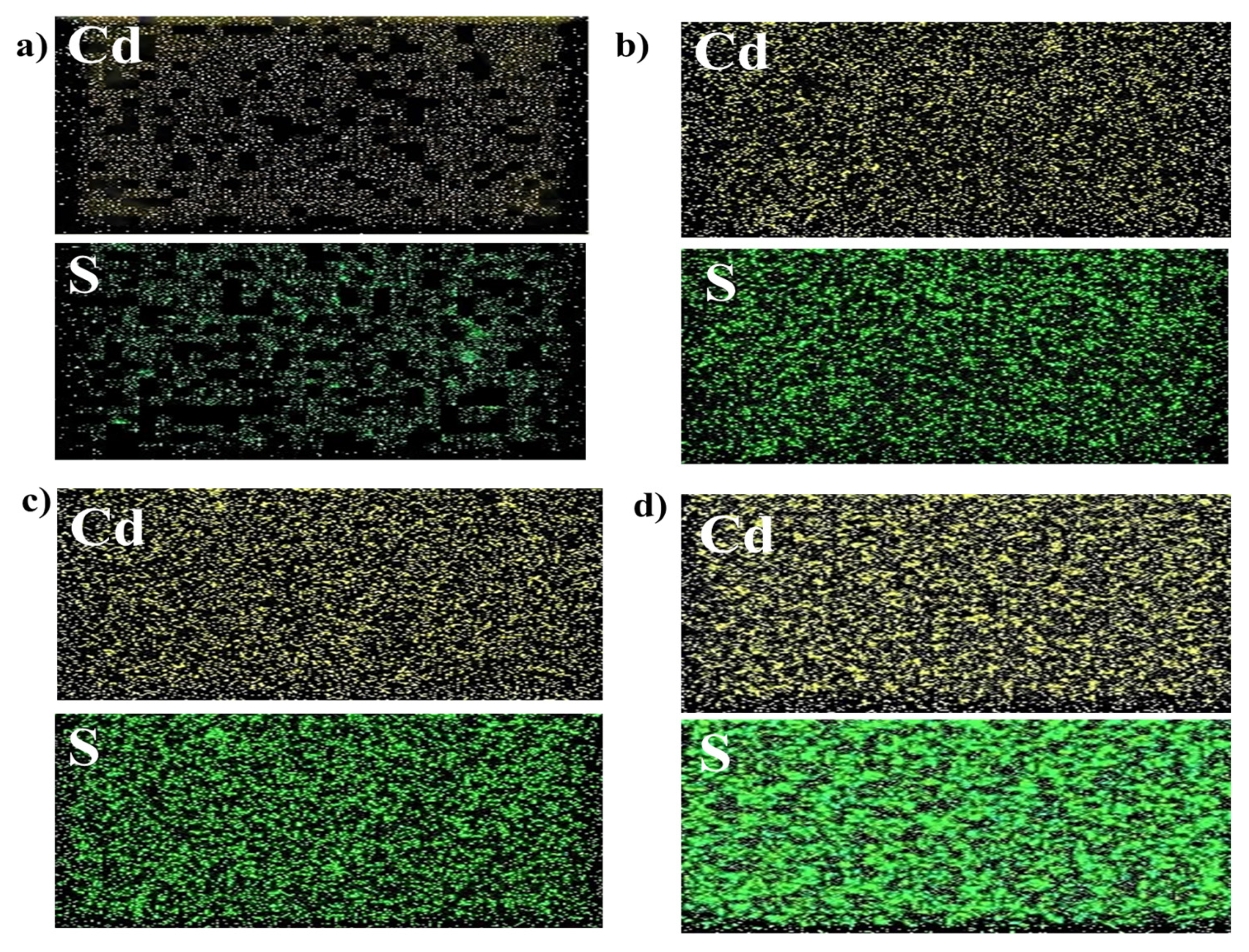
3.3. Surface Morphology
3.4. Electrical Measurements
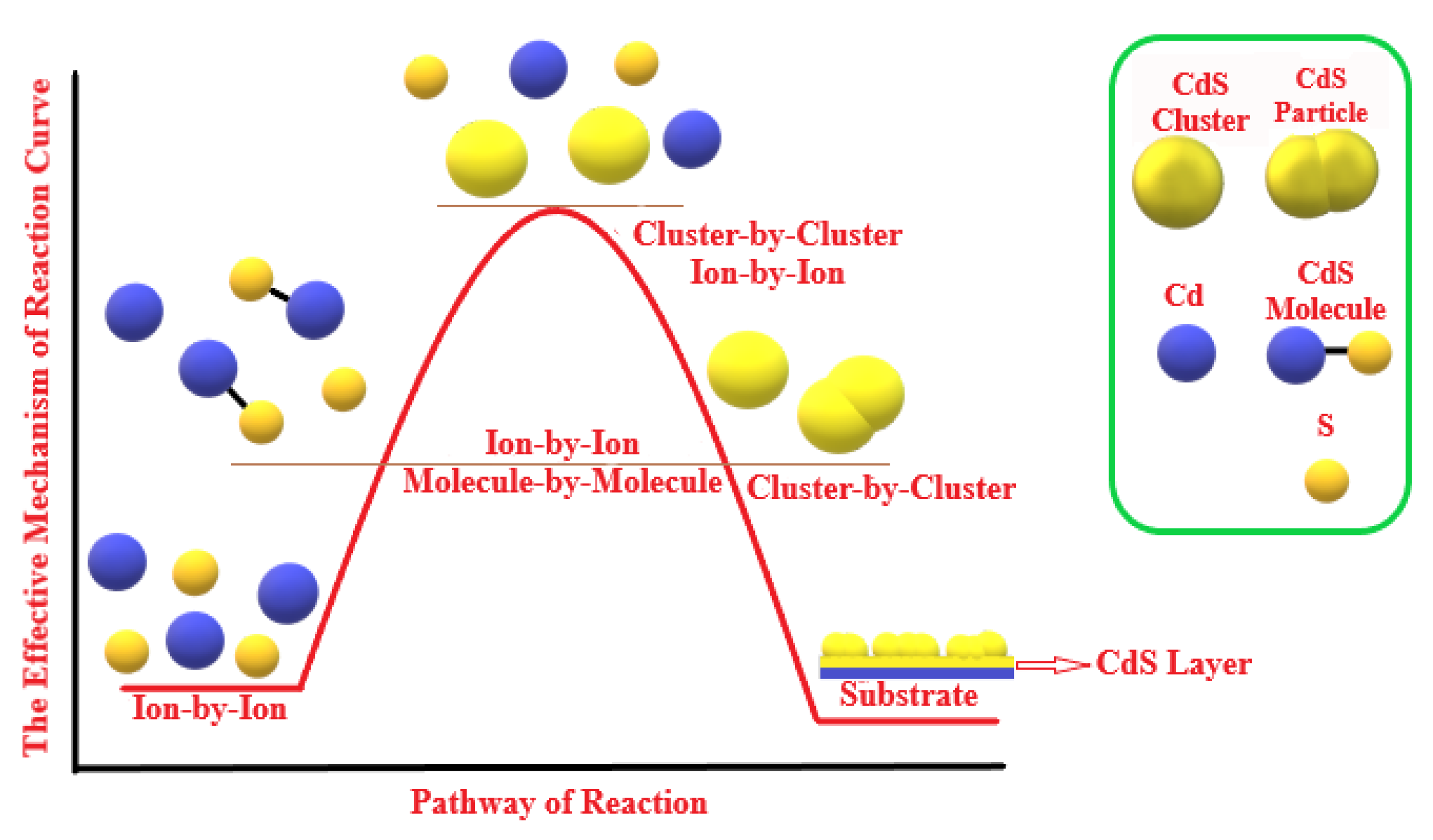
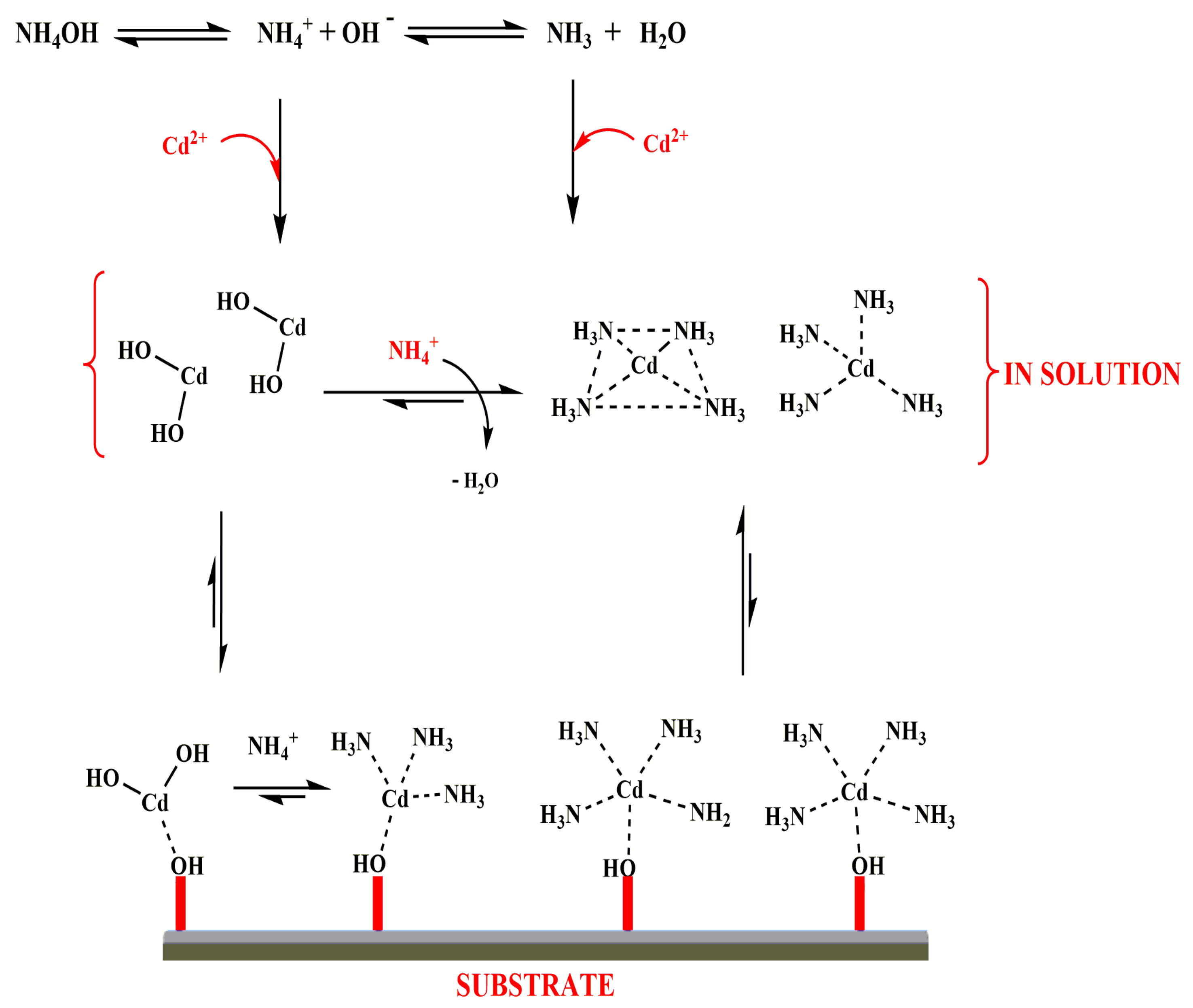
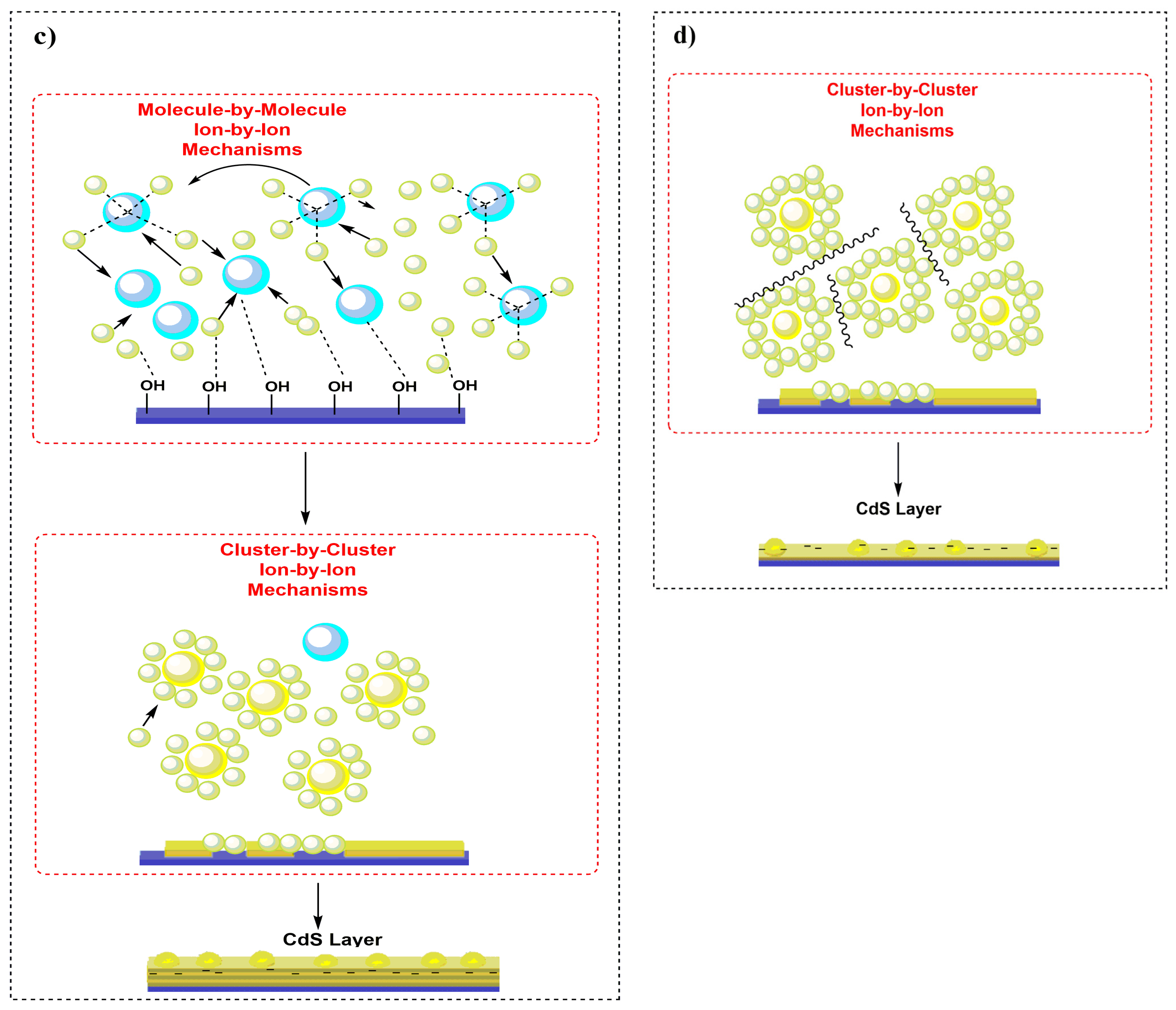
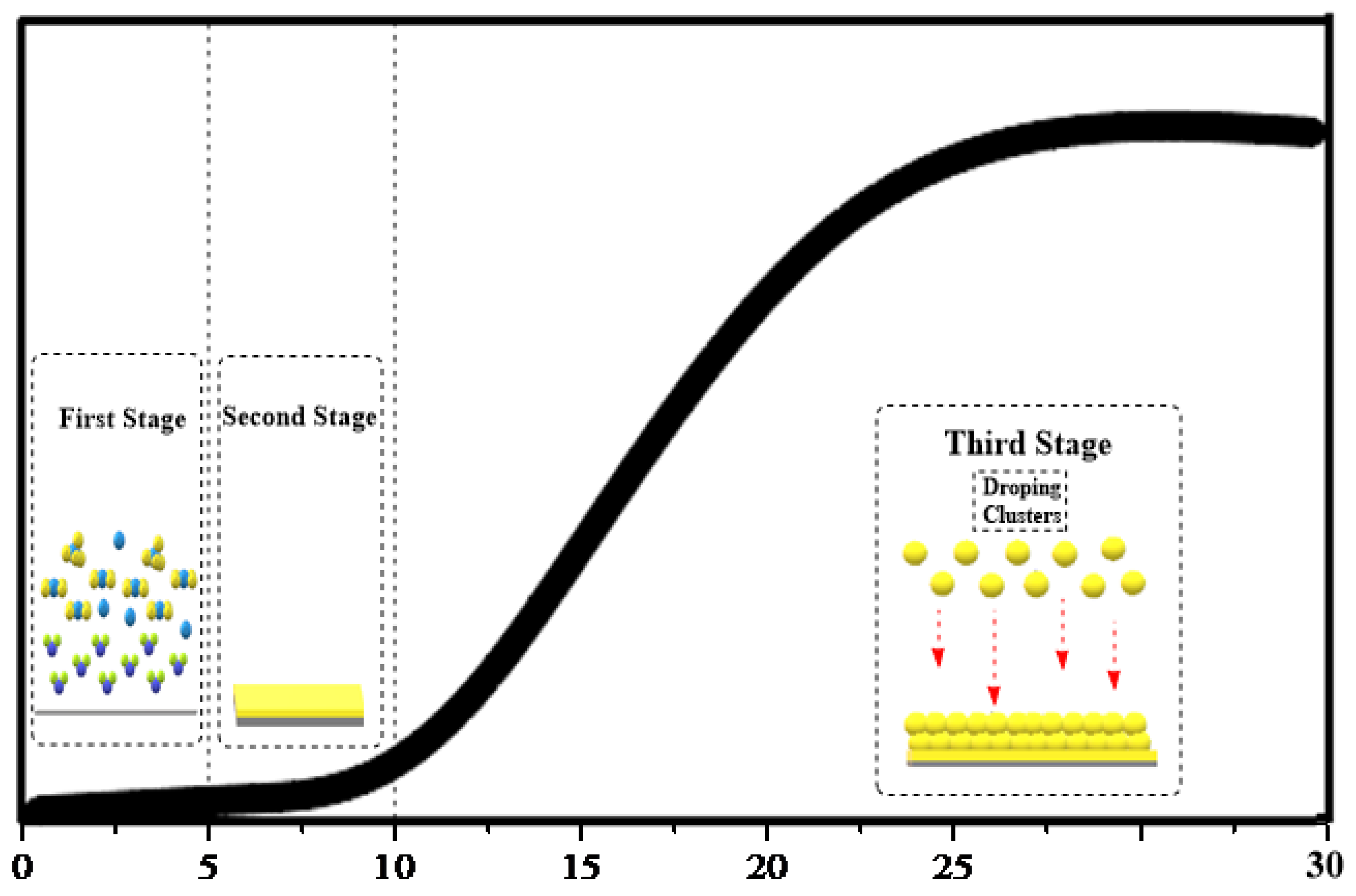
3.5. Mechanism
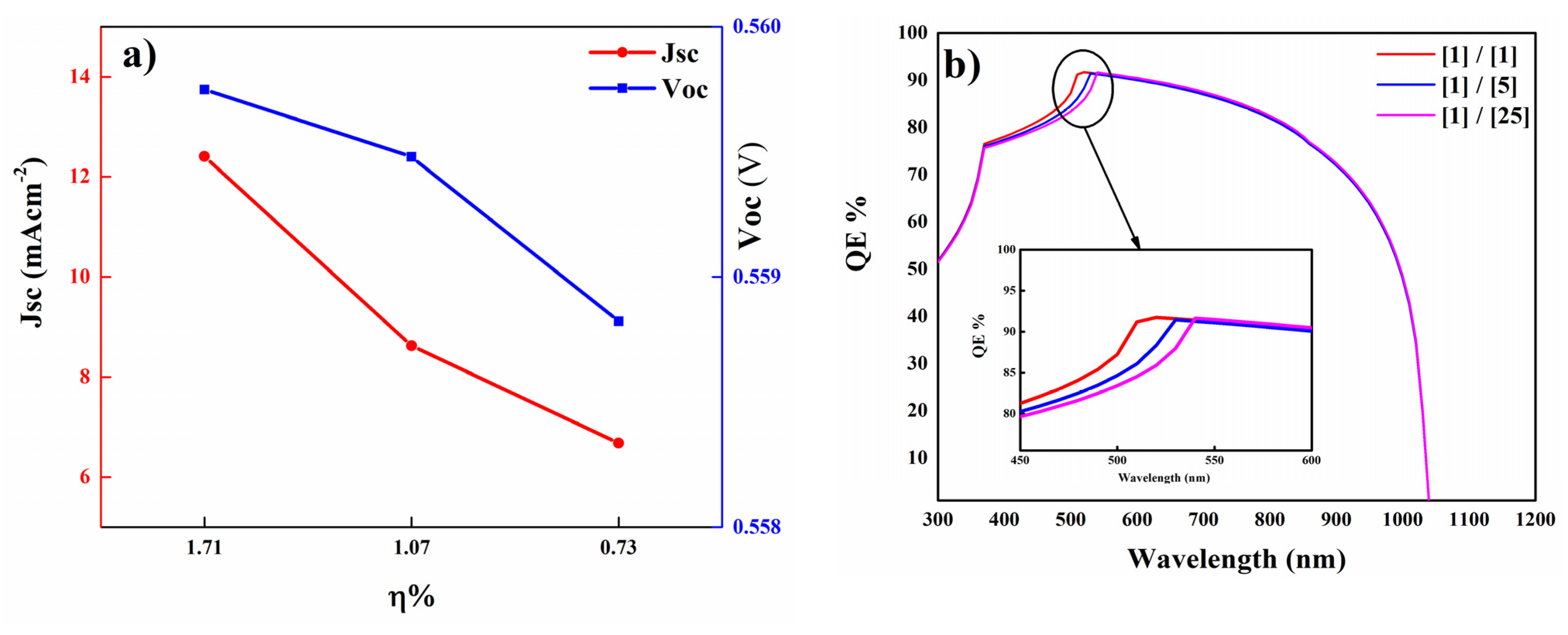
3.6. The Influence of [Cd/S] Ratio on Cell Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabbir, E.; Chelvanathan, P.; Ahmad, S.; Bais, B.; Kiong, S.; Sopian, K. Fabrication of Cu2SnS3 thin film solar cells by sulphurization of sequentially sputtered Sn/CuSn metallic stacked precursors. Sol. Energy 2019, 177, 262–273. [Google Scholar]
- Lv, B.; Yan, B.; Cai, P.; Li, Y.; Gao, F.; Ye, Z.; Lu, Z.; Sui, C.; Huang, P. Improving the structural and optical properties of CdS films grown by Chemical Bath Deposition with adding H2O2. Mater. Lett. 2018, 225, 42–45. [Google Scholar] [CrossRef]
- Willars-Rodríguez, F.J.; Chávez-Urbiola, I.R.; Hernández-Landaverde, M.A.; Vorobiev, P.; Ramírez-Bon, R.; Vorobiev, Y.V. Effects of tin-doping on cadmium sulfide (CdS:Sn) thin-films grown by light-assisted chemical bath deposition process for solar photovoltaic cell. Thin Solid Film. 2018, 653, 341–349. [Google Scholar] [CrossRef]
- Lu, Z.; Jin, R.; Liu, Y.; Guo, L.; Liu, X.; Liu, J.; Cheng, K.; Du, Z. Optimization of chemical bath deposited cadmium sulfide buffer layer for high-efficient CIGS thin film solar cells. Mater. Lett. 2017, 204, 53–56. [Google Scholar] [CrossRef]
- Swathi, S.; Yuvakkumar, R.; Ravi, G.; Babu, E.S.; Velauthapillai, D.; Syed, A.; Dawoud, T.M.S. Silver-doped cadmium sulfide for electrochemical water oxidation. Appl. Nanosci. 2020, 10, 4351–4358. [Google Scholar] [CrossRef]
- Mehmood, I.; Huang, J.; Khan, S.A.; Shah, A.H.; Khan, Q.U.; Kiani, M.; Zhou, D.; Li, G. Investigation of silver doped CdS co-sensitized TiO2/CISe/Ag–CdS heterostructure for improved optoelectronic properties. Opt. Mater. 2020, 111, 110645. [Google Scholar] [CrossRef]
- Zia, R.; Riaz, M.; Anjum, S. Study the effect of thiourea concentration on optical and structural properties of CdS-nanocrystalline thin films prepared by CBD technique. Optik 2016, 127, 5407–5412. [Google Scholar] [CrossRef]
- Beggas, A.; Benhaoua, B.; Attaf, A.; Aida, M.S. Growth study of CdS thin films deposited by chemical bath. Optik 2016, 127, 8423–8430. [Google Scholar] [CrossRef]
- Samir, P.; Raval, K. Investigation of structural, morphological and optical properties of cadmium sulphide (CdS) thin films at different Cd/S concentration deposited by chemical technique. J. Mater. Sci. Mater. Electron. 2017, 28, 18031–18039. [Google Scholar]
- Kwon, J.; Ahn, J.; Yang, H. Chemical bath deposition of CdS channel layer for fabrication of low temperature-processed thin-film-transistors. Curr. Appl. Phys. 2013, 13, 84–89. [Google Scholar] [CrossRef]
- Aliyu, M.; Diso, D.G.; Musa, A.O.; Abubakar, A.I. Effect of growth voltage on electrodeposited CdS thin films. Bayero J. Pure Appl. Sci. 2022, 13, 539–545. [Google Scholar]
- Shanmugapriya, S.; Sakthivel, B.; Nandhabalaji, S. ZnSe/CdS Thin Films Prepared with Physical Vapour Deposition Technique. ADALYA J. 2020, 9, 635–640. [Google Scholar]
- Shaikh, S.S.; Shkir, M.; Masumdar, E.U. Facile fabrication and characterization of modified spray deposited cadmium sulphide thin films. Phys. B Condens. Matter 2019, 571, 64–70. [Google Scholar] [CrossRef]
- Holi, A.M.; Al-Zahrani, A.A.; Najm, A.S.; Chelvanathan, P.; Amin, N. PbS/CdS/ZnO Nanowire Arrays: Synthesis, Structural, Optical, Electrical, and Photoelectrochemical Properties. Chem. Phys. Lett. 2020, 750, 137486. [Google Scholar] [CrossRef]
- Najm, A.S.; Chowdhury, M.S.; Munna, F.T.; Chelvanathan, P.; Selvanathan, V.; Aminuzzaman, M.; Techato, K.; Amin, N.; Akhtaruzzaman, M.D. Impact of cadmium salt concentration on cds nanoparticles synthesized by chemical precipitation method. Chalcogenide Lett. 2020, 17, 537–547. [Google Scholar]
- Najm, A.S.; Chelvanathan, P.; Tiong, S.K.; Ferdaous, M.T. Numerical insights into the influence of electrical properties of n-CdS buffer layer on the performance of SLG/Mo/p-absorber/n-CdS/n-ZnO/Ag configured thin film photovoltaic devices. Coating 2021, 11, 52. [Google Scholar] [CrossRef]
- Yılmaz, S.; Ünverdi, A.; Tomakin, M.; Polat, I.; Bacaksız, E. Surface modification of CBD-grown CdS thin films for hybrid solar cell applications. Optik 2019, 185, 256–263. [Google Scholar] [CrossRef]
- Nieto-Zepeda, K.E.; Guillén-Cervantes, A.; Rodríguez-Rosales, K.; Santos-Cruz, J.; Santos-Cruz, D. Effect of the sulfur and fluorine concentration on physical properties of CdS films grown by chemical bath deposition. Results Phys. 2017, 7, 1971–1975. [Google Scholar] [CrossRef]
- Kamble, A.; Sinha, B.; Agawane, G.; Vanalakar, S.; Kim, I.; Kim, J.Y.; Kale, S.S.; Patil, P.; Kim, J.H. Sulfur ion concentration dependent morphological evolution of CdS thin films and its subsequent effect on photo-electrochemical performance. Phys. Chem. Chem. Phys. 2016, 18, 28024–28032. [Google Scholar] [CrossRef]
- Chen, F.; Jie, W.; Cai, X. Effects of supersaturation on CdS film growth from dilute solutions on glass substrate in chemical bath deposition process. Thin Solid Film. 2008, 516, 2823–2828. [Google Scholar] [CrossRef]
- Azmi, N.; Chelvanathan, P.; Yusoff, Y.; Shahahmadi, S.A.; Tiong, S.K.; Sopian, K.; Amin, N. A comprehensive study on the effects of alternative sulphur precursor on the material properties of chemical bath deposited CdS thin films. Ceram. Int. 2020, 46, 18716–18724. [Google Scholar] [CrossRef]
- Munna, F.; Chelvanathan, P.; Sobayel, K.; Nurhafiza, K.; Sarkar, D.; Nour, M.; Sindi, H.; Rawa, M.; Sopian, K.; Amin, N.; et al. Effect of zinc doping on the optoelectronic properties of cadmium sulphide (CdS) thin films deposited by chemical bath deposition by utilising an alternative sulphur precursor. Optik 2020, 218, 165197. [Google Scholar] [CrossRef]
- Hsu, W.-T.; Chiang, C.-C.; Yeh, T.-K.; Lan, C.-W.; Tsai, S.-Y. CdS Thin Film Prepared by Shallow Chemical Bath Deposition for Low Cost CIGS Thin Film Solar Cell. In Proceedings of the 2011 37th IEEE Photovoltaic Specialists Conference, Seattle, WA, USA, 19–24 June 2011; pp. 2723–2726. [Google Scholar]
- Kaur, I.; Pandya, D.K.; Chopra, K.L.; Soc, J.E. Growth Kinetics and Polymorphism of Chemically Deposited CdS Films Growth Kinetics and Polymorphism of Chemically Deposited CdS Films. J. Electrochem. Soc. 1980, 127, 943–948. [Google Scholar] [CrossRef]
- Sengupta, S.; Aggarwal, R.; Raula, M. A review on chemical bath deposition of metal chalcogenide thin films for heterojunction solar cells. J. Mater. Res. 2022, 1–12. [Google Scholar] [CrossRef]
- Jadhav, U.S.; Kale, S.S.; Lokhande, C.D. Effect of Cd: S ratio on the photoconducting properties of chemically deposited CdS films. Mater. Chem. Phys. 2001, 69, 125–132. [Google Scholar] [CrossRef]
- Xiao, Q.; Xiao, C. Surface-defect-states photoluminescence in CdS nanocrystals prepared by one-step aqueous synthesis method. Appl. Surf. Sci. 2009, 255, 7111–7114. [Google Scholar] [CrossRef]
- Yuan, X.; Li, M.; Ruan, H.; Yang, Y.; Liu, Y.; Zhang, L. CdS nanoparticles with high photocatalytic property synthesized by a new liquid—Liquid method. J. Mater. Sci. Mater. Electron. 2016, 27, 6030–6034. [Google Scholar] [CrossRef]
- Kakhaki, Z.M.; Youzbashi, A.A.; Sangpour, P.; Naderi, N.; Orooji, Y. Influence of Cd salt concentration on the photoconductivity of CdS thin films prepared by chemical bath technique. Mater. Sci. Semicond. Process. 2022, 148, 106773. [Google Scholar] [CrossRef]
- Najm, A.S.; Naeem, H.S.; Alabboodi, K.O.; Hasbullah, S.A. New systematic study approach of green synthesis CdS thin film via Salvia dye. Sci. Rep. 2022, 12, 12521. [Google Scholar] [CrossRef]
- Burgelman, M.; Nollet, P.; Degrave, S. Modelling polycrystalline semiconductor solar cells. Thin Solid Film. 2000, 362, 527–532. [Google Scholar] [CrossRef]
- Jaymin, R.; Tapas, K.C.; Chetan, P.; Kinjal, P.; Keyur, P.; Gopal, B.; Priya, S. PbS-ZnO solar cell: A numerical simulation. J. Nano-Electron. Phys. 2017, 9, 03041. [Google Scholar]
- Chelvanathan, P.; Istiaque, M.; Amin, N. Performance analysis of copper–indium–gallium–diselenide (CIGS) solar cells with various buffer layers by SCAPS. Curr. Appl. Phys. J. 2010, 10, 387–391. [Google Scholar] [CrossRef]
- Akter, F.; Mohammad, J.; Rashid, J. Simulation study to find suitable dopants of CdS buffer layer for CZTS solar cell. J. Theor. Appl. Phys. 2020, 14, 75–84. [Google Scholar]
- Najm, A.S.; Moria, H.; Ludin, N.A. Areca catechu as photovoltaic sensitizer for dye-sensitized solar cell (DSSC). Biointerface Res. Appl. Chem. 2020, 10, 5636–5639. [Google Scholar]
- Nakanishi, T.; Ito, K. Properties of chemical bath deposited CdS thin films. Sol. Energy Mater. Sol. Cells 1994, 35, 171–178. [Google Scholar] [CrossRef]
- Alexander, J.N.; Higashiya, S.; Caskey, D.; Efstathiadis, H. Deposition and characterization of cadmium sulfide (CdS) by chemical bath deposition using an alternative chemistry cadmium precursor. Sol. Energy Mater. Sol. Cells 2014, 125, 47–53. [Google Scholar] [CrossRef]
- Graf, A.; Maticiuc, N.; Spalatu, N.; Mikli, V.; Mere, A.; Gavrilov, A.; Hiie, J. Electrical characterization of annealed chemical-bath-deposited CdS fi lms and their application in superstrate con fi guration CdTe/CdS solar cells. Thin Solid Film. 2014, 582, 351–355. [Google Scholar] [CrossRef]
- Subba, K.; Pilkington, R.D.; Hill, A.E.; Tomlinson, R.D.; Bhatnagar, A.K. Structural and optical investigations on CdS thin films grown by chemical bath technique. Mater. Chem. Phys. 2001, 68, 22–30. [Google Scholar] [CrossRef]
- Ahmed, N.M.; Sulaiman, N.H.; Abdullah, M.F.; Najm, A.S.; Afzal, N.; Altowyan, A.S.; Rafique, M. Improvement in structural, optical and electrical properties of ito film through aln and HfO2 buffer layers. Surf. Rev. Lett. 2021, 28, 2150094. [Google Scholar] [CrossRef]
- Munna, F.; Selvanathan, V.; Sobayel, K.; Muhammad, G.; Asim, N.; Amin, N.; Sopian, K.; Akhtaruzzaman, M. Diluted chemical bath deposition of CdZnS as prospective buffer layer in CIGS solar cell. Ceram. Int. 2020, 47, 11003–11009. [Google Scholar] [CrossRef]
- Mohammadi, B.; Yousefi, A.A.; Bellah, S.M. Effect of tensile strain rate and elongation on crystalline structure and piezoelectric properties of PVDF thin films. Polym. Test. 2007, 26, 42–50. [Google Scholar] [CrossRef]
- Pandya, S.G. Preparation and Characterization of Cadmium Sulphide Nanocrystalline Thin Film Grown by Chemical Method. Int. J. Recent Sci. Res. 2016, 7, 14887–14890. Available online: http://www.recentscientific.com (accessed on 1 January 2020).
- Öztas, M.; Bedir, M.; Öztürk, Z.; Korkmaz, D.; Sur, S. Structural and Optical Properties of Nanocrystal In2O3 Films by Thermal Oxidation of In2S3 Films. Chin. Phys. Lett. 2006, 23, 1610. [Google Scholar] [CrossRef]
- Najm, A.S.; Mohamad, A.B.; Ludin, N.A. The extraction and absorption study of natural dye from Areca catechu for dye sensitized solar cell application. Am. Inst. Phys. 2017, 1838, 020019. [Google Scholar]
- Zanatta, A.R. Revisiting the optical bandgap of semiconductors and the proposal of a unified methodology to its determination. Sci. Rep. 2019, 9, 11225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Wei, A.X.; Zhao, Y.; Liu, J. Structural and optical properties of CdS thin films prepared by chemical bath deposition at different ammonia concentration and S/Cd molar ratios. J. Mater. Sci. Mater. Electron. 2013, 24, 457–462. [Google Scholar] [CrossRef]
- Ouachtari, F.; Rmili, A.; Elidrissi, B.; Bouaoud, A.; Erguig, H.; Elies, P. Influence of Bath Temperature, Deposition Time and S/Cd Ratio on the Structure, Surface Morphology, Chemical Composition and Optical Properties of CdS Thin Films Elaborated by Chemical Bath Deposition. J. Mod. Phys. 2011, 2, 1073–1082. [Google Scholar] [CrossRef]
- Najm, A.S.; Ludin, N.A.; Abdullah, M.F.; Almessiere, M.A.; Ahmed, N.M.; Al-Alwani, M.A.M. Areca catechu extracted natural new sensitizer for dye-sensitized solar cell: Performance evaluation. J. Mater. Sci. Mater. Electron. 2020, 31, 3564–3575. [Google Scholar] [CrossRef]
- Najm, A.S.; Ludin, N.A.; Hamid, N.H.; Ibrahim, M.A.; Teridi, M.A.M.; Sopian, K.; Moria, H.; Holi, A.M.; Al-Zahrani, A.A.; Naeem, H.S. Effect of Chenodeoxycholic Acid on the Performance of Dye-sensitized Solar Cells utilizing Pinang Palm (Areca catechu) Dye. Sains Malays. 2020, 49, 2971–2982. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Akl, A.A. Effect of Se addition on optical and electrical properties of chalcogenide CdSSe thin films Superlattices and Microstructures Effect of Se addition on optical and electrical properties of chalcogenide CdSSe thin films. Superlattices Microstruct. 2016, 89, 153–169. [Google Scholar] [CrossRef]
- Ikhmayies, S.; Ahmad-Bitar, R.N. Temperature dependence of the photoluminescence spectra of CdS: In thin fi lms prepared by the spray pyrolysis technique. J. Lumin. 2013, 142, 40–47. [Google Scholar] [CrossRef]
- Ikhmayies, S.J.; Ahmad-Bitar, R.N. Thickness Dependence of the Bandgap Energy and Urbach Tail for CdS Thin Films Prepared by Vacuum Evaporation. Proc. Elev. World Renew. Energy Congr. Exhib. 2010, 979–984. [Google Scholar]
- Faris Abdullah, M.; Zulkifli, R.; Moria, H.; Soheil Najm, A.; Harun, Z.; Abdullah, S.; Wan Ghopa, W.A.; Sulaiman, N.H. Assessment of TiO2 Nanoconcentration and Twin Impingement Jet of Heat Transfer Enhancement—A Statistical Approach Using Response Surface Methodology. Energies 2021, 14, 595. [Google Scholar] [CrossRef]
- Faris Abdullah, M.; Zulkifli, R.; Harun, Z.; Abdullah, S.; Wan Ghopa, W.A.; Soheil Najm, A.; Humam Sulaiman, N. Impact of the TiO2 nanosolution concentration on heat transfer enhancement of the twin impingement jet of a heated aluminum plate. Micromachines 2019, 10, 176. [Google Scholar] [CrossRef]
- Carreón-Moncada, L.; González, A.; Rodríguez-Galicia, J.L.; Rendón-Angeles, J.C. Chemical deposition of CdS films by an ammonia-free process with amino acids as complexing agents. Thin Solid Film. 2016, 599, 166–173. [Google Scholar] [CrossRef]
- Ashith, V.; Rao, G.K. Bandgap tailoring in CdxZn1−xS alloy films deposited by successive ionic layer adsorption and reaction. Thin Solid Film. 2017, 626, 1–8. [Google Scholar]
- Yilmaz, S.; Tomakin, M.; Unverdi, A.; Aboghalon, A. A Study on Hydrothermal Grown CdS Nanospheres: Effects of Cd/S Molar Ratio. J. Sci. 2019, 32, 1271–1281. [Google Scholar] [CrossRef]
- Al-Sajad, G.A.; Holi, A.M.; L-Zahrani, A.A.A.; Najm, A.S. Titania Nanotubes Arrays Based-Gas Sensor: NO2-Oxidizing Gas and H2 Reducing Gas. Nano Biomed. Eng. 2020, 12, 191–196. [Google Scholar] [CrossRef]
- Cruz, J.S.; Pérez, R.C.; Delgado, G.T.; Angel, O.Z. CdS thin films doped with metal-organic salts using chemical bath deposition. Thin Solid Film. 2010, 518, 1791–1795. [Google Scholar] [CrossRef]
- Hop, B.X.; van Trinh, H.; Dat, K.Q.; Bao, P.Q. Growth o f CdS thin films by chemical bath deposition technique. J. Sci. Math. 2008, 24, 119–123. [Google Scholar]
- Kim, M.J.; Kim, H.T.; Kang, J.K.; Kim, D.H. Molecular Crystals and Liquid Crystals Effects of the Surface Roughness on Optical Properties of CdS Thin Films. Mol. Cryst. Liq. Cryst. 2010, 532, 437–444. [Google Scholar] [CrossRef]
- Mohammed, I.M.S.; Gubari, G.M.M.; Huse, N.P.; Dive, A.S.; Han, S.H. Effect of Cd/S ratio on growth and physical properties of CdS thin films for photosensor application. J. Mater. Sci. Mater. Electron. 2020, 31, 9989–9996. [Google Scholar] [CrossRef]
- Mugdur, P.H.; Chang, Y.J.; Su, Y.W.; Morrone, A.A.; Ryu, S.O.; Lee, T.J.; Chang, C.H. A Comparison of Chemical Bath Deposition of CdS from a Batch Reactor and a Continuous-Flow Microreactor. J. Electrochem. Soc. 2007, 154, D482. [Google Scholar] [CrossRef]
- Ouafi, M.; Jaber, B.; Atourki, L.; Zayyoun, N.; Ihlal, A.; Mzerd, A.; Laânab, L. In Situ Low-Temperature Chemical Bath Deposition of CdS Thin Films without Thickness Limitation: Structural and Optical Properties. Int. J. Photoenergy 2018, 2018, 4549154. [Google Scholar] [CrossRef]
- Djelloul, A.; Adnane, M.; Larbah, Y.; Zerdali, M.; Zegadi, C.; Messaoud, A. Effect of Annealing on the Properties of Nanocrystalline CdS Thin Films Prepared by CBD Method. J. Nano-Electron. Phys. 2016, 8, 02005–1–02005-7. [Google Scholar] [CrossRef]
- Senthil, K.; Mangalaraj, D.; Narayandass, S.K. Structural and optical properties of CdS thin Flms. Appl. Surf. Sci. 2001, 170, 476–479. [Google Scholar] [CrossRef]
- Slonopas, A.; Ryan, H.; Foley, B.; Sun, Z.; Sun, K.; Globus, T.; Norris, P. Growth mechanisms and their effects on the opto-electrical properties of CdS thin fi lms prepared by chemical bath deposition. Mater. Sci. Semicond. Process. 2016, 52, 24–31. [Google Scholar] [CrossRef]
- Sandoval-paz, M.G.; Ramírez-bon, R. Analysis of the early growth mechanisms during the chemical deposition of CdS thin fi lms by spectroscopic ellipsometry. Thin Solid Film. 2009, 517, 6747–6752. [Google Scholar] [CrossRef]
- Choi, C.; Paul, B.K.; Chang, C. Microreactor-Assisted Solution Deposition for Compound Semiconductor Thin Films. Processes 2014, 2, 441–465. [Google Scholar] [CrossRef]
- Challa, K.K.; Magnone, E.; Kim, E.T. Highly photosensitive properties of CdS thin films doped with boron in high doping levels. Mater. Lett. 2012, 85, 135–137. [Google Scholar] [CrossRef]
- Daniel-Umeri, R.A. Effect of pH on the Optical Properties of Cadmium Sulphide (CdS) Thin Film Deposited Using Chemical Bath Method. Int. J. Mater. Sci. Appl. 2015, 4, 138–142. [Google Scholar] [CrossRef][Green Version]
- Pawar, S.M.; Pawar, B.S.; Kim, J.H.; Joo, O.; Lokhande, C.D. Recent status of chemical bath deposited metal chalcogenide and metal oxide thin fi lms. Curr. Appl. Phys. 2011, 11, 117–161. [Google Scholar] [CrossRef]
- Singaevsky, A.F.; Piryatinski, Y.; Grynko, D.O.; Dimitriev, O.P. Asymmetric effect of (000) and (0001) facets on surface and interface properties of CdS single crystal. Appl. Phys. A 2011, 104, 493–502. [Google Scholar]
- Dhere, N.G.; Waterhouse, D.L.; Sundaram, K.B.; Melendez, O.; Parikh, N.R.; Patnaik, B. Studies on chemical bath deposited cadmium sulphide films by buffer solution technique. J. Mater. Sci. Mater. Electron. 1995, 6, 52–59. [Google Scholar] [CrossRef]
- Regmi, A.A.G.; Núñez, A.R.; López, M.S.; Castaneda, H. Comparative studies of CdS thin films by chemical bath deposition techniques as a buffer layer for solar cell applications. J. Mater. Sci. Mater. Electron. 2020, 31, 7499–7518. [Google Scholar]
- Voss, C.; Subramanian, S.; Ryu, S.O. Growth Kinetics of Thin-Film Cadmium Sulfide by Ammonia-Thiourea Based CBD. J. Electrochem. Soc. 2014, 151, 8–13. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, J.; Wang, W.; Huang, X.; Yuan, Q.; Hong, R.; Cha, L. Growth process and properties of CdS thin films prepared by chemical bath deposition at different pH values. J. Mater. Sci. Mater. Electron. 2018, 29, 7637–7643. [Google Scholar] [CrossRef]
- Hodes, G. Chemical Solution Deposition of Semiconductor Films; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Kariper, A.; Güneri, E.M.; Göde, F.; Gümüş, C.E. Effect of pH on the physical properties of CdS thin films deposited by CBD. Chalcogenide Lett. 2012, 9, 27–40. [Google Scholar]
- Ikhmayies, S.J. A Study of the Absorption Edge of ZnO Thin Films Prepared by the Spray Pyrolysis Method. Miner. Met. Mater. Ser. 2018, 8, 83–92. [Google Scholar]
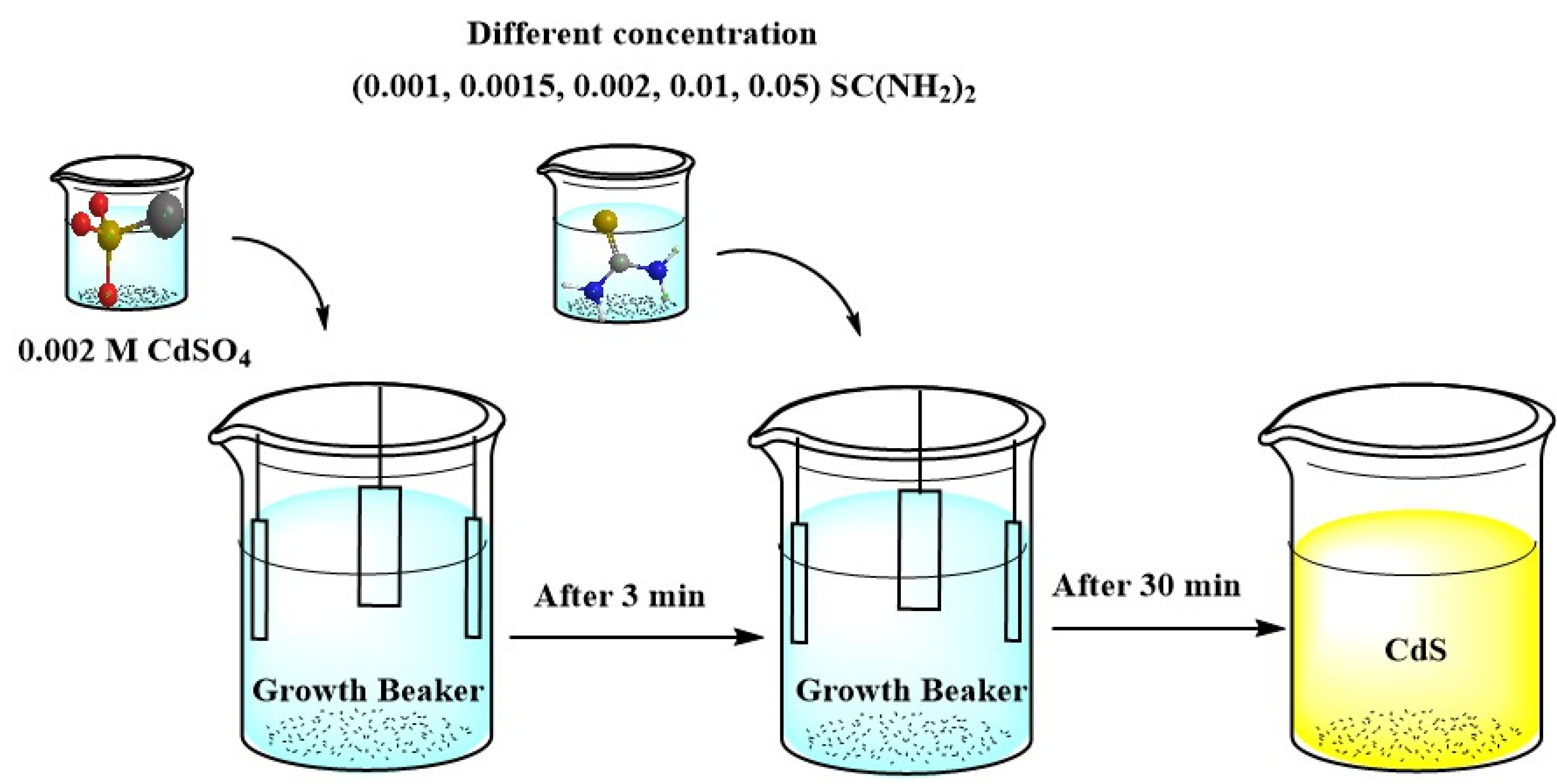
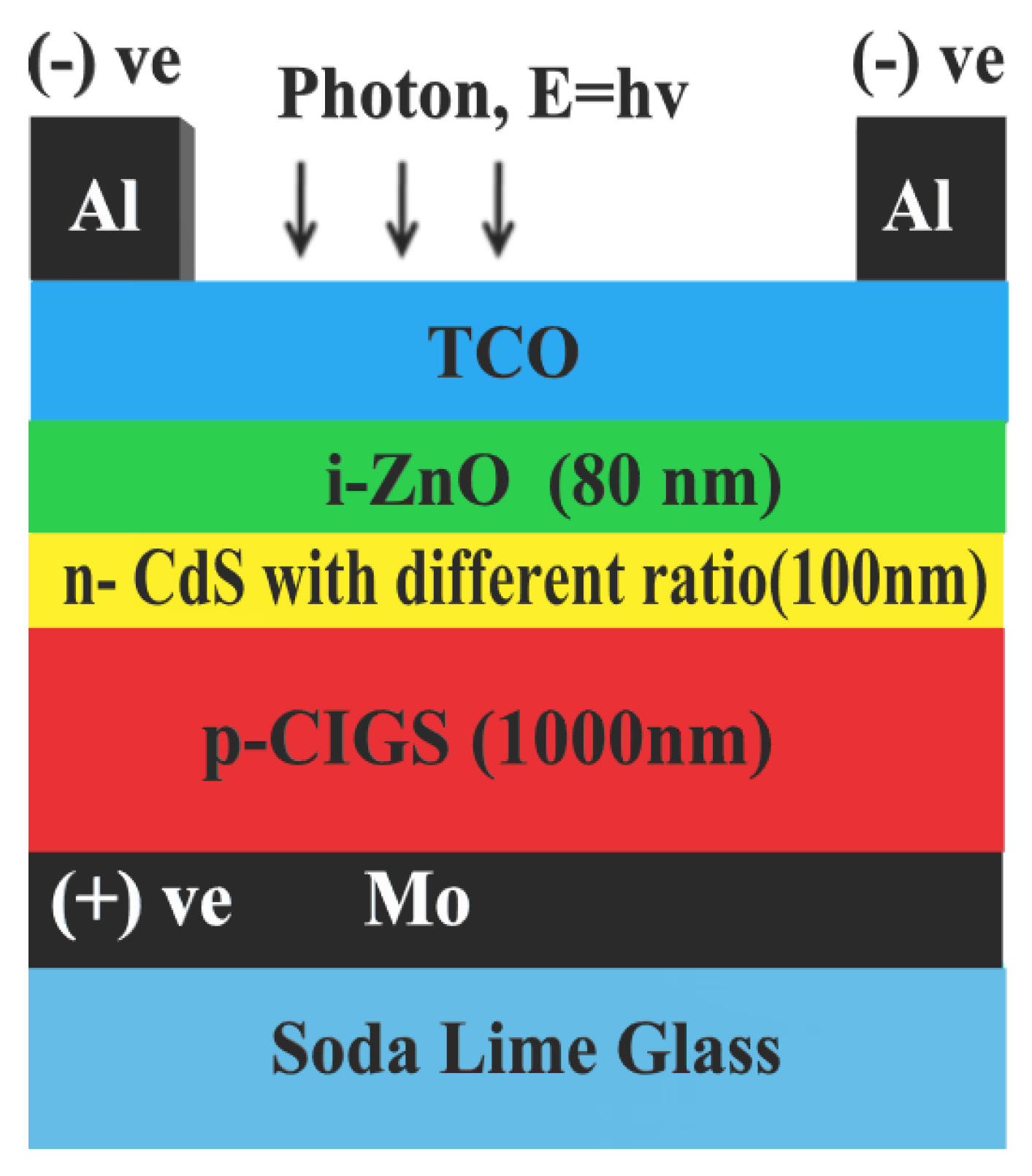


| Purpose | Chemical | Quantity |
|---|---|---|
| Stabilizer | DIW water | 360 mL |
| Cadmium source | CdSO4 | 0.002 m |
| Sulphur source | SC(NH2)2 | 0.001–0.05 m |
| Buffer Solution and pH Control | NH4OH | 3.5 m (40 mL) |
| Layers’ Characteristics | n-ZnO | n-CdS (1/1) | n-CdS (1/5) | n-CdS (1/25) | p-CIGS |
|---|---|---|---|---|---|
| Layer thickness (nm) | 80 | 100 | 100 | 100 | 1000 |
| Dielectric constant, εr | 10 | 10 | 10 | 10 | 10 |
| Electron mobility, µn (cm²/V·s) | 50 | Experiment | Experiment | Experiment | 50 |
| Hole mobility, µp (cm²/V·s) | 20 | 20 | 20 | 20 | 20 |
| Acceptor concentration, NA (cm−3) | 0 | 0 | 0 | 0 | 5.5 × 1015 |
| Donor concentration, ND (cm−3) | 5 × 1017 | Experiment | Experiment | Experiment | 0 |
| Band gap, Eg (eV) | 3.4 | Experiment | Experiment | Experiment | 1.2 |
| Electron affinity, χ (eV) | 4.55 | 4.45 | 4.45 | 4.45 | 4.5 |
| Conduction band density of states, NC (cm−3) | 4 × 1018 | 2 × 1018 | 2 × 1018 | 2 × 1018 | 2 × 1018 |
| Valance band density of states, NV (cm−3) | 9 × 1018 | 1.5 × 1019 | 1.5 × 1019 | 1.5 × 1019 | 2 × 1018 |
| Electron thermal velocity (cm s−1) | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 |
| Hole thermal velocity (cm s−1) | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 |
| Layers’ Characteristics | n-ZnO | n-Buffer | CIGS |
|---|---|---|---|
| Defect Type | Neutral | Neutral | Neutral |
| Electron capture cross section (cm2) | 10−12 | 10−13 | 10−15 |
| Hole capture cross section (cm2) | 10−12 | 10−13 | 10−13 |
| Energetic distribution | Gauẞ | Gauẞ | Gauẞ |
| Reference for defect energy level Et | Above Ev | Above Ev | Above Ev |
| Energy level with respect to reference (eV) | 1.650 | 1.200 | (0.6/1.1) × Eg-Absorber |
| Characteristic energy (eV) | 0.100 | 0.100 | 0.100 |
| Total defect density, NT-total (cm−3) | 1.772 × 1016 | 1.772 × 1017 | (0–1.772) × 1017 |
| Peak defect density NT-peak (eV−1·cm−3) | 1 × 1017 | 1 × 1018 | (0–1) × 1018 |
| Sample | Angle (2θ Degree) | The Interplane Spacing ‘d’ (nm) | FWHM ‘B’ Peak Width (°) | Lattice Strain, ε (%) | Crystallite Size (nm) ‘D’ |
|---|---|---|---|---|---|
| [1/1] | 27.23° | 0.326 | 0.325 | 0.585 | 25.80 |
| [1/5] | 27.06° | 0.328 | 0.470 | 0.852 | 17.70 |
| [1/25] | 27.20° | 0.327 | 0.576 | 1.039 | 14.40 |
| Sample | Resistivity, (Ω cm) | Carrier Concentration, (1/cm3) | Mobility, (cm2 Vs) | Hall Coefficient (cm3/C) |
|---|---|---|---|---|
| [1]/[1] | 1843.9 | 3.210 × 1014 | 12.86 | −2.19 × 104 |
| [1]/[5] | 3937.1 | 1.406 × 1014 | 10.39 | −5.13 × 104 |
| [1]/[25] | 6595.1 | 2.199 × 1014 | 17.24 | −3.79 × 104 |
| Sample | JSC (mA/cm2) | VOC (V) | FF (%) | Efficiency (%) |
|---|---|---|---|---|
| [1/1] | 12.40 | 0.559 | 24.71 | 1.71 |
| [1/5] | 8.62 | 0.559 | 22.32 | 1.07 |
| [1/25] | 6.67 | 0.558 | 19.61 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najm, A.S.; Naeem, H.S.; Alwarid, D.A.R.M.; Aljuhani, A.; Hasbullah, S.A.; Hasan, H.A.; Sopian, K.; Bais, B.; Al-Iessa, H.J.; Majdi, H.S.; et al. Mechanism of Chemical Bath Deposition of CdS Thin Films: Influence of Sulphur Precursor Concentration on Microstructural and Optoelectronic Characterizations. Coatings 2022, 12, 1400. https://doi.org/10.3390/coatings12101400
Najm AS, Naeem HS, Alwarid DARM, Aljuhani A, Hasbullah SA, Hasan HA, Sopian K, Bais B, Al-Iessa HJ, Majdi HS, et al. Mechanism of Chemical Bath Deposition of CdS Thin Films: Influence of Sulphur Precursor Concentration on Microstructural and Optoelectronic Characterizations. Coatings. 2022; 12(10):1400. https://doi.org/10.3390/coatings12101400
Chicago/Turabian StyleNajm, Asmaa Soheil, Hasanain Salah Naeem, Duaa Abdul Rida Musa Alwarid, Abdulwahab Aljuhani, Siti Aishah Hasbullah, Hiba Ali Hasan, Kamaruzzaman Sopian, Badariah Bais, Heidar J. Al-Iessa, Hasan Sh. Majdi, and et al. 2022. "Mechanism of Chemical Bath Deposition of CdS Thin Films: Influence of Sulphur Precursor Concentration on Microstructural and Optoelectronic Characterizations" Coatings 12, no. 10: 1400. https://doi.org/10.3390/coatings12101400
APA StyleNajm, A. S., Naeem, H. S., Alwarid, D. A. R. M., Aljuhani, A., Hasbullah, S. A., Hasan, H. A., Sopian, K., Bais, B., Al-Iessa, H. J., Majdi, H. S., Sultan, A. J., & Moria, H. (2022). Mechanism of Chemical Bath Deposition of CdS Thin Films: Influence of Sulphur Precursor Concentration on Microstructural and Optoelectronic Characterizations. Coatings, 12(10), 1400. https://doi.org/10.3390/coatings12101400












