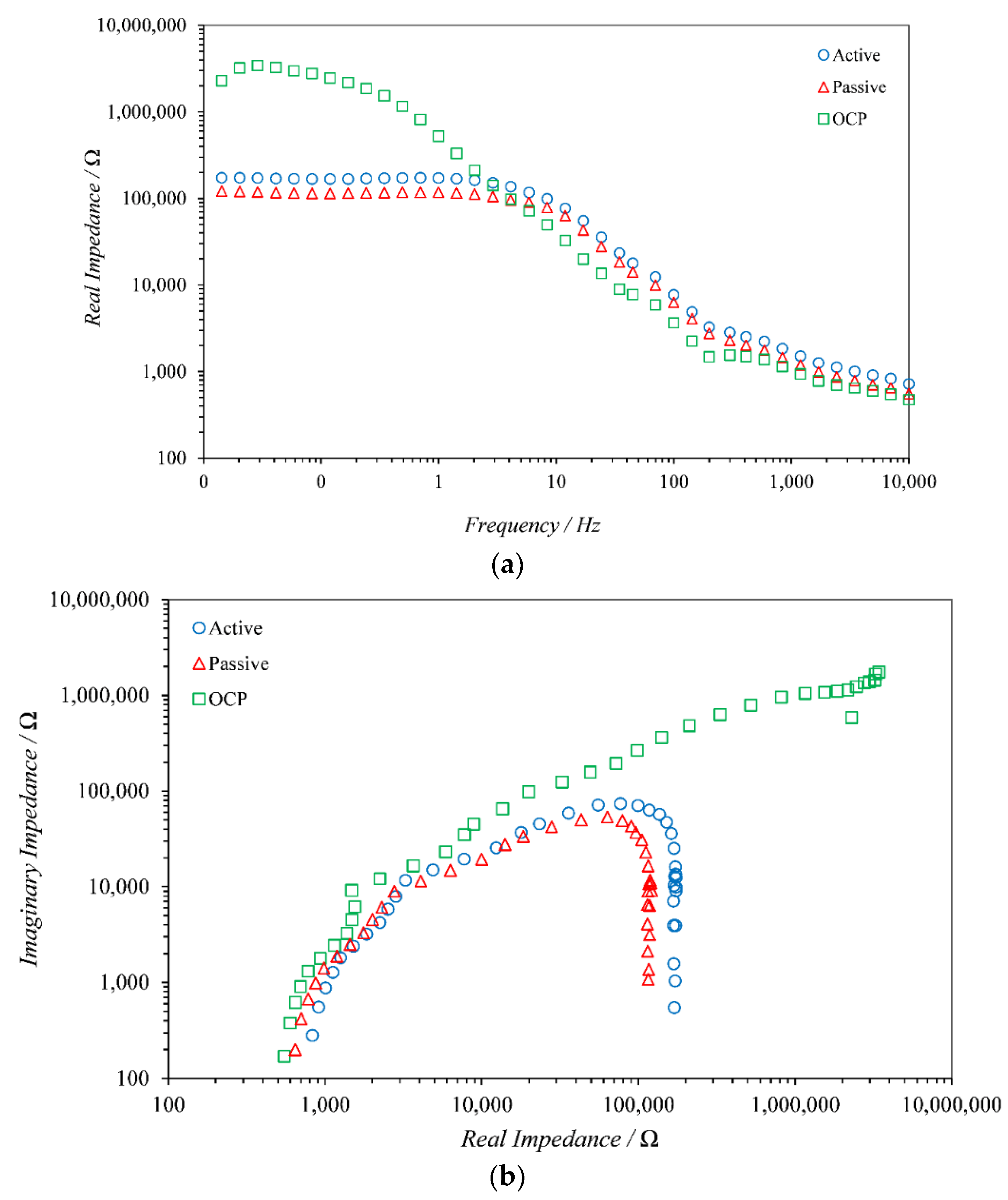
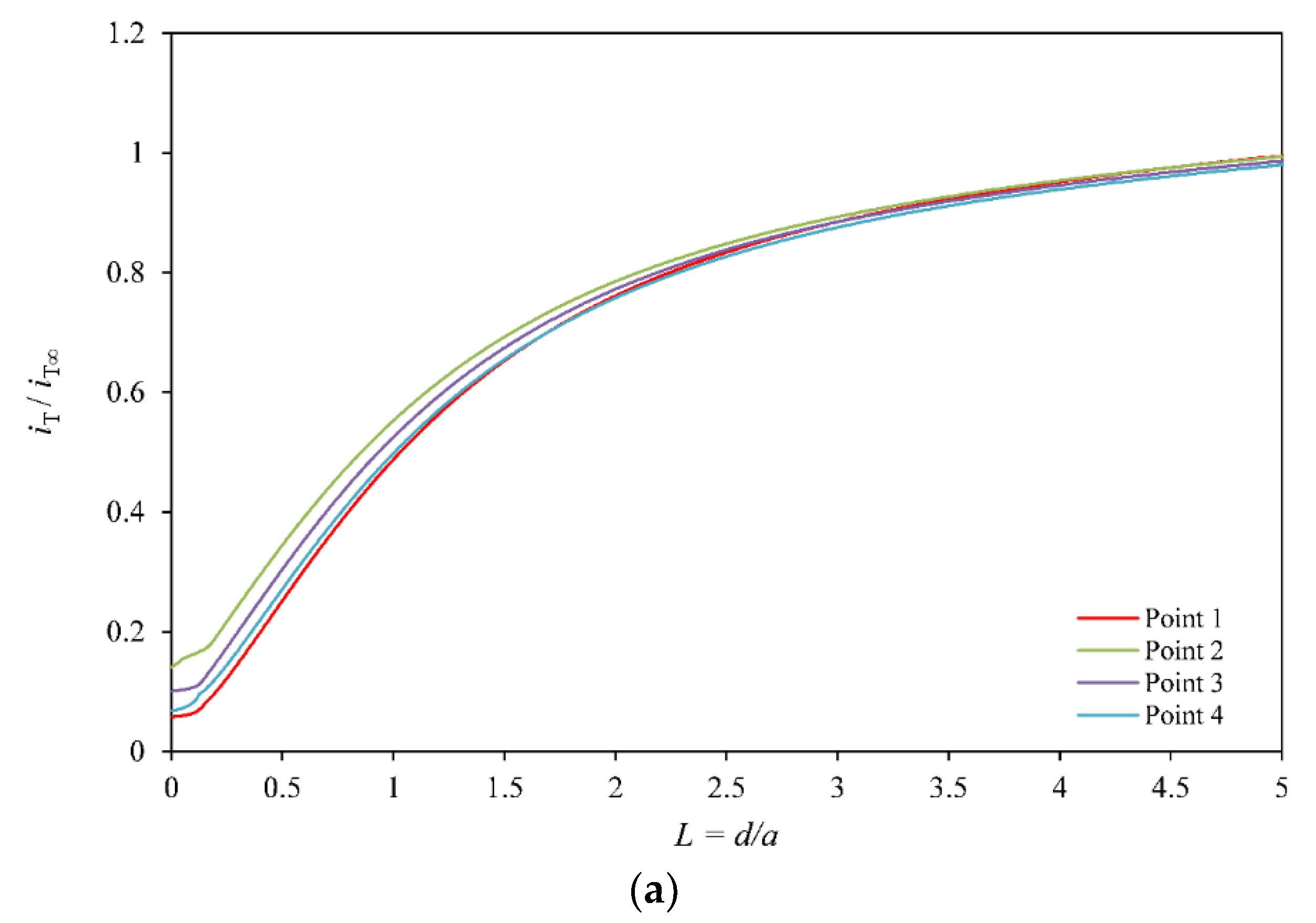
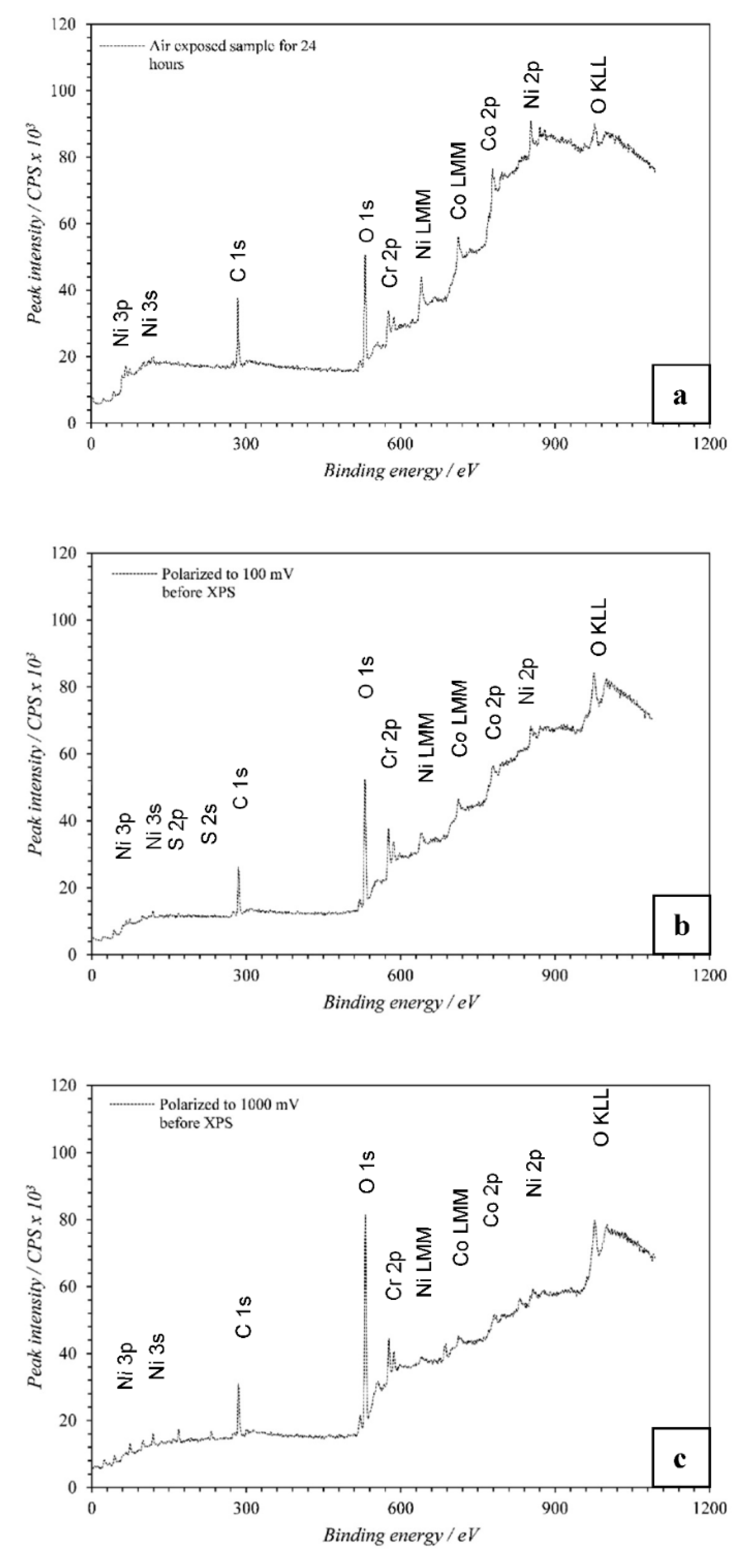
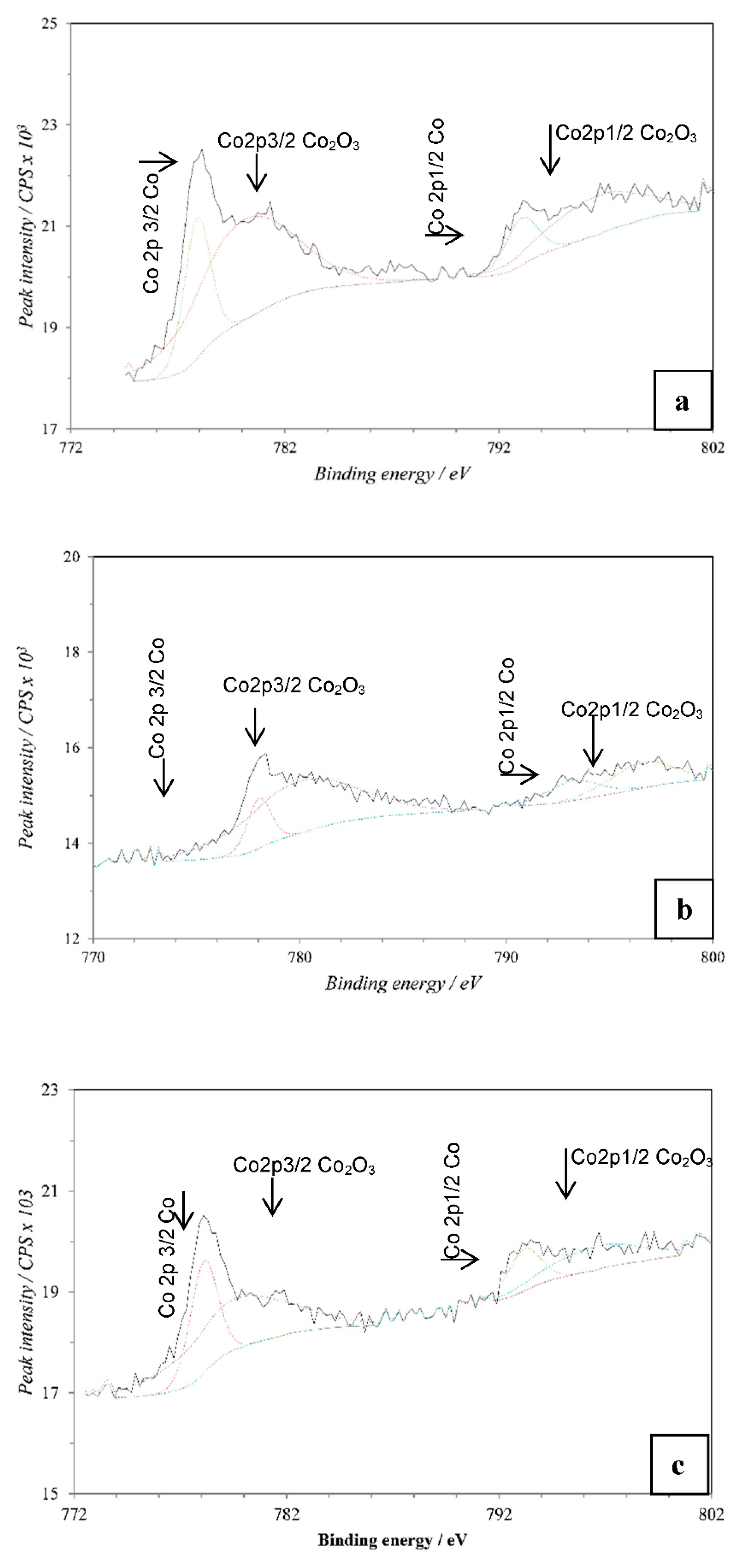
1. Introduction
To maintain thermal insulation and integrity at very high temperatures, thermal barrier coatings are made from multiple materials [
1,
2,
3,
4]. This multiple-component system provides unique insulation properties but also causes corrosion degradation problems. If these corrosion problems get worse with time, they severely hamper the integrity of coatings [
5,
6]. Coatings for high-temperature applications consist of a heat-insulating top ceramic coat and an intermediate bond coat of MCrAlY. The bond coat improves the adherence with the substrate on one side, while improving the bond with the insulating top coat on the other side [
7,
8]. A number of experimental sequences are reported to study coating microstructure, composition, and corrosion degradation properties. Among the reported work, researchers used electron microscopy, X-ray diffraction, phases/composition analysis, and electrochemical testing techniques [
8,
9,
10,
11]. A detailed standalone electrochemical aspect of the corrosion degradation is missing. In this study, we will use traditionally reported electrochemical testing techniques alongside advanced scanning electrochemical microscopy to study the corrosion process in the closest vicinity of the coating surface. The air-grown and voltage-assisted passive layer is studied by X-ray photoelectron spectroscopy to find the types of oxides formed with the severity of the environment. Although different aspects of coatings have been studied by researchers in the past, only the most relevant work will be presented here.
M.S. Ali et al. studied thermally grown oxide (TGO) on thermal barrier coatings as a function of temperature [
12]. An intermediate bond coat of NiCrAlY was deposited on a high nickel–chromium superalloy. The coated samples were heated from 700 °C to 1200 °C for a period from 1 h to 1000 h. The samples were studied by scanning electron microscope (SEM), X-ray diffraction (XRD), and electrochemical impedance spectroscopy (EIS) to record microstructural and electrical properties changes. Their results showed the rise of impedance and capacitance up to 100 h of thermal cycles. After thermal cycling beyond 100 h, they found a fall in the impedance values. They attributed the initial rise in the impedance to the thin Al
2O
3 growth, while later impedance drop to the mixed oxide growth [
12]. The low number of thermal cycles grows an adherent oxide which blocks/slows down the electronic and ionic mobility across the Al
2O
3. At a higher number of cycles, cobalt, chromium, and nickel get oxidized and mixed with already present Al
2O
3 [
12]. The adherent oxide transfer to a porous spinel oxide causes increased thickness and decreases the impedance and capacitance of the coatings. The XRD and microstructure results also support the presentence of multicomponent oxides at higher thermal cycles.
The formation of the adherent alumina layer also depends on the amount of aluminum present in the coatings. The low percentage of aluminum in the coating does not allow the formation of a uniform layer. F. Tang et al. added 2% of finely divided (≤40 µm) powder to the commercially available CoNiCrAlY powder [
13]. A 100 µm coating layer was produced on mild steel samples. Thermal cycling was carried out on polished and unpolished samples at 1000 °C for a period of 1 h to 24 h. The polished surface formed an alumina layer all over the surface while the unpolished surface formed a mixed oxide layer. They suggested that oxide concentration in the coating, as well as surface roughness, also contribute to forming a uniform oxide layer [
14]. The rough surface predominantly forms a mixed oxide at high temperatures. The formation of mixed spinal oxide can be related to the rough surface which allows other coating constituents to be exposed to the air and form their oxides.
In a separate study, F. Tang et al. also studied the effect of coating process parameters on the formation of the oxide [
13,
14]. Low oxygen to fuel ratio formed an enriched alumina dense oxide layer while high oxygen to fuel ratio formed a spinel mixed oxide layer. The higher oxygen concentration allows oxidation of all coating powder constituents rather than a highly oxidizing element [
13]. The process of formation of spinel oxide during the coating process increases multi-oxide growth during thermal cycling. The porous structure of coatings also promotes oxidation and oxygen can seep easily through the coating surface.
The oxidation of the coating material before hitting the target material contributes to the formation of spinel oxide. The problems occur due to direct contact of the metallic powder with the oxygen during the particle flight time. To avoid oxidation during particle flight W. Guo et al., manufactured a mechanically alloyed CoNiCrAlY powder [
15]. The shell of alumina, approximately 1 µm was formed on CoNiCrAlY by ball milling. The coatings produced by core-shell structure powder exhibit low oxide formation in the thermal spraying process. The low oxide content during HVOF process assists the formation of a dense alumina layer during thermal cycling and has good corrosion resistance [
15]. The partial drawback of the core-shell powder coating is the poor deformability of the powder while hitting the target material which causes excessive porosity and some hard Al-Ni phases in the final coating.
Another modification is the use of different thermal spraying guns to produce the coatings. One such study is carried out by A. C Karaoglanli, where they used atmospheric plasma spraying (APS) gun, supersonic atmospheric spraying (SAPS) gun, high-velocity oxygen fuel (HVOF) gun, detonation spraying (DS) gun, and cold gas dynamic spraying (CGDS) gun [
16]. The coating was prepared on 718 supper alloy and isothermally heated to 1000 °C to study changes in microstructure and thermally grown oxide. The coating produced from the CGDS gun produces a dense uniform oxide during thermal cycling with higher corrosion resistance performance [
16].
Microstructure and isothermal oxidation of CoNiCrAlY coatings was also studied by H. Luo et al. [
2]. The coating was produced on IN718 alloy by using laser powder bed fusion (LPBF) and heated to 1100 °C for a time period of 1 h to 100 h. The LPBF is an additive manufacturing process, where the powder can be deposited in line with the path defined by computer-aided design. They reported the formation of homogenized phases in coating microstructure. Under low thermal cycling, the alumina layer was also found dense and crack-free. Improved microstructural and oxidation properties are attributed to the LPBF process which build-up columnar dendrite and equated grains that helps in producing a thin uniform oxide [
2]. The increase in time of oxidation promotes the formation of spinel oxide which degrade corrosion resistance. Some other researchers have also studied the improvement in insulation, corrosion resistance, and tribo-properties improvement by using additive manufacturing, as in ref. [
1,
2,
3,
4], using nano-particle powder [
12,
16,
17].
It can be summarized from the above literature review that the majority of the reported work exists in the field of microstructure study, thermally grown oxide as a function of change in the process parameters, coating deposition technique, and coating powder composition. The focus of this study is the corrosion performance evaluation by using electrochemical testing techniques and characterization of surface oxides by using X-ray photoelectron spectroscopy. The corrosion testing will be carried out by using the potentiodynamic polarization method, electrochemical impedance spectroscopy, and scanning electrochemical microscopy (SECM). By using SECM, the current distance response is recorded in the closest vicinity of the coating surface (0–60 µm). The oxide growth on the surface is simulated by potentiostatically polarizing the sample to 100 mV, 500 mV, and 1000 mV. The analysis of the oxide surface will be carried out by using X-ray photoelectron spectroscopy. The use of this experimental technique contributes to enhancing understating of corrosion performance of CoNiCrAlY coating.