Electrochemical Surface Biofunctionalization of Titanium through Growth of TiO2 Nanotubes and Deposition of Zn Doped Hydroxyapatite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Duplex Electrochemical Treatment
2.2.1. Anodization
2.2.2. Coating Deposition
2.3. Characterization
3. Results and Discussions
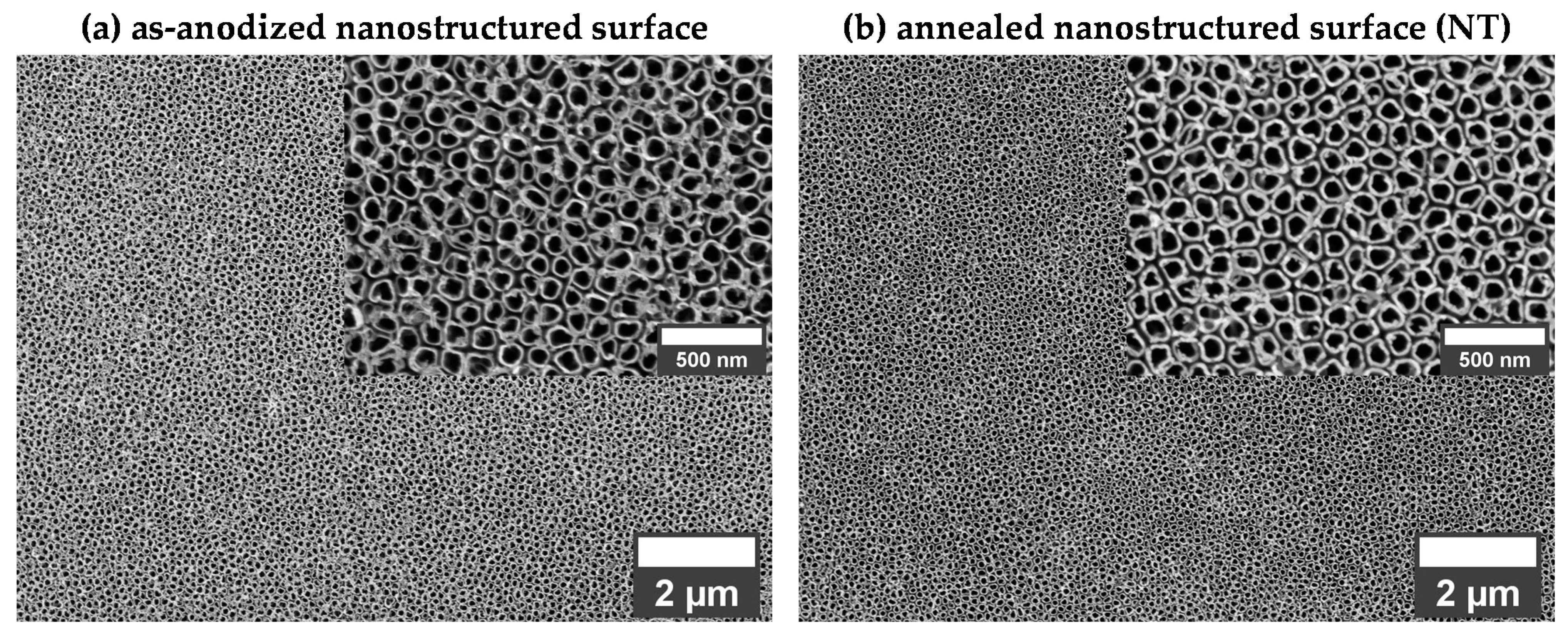
3.1. Morphology
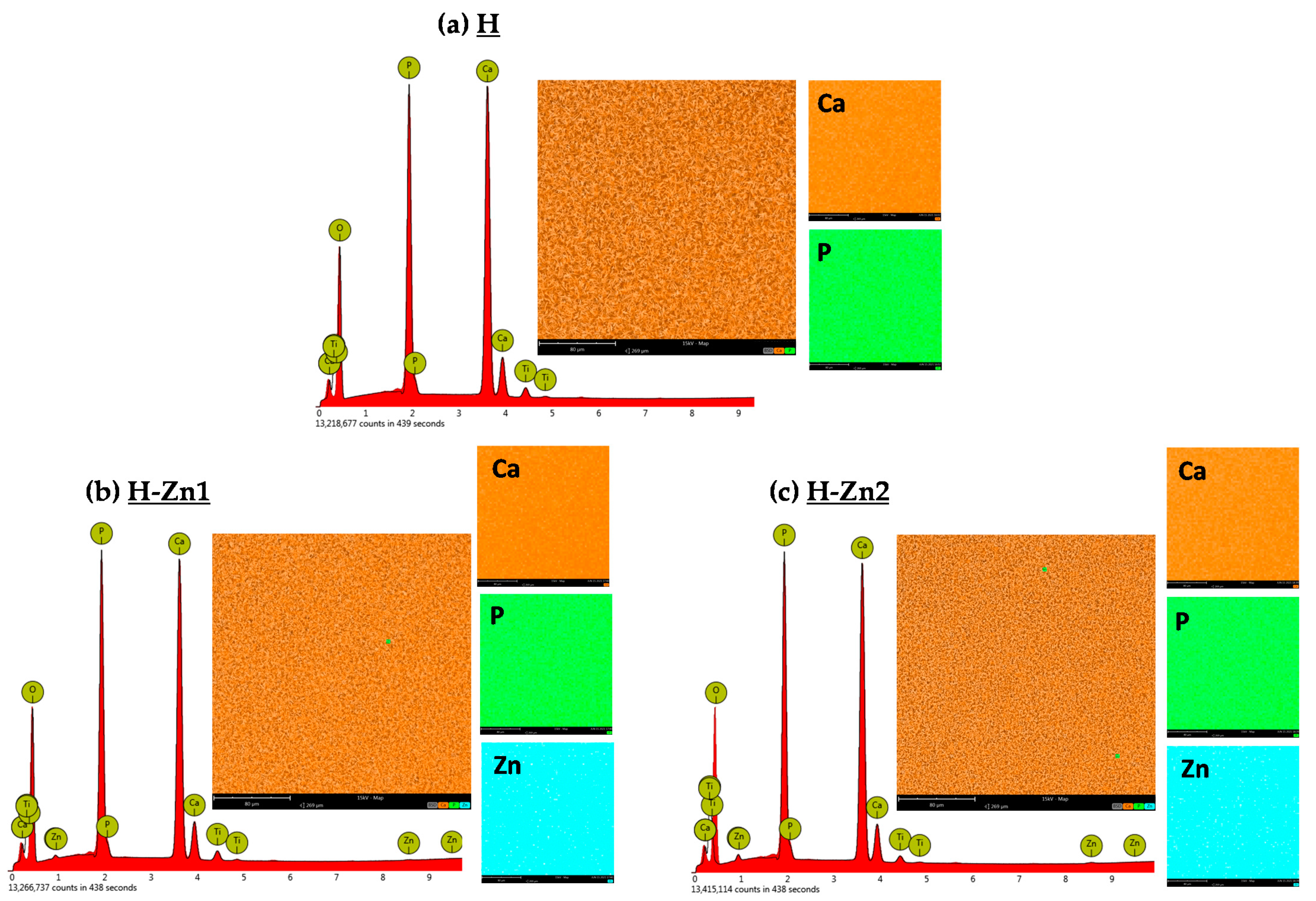
3.2. Elemental Composition
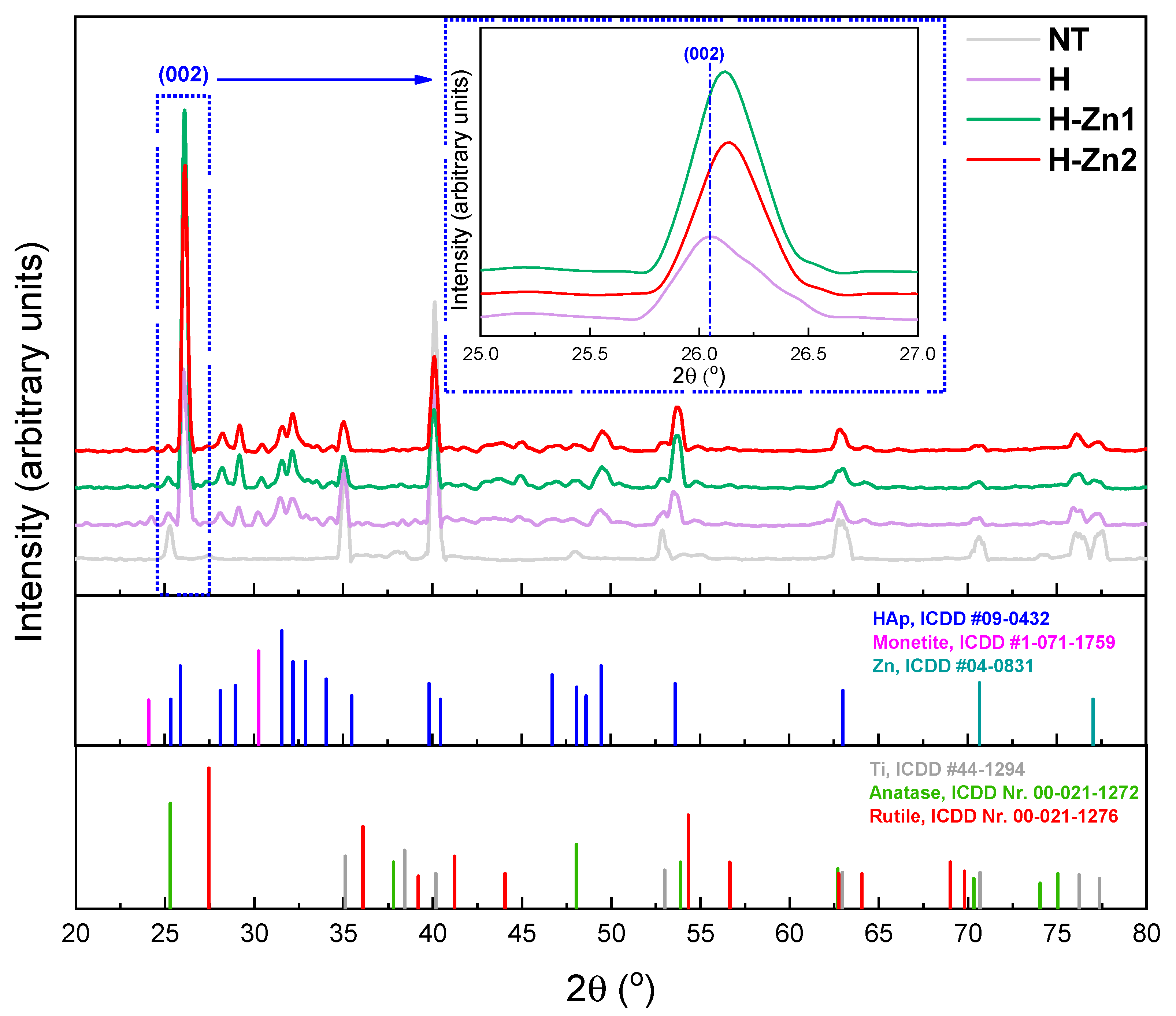
3.3. Phase Composition
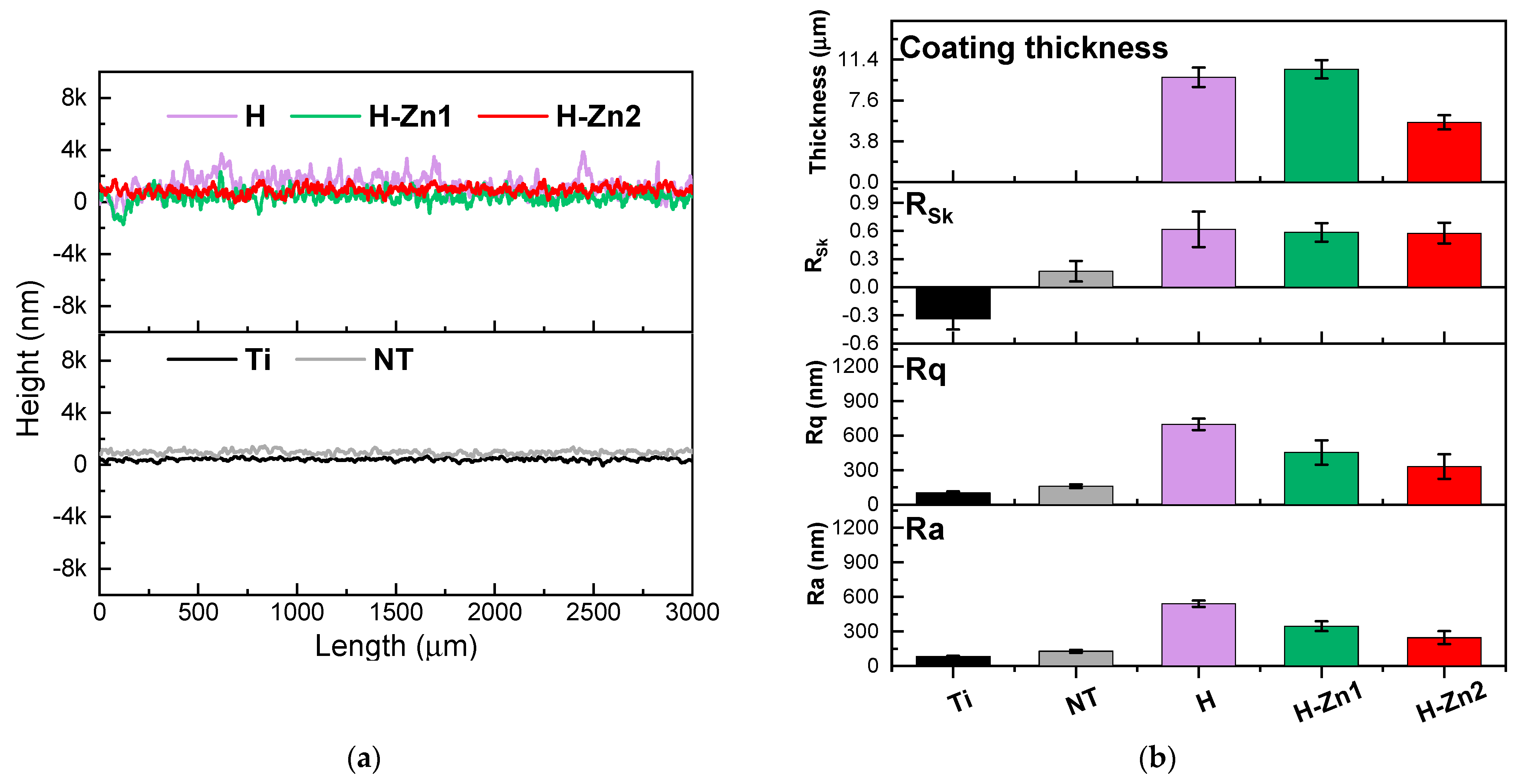
3.4. Roughness
3.5. Chemical Bonds
3.6. Adhesion
4. Conclusions
- The electrochemical surface functionalization of Ti by the growth of nanotubes with an average diameter of ~70 nm and deposition of undoped HAp doped with Zn in different concentrations was successfully achieved by electrochemical techniques. The present study highlighted that the addition of Zn in even a small amount can enhance the characteristics of HAp. All coatings presented a morphology consisting of ribbon-like crystals, which suffers some alterations by the addition and increment of Zn content. The XRD and FTIR investigations have confirmed that all coatings consist in HAp as a main phase. Irrespective of the Zn content, the crystallinity of the HAp coatings was enhanced after the addition of Zn.
- Regarding the effect of Zn content on the properties of HAp, it can be said that even though the differences between the two selected concentrations is minor, the coating H-Zn1 (with Zn of 0.78 at.%) has met most of the requirements, being more suitable for medical applications. Thus, the coatings with a smaller Zn amount will be further evaluated in terms of electrochemical behavior in synthetic media, along with their biomineralization ability and in vitro cell behavior, to establish if the proposed coatings can impart a good biocompatibility with a suitable bioactive character and antibacterial efficiency, without inducing a cytotoxic effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Quinn, J.; McFadden, R.; Chan, C.W.; Carson, L. Titanium for orthopedic applications: An overview of surface modification to improve biocompatibility and prevent bacterial biofilm formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign bodies or tuners for the immune response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium alloys for dental implants: A review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Prasad, S.; Ehrensberger, M.; Gibson, M.P.; Kim, H.; Monaco, E.A. Biomaterial properties of titanium in dentistry. J. Oral Biosci. 2015, 57, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Szmukler-Moncler, S.; Salama, H.; Reingewirtz, Y.; Dubruille, J.H. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J. Biomed. Mater. Res. 1998, 43, 192–203. [Google Scholar] [CrossRef]
- Irandoust, S.; Müftü, S. The interplay between bone healing and remodeling around dental implants. Sci. Rep. 2020, 10, 4335. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Lu, Y.; Ren, J.; Wang, Z.; Huang, J.; Zhu, L.; Wang, K. Decreased fibrous encapsulation and enhanced osseointegration in vitro by decorin-modified titanium surface. Colloids Surf. B Biointerfaces 2017, 155, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface modification techniques of titanium and titanium alloys for biomedical dental Applications: A review. Mater. Today Proc. 2020, 39, 84–90. [Google Scholar] [CrossRef]
- Xue, T.; Attarilar, S.; Liu, S.; Liu, J.; Song, X.; Li, L.; Zhao, B.; Tang, Y. Surface modification techniques of titanium and its alloys to functionally optimize their biomedical properties: Thematic review. Front. Bioeng. Biotechnol. 2020, 8, 1261. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, P.; Attarilar, S.; Shi, H. Innovative surface modification procedures to achieve micro/nano-graded Ti-based biomedical alloys and implants. Coatings 2021, 11, 647. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Ortiz-García, I.; Jiménez-Guerra, A.; Monsalve-Guil, L.; Muñoz-Guzón, F.; Perez, R.A.; Gil, F.J. Comparison between sandblasted acid-etched and oxidized titanium dental implants: In vivo study. Int. J. Mol. Sci. 2019, 20, 3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartoretto, S.C.; Alves, A.T.N.N.; Resende, R.F.B.; Calasans-Maia, J.; Granjeiro, J.M.; Calasans-Maia, M.D. Early osseointegration driven by the surface chemistry and wettability of dental implants. J. Appl. Oral Sci. 2015, 23, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Rama, M.; Chetan, U.; Vijayalakshmi, U. Bioactive coating as a surface modification technique for biocompatible metallic implants: A review. J. Asian Ceram. Soc. 2019, 7, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Mitrichenko, D.V.; Prosolov, A.B.; Nikolaeva, O.O.; Lastovka, V.V.; Belyavskaya, O.A.; Chebodaeva, V.A.; Glukhov, I.A.; Litvinova, L.S.; Shupletsova, V.V.; et al. Zn-doped CaP-based coatings on Ti–6Al–4V and Ti–6Al–7Nb alloys prepared by magnetron sputtering: Controllable biodegradation, bacteriostatic, and osteogenic activities. Coatings 2021, 11, 809. [Google Scholar] [CrossRef]
- Popa, C.L.; Deniaud, A.; Michaud-Soret, I.; Guégan, R.; Motelica-Heino, M.; Predoi, D. Structural and biological assessment of zinc doped hydroxyapatite nanoparticles. J. Nanomater. 2016, 2016, 1062878. [Google Scholar] [CrossRef] [Green Version]
- Sergi, R.; Bellucci, D.; Candidato, R.T.; Lusvarghi, L.; Bolelli, G.; Pawlowski, L.; Candiani, G.; Altomare, L.; de Nardo, L.; Cannillo, V. Bioactive Zn-doped hydroxyapatite coatings and their antibacterial efficacy against Escherichia coli and Staphylococcus aureus. Surf. Coat. Technol. 2018, 352, 84–91. [Google Scholar] [CrossRef]
- Begam, H.; Kundu, B.; Chanda, A.; Nandi, S.K. MG63 osteoblast cell response on Zn doped hydroxyapatite (HAp) with various surface features. Ceram. Int. 2017, 43, 3752–3760. [Google Scholar] [CrossRef]
- Wang, M.; Tang, T. Surface treatment strategies to combat implant-related infection from the beginning. J. Orthop. Transl. 2019, 17, 42–54. [Google Scholar] [CrossRef]
- Uysal, I.; Yilmaz, B.; Evis, Z. Zn-doped hydroxyapatite in biomedical applications. J. Aust. Ceram. Soc. 2021, 57, 869–897. [Google Scholar] [CrossRef]
- Ito, A.; Ojima, K.; Naito, H.; Ichinose, N.; Tateishi, T. Preparation, solubility, and cytocompatibility of zinc-releasing calcium phosphate ceramics. J. Biomed. Mater. Res. 2000, 50, 178–183. [Google Scholar] [CrossRef]
- Tank, K.P.; Chudasama, K.S.; Thaker, V.S.; Joshi, M.J. Pure and zinc doped nano-hydroxyapatite: Synthesis, characterization, antimicrobial and hemolytic studies. J. Cryst. Growth 2014, 401, 474–479. [Google Scholar] [CrossRef]
- Candidato, R.T.; Thouzellier, C.; Pawłowski, L. Evaluation of the in-vitro behavior of nanostructured hydroxyapatite and zinc doped hydroxyapatite coatings obtained using solution precursor plasma spraying. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2101–2108. [Google Scholar] [CrossRef]
- Codescu, M.M.; Vladescu, A.; Geanta, V.; Voiculescu, I.; Pana, I.; Dinu, M.; Kiss, A.E.; Braic, V.; Patroi, D.; Marinescu, V.E.; et al. Zn based hydroxyapatite based coatings deposited on a novel FeMoTaTiZr high entropy alloy used for bone implants. Surf. Interfaces 2021, 101591. [Google Scholar] [CrossRef]
- Ke, D.; Banerjee, D.; Bose, S. In vitro characterizations of Si4+ and Zn2+ doped plasma sprayed hydroxyapatite coatings using osteoblast and osteoclast coculture. ACS Biomater. Sci. Eng. 2019, 5, 1302–1310. [Google Scholar] [CrossRef]
- Hidalgo-Robatto, B.M.; López-Álvarez, M.; Azevedo, A.S.; Dorado, J.; Serra, J.; Azevedo, N.F.; González, P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Prodan, A.M.; Buton, N.; Predoi, D. Structural characterization and antifungal studies of zinc-doped hydroxyapatite coatings. Molecules 2017, 22, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanh, T.T.; Cotrut, C.M.; Vranceanu, M.D.; Ungureanu, E.; Tarcolea, M. Studies of microstructure and composition of modified hydroxyapatite coatings via Sem investigations. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2020, 82, 145–154. [Google Scholar]
- Safavi, M.S.; Walsh, F.C.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. Electrodeposited hydroxyapatite-based biocoatings: Recent progress and future challenges. Coatings 2021, 11, 110. [Google Scholar] [CrossRef]
- Vladescu, A.; Vranceanu, D.M.; Kulesza, S.; Ivanov, A.N.; Bramowicz, M.; Fedonnikov, A.S.; Braic, M.; Norkin, I.A.; Koptyug, A.; Kurtukova, M.O.; et al. Influence of the electrolyte’s PH on the properties of electrochemically deposited hydroxyapatite coating on additively manufactured Ti64 alloy. Sci. Rep. 2017, 7, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Ling, L.; Lin, M.C.; Peng, H.K.; Ren, H.T.; Lou, C.W.; Lin, J.H. Recent advances in multifunctional hydroxyapatite coating by electrochemical deposition. J. Mater. Sci. 2020, 55, 6352–6374. [Google Scholar] [CrossRef]
- Blackwood, D.J.; Seah, K.H.W. Electrochemical cathodic deposition of hydroxyapatite: Improvements in adhesion and crystallinity. Mater. Sci. Eng. C 2009, 29, 1233–1238. [Google Scholar] [CrossRef]
- Yan, Y.; Ding, Q.; Huang, Y.; Han, S.; Pang, X. Magnesium substituted hydroxyapatite coating on titanium with nanotublar TiO2 intermediate layer via electrochemical deposition. Appl. Surf. Sci. 2014, 305, 77–85. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A review on TiO2 nanotubes: Influence of anodization parameters, formation mechanism, properties, corrosion behavior, and biomedical applications. J. Bio-Tribo-Corros. 2015, 1, 28. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Marquez, D.; Gulati, K.; Carty, C.P.; Stewart, R.A.; Ivanovski, S. Determining the relative importance of titania nanotubes characteristics on bone implant surface performance: A quality by design study with a fuzzy approach. Mater. Sci. Eng. C 2020, 114, 110995. [Google Scholar] [CrossRef] [PubMed]
- Shahmohammadi, P.; Khazaei, B.A. Characterization of Zn/Mg-enriched calcium phosphate coating produced by the two-step pulsed electrodeposition method on titanium substrate. Surf. Interfaces 2021, 22, 110819. [Google Scholar] [CrossRef]
- Vranceanu, D.M.; Ionescu, I.C.; Ungureanu, E.; Cojocaru, M.O.; Vladescu, A.; Cotrut, C.M. Magnesium doped hydroxyapatite-based coatings obtained by pulsed galvanostatic electrochemical deposition with adjustable electrochemical behavior. Coatings 2020, 10, 727. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S. Densification behaviour and mechanisms of synthetic hydroxyapatites. J. Eur. Ceram. Soc. 2000, 20, 2377–2387. [Google Scholar] [CrossRef]
- Gopi, D.; Kanimozhi, K.; Bhuvaneshwari, N.; Indira, J.; Kavitha, L. Novel banana peel pectin mediated green route for the synthesis of hydroxyapatite nanoparticles and their spectral characterization. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Rusu, V.; Chuen-How, N.; Wilke, M.; Tiersch, B.; Fratzl, P.; Peter, M. Size-controlled hydroxyapatite nanoparticles as self-organized organic-inorganic composite materials. Biomaterials 2005, 26, 5414–5426. [Google Scholar] [CrossRef]
- Li, Z.Y.; Lam, W.M.; Yang, C.; Xu, B.; Ni, G.X.; Abbah, S.A.; Cheung, K.M.C.; Luk, K.D.K.; Lu, W.W. Chemical composition, crystal size and lattice structural changes after incorporation of strontium into biomimetic apatite. Biomaterials 2007, 28, 1452–1460. [Google Scholar] [CrossRef]
- Narayanan, R.; Kwon, T.-Y.; Kim, K.-H. Direct nanocrystalline hydroxyapatite formation on titanium from ultrasonated electrochemical bath at physiological PH. Mater. Sci. Eng. C 2008, 28, 1265–1270. [Google Scholar] [CrossRef]
- ASTM International. ASTM D3359-17 Standard Test Methods for Rating Adhesion by Tape Test; ASTM International: Calw, Germany, 2017; pp. 1–9. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Ionescu, I.C.; Ungureanu, E.; Berbecaru, A.; Zamfir, R.I.; Vladescu, A.; Vranceanu, D.M. Evaluation of surface modification techniques on the ability of apatite formation and corrosion behavior in synthetic body fluid: An in vitro study. Surf. Interfaces 2021, 22, 100866. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B.; Li, L.; Liu, Y.; Zheng, Q.; Shao, J.; Zhu, X.; Cai, W.; Liao, J.; Zou, L. The formation mechanism of titania nanotube arrays in hydrofluoric acid electrolyte. J. Mater. Sci. 2008, 43, 1880–1884. [Google Scholar] [CrossRef]
- Lai, Y.K.; Sun, L.; Chen, C.; Nie, C.G.; Zuo, J.; Lin, C.J. Optical and electrical characterization of TiO2 nanotube arrays on titanium substrate. Appl. Surf. Sci. 2005, 252, 1101–1106. [Google Scholar] [CrossRef]
- Zielinski, A.; Bartmanski, M. Electrodeposited biocoatings, their properties and fabrication technologies: A review. Coatings 2020, 10, 782. [Google Scholar] [CrossRef]
- Bigi, A.; Foresti, E.; Gandolfi, M.; Gazzano, M.; Roveri, N. Inhibiting effect of zinc on hydroxylapatite crystallization. J. Inorg. Biochem. 1995, 58, 49–58. [Google Scholar] [CrossRef]
- Kanzaki, N.; Onuma, K.; Treboux, G.; Tsutsumi, S.; Ito, A. Inhibitory effect of magnesium and zinc on crystallization kinetics of hydroxyapatite (0001) face. J. Phys. Chem. B 2000, 104, 4189–4194. [Google Scholar] [CrossRef]
- Kaseem, M.; Choe, H.-C. Triggering the hydroxyapatite deposition on the surface of PEO-coated Ti–6Al–4V alloy via the dual incorporation of Zn and Mg ions. J. Alloy. Compd. 2020, 819, 153038. [Google Scholar] [CrossRef]
- Mavropoulos, E.; Rossi, A.M.; da Rocha, N.C.C.; Soares, G.A.; Moreira, J.C.; Moure, G.T. Dissolution of calcium-deficient hydroxyapatite synthesized at different conditions. Mater. Charact. 2003, 50, 203–207. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. A review on the dissolution models of calcium apatites. Prog. Cryst. Growth Charact. Mater. 2002, 44, 45–61. [Google Scholar] [CrossRef]
- Lin, J.H.; Kuo, K.H.; Ding, S.J.; Ju, C.P. Surface reaction of stoichiometric and calcium-deficient hydroxyapatite in simulated body fluid. J. Mater. Sci. Mater. Med. 2001, 12, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, F.; Kono, Y.; Suyama, Y. Formation and structure of zinc-substituted calcium hydroxyapatite. Mater. Res. Bull. 2005, 40, 209–220. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Park, H.H.; Lee, M.H.; Bae, T.S.; Duncan, W.; Swain, M. The effect of annealing temperatures on surface properties, hydroxyapatite growth and cell behaviors of TiO2 nanotubes. Surf. Interface Anal. 2011, 43, 998–1005. [Google Scholar] [CrossRef]
- He, J.; Zhou, W.; Zhou, X.; Zhong, X.; Zhang, X.; Wan, P.; Zhu, B.; Chen, W. The anatase phase of nanotopography titania plays an important role on osteoblast cell morphology and proliferation. J. Mater. Sci. Mater. Med. 2008, 19, 3465–3472. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, J.; Tang, H.; Mao, D.; Wang, C.; Liu, R. TiO2 nanotube arrays induced deposition of hydroxyapatite coating by hydrothermal treatment. Mater. Chem. Phys. 2013, 138, 695–702. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Zhou, Z.; Guo, X.; Liu, Y.; Hou, W.; Li, H. In vitro bioactivity and antibacterial performances of atmospheric plasma sprayed C-axis preferential oriented hydroxyapatite coatings. Surf. Coat. Technol. 2021, 417, 127209. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Zhou, Z.; Wang, G.; Wang, Z.; Wu, X.; Tan, Z. Characteristics of (002) oriented hydroxyapatite coatings deposited by atmospheric plasma spraying. Coatings 2018, 8, 258. [Google Scholar] [CrossRef] [Green Version]
- Bi, Q.; Song, X.; Chen, Y.; Zheng, Y.; Yin, P.; Lei, T. Zn-HA/Bi-HA biphasic coatings on titanium: Fabrication, characterization, antibacterial and biological activity. Colloids Surf. B Biointerfaces 2020, 189, 110803. [Google Scholar] [CrossRef] [PubMed]
- Motameni, A.; Alshemary, A.Z.; Evis, Z. A review of synthesis methods, properties and use of monetite cements as filler for bone defects. Ceram. Int. 2021, 47, 13245–13256. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. Monetite, an important calcium phosphate compound—Its synthesis, properties and applications in orthopedics. Acta Biomaterialia 2021, 127, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Uysal, I.; Severcan, F.; Tezcaner, A.; Evis, Z. Co-doping of hydroxyapatite with zinc and fluoride improves mechanical and biological properties of hydroxyapatite. Prog. Nat. Sci. Mater. Int. 2014, 24, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Bulina, N.V.; Vinokurova, O.B.; Eremina, N.V.; Prosanov, I.Y.; Khusnutdinov, V.R.; Chaikina, M.V. Features of solid-phase mechanochemical synthesis of hydroxyapatite doped by copper and zinc ions. J. Solid State Chem. 2021, 296, 121973. [Google Scholar] [CrossRef]
- Grzesik, W. Prediction of the functional performance of machined components based on surface topography: State of the art. J. Mater. Eng. Perform. 2016, 25, 4460–4468. [Google Scholar] [CrossRef] [Green Version]
- Vranceanu, D.M.; Parau, A.C.; Cotrut, C.M.; Kiss, A.E.; Constantin, L.R.; Braic, V.; Vladescu, A. In vitro evaluation of Ag doped hydroxyapatite coatings in acellular media. Ceram. Int. 2019, 45, 11050–11061. [Google Scholar] [CrossRef]
- Monsees, T.K.; Azem, F.A.; Cotrut, C.M.; Braic, M.; Abdulgader, R.; Pana, I.; Birlik, I.; Kiss, A.; Booysen, R.; Vladescu, A. Biodegradable ceramics consisting of hydroxyapatite for orthopaedic implants. Coatings 2017, 7, 184. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Doyle, C.S.; Yonekura, D.; James, B.J. Effect of surface roughness parameters on thermally sprayed PEEK coatings. Surf. Coat. Technol. 2010, 204, 3567–3572. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, Q.; Han, S.; Yan, Y.; Pang, X. Characterisation, corrosion resistance and in vitro bioactivity of manganese-doped hydroxyapatite films electrodeposited on titanium. J. Mater. Sci. Mater. Med. 2013, 24, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H. Calcium phosphate-based coatings on titanium and its alloys. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2008, 85, 279–299. [Google Scholar] [CrossRef]
- Zhu, X.; Son, D.W.; Ong, J.L.; Kim, K. Characterization of hydrothermally treated anodic oxides containing Ca and P on titanium. J. Mater. Sci. Mater. Med. 2003, 14, 629–634. [Google Scholar] [CrossRef]
- Gopi, D.; Murugan, N.; Ramya, S.; Shinyjoy, E.; Kavitha, L. Ball flower like manganese, strontium substituted hydroxyapatite/cerium oxide dual coatings on the AZ91 Mg alloy with improved bioactive and corrosion resistance properties for implant applications. RSC Adv. 2015, 5, 27402–27411. [Google Scholar] [CrossRef]
- Dascălu, C.-A.; Maidaniuc, A.; Pandele, A.M.; Voicu, S.I.; Machedon-Pisu, T.; Stan, G.E.; Cîmpean, A.; Mitran, V.; Antoniac, I.V.; Miculescu, F. Synthesis and characterization of biocompatible polymer-ceramic film structures as favorable interface in guided bone regeneration. Appl. Surf. Sci. 2019, 494, 335–352. [Google Scholar] [CrossRef]
- Meejoo, S.; Maneeprakorn, W.; Winotai, P. Phase and thermal stability of nanocrystalline hydroxyapatite prepared via microwave heating. Thermochimica Acta 2006, 447, 115–120. [Google Scholar] [CrossRef]
- Gopi, D.; Murugan, N.; Ramya, S.; Kavitha, L. Electrodeposition of a porous strontium-substituted hydroxyapatite/zinc oxide duplex layer on AZ91 magnesium alloy for orthopedic applications. J. Mater. Chem. B 2014, 2, 5531–5540. [Google Scholar] [CrossRef]
- Gopi, D.; Karthika, A.; Rajeswari, D.; Kavitha, L.; Pramod, R.; Dwivedi, J. Investigation on corrosion protection and mechanical performance of minerals substituted hydroxyapatite coating on HELCDEB-treated titanium using pulsed electrodeposition method. RSC Adv. 2014, 4, 34751–34759. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, F.; Ciocoiu, R.C.; Butte, T.M.; Pasuk, I.; Stan, G.E.; Voicu, S.I.; Ciocan, L.T. Effect of the processing parameters on surface, physico-chemical and mechanical features of bioceramics synthesized from abundant carp fish bones. Ceram. Int. 2020, 46, 10159–10171. [Google Scholar] [CrossRef]
- Robinson, L.; Salma-Ancane, K.; Stipniece, L.; Meenan, B.J.; Boyd, A.R. The deposition of strontium and zinc co-substituted hydroxyapatite coatings. J. Mater. Sci. Mater. Med. 2017, 28, 51. [Google Scholar] [CrossRef] [PubMed]
- Parcharoen, Y.; Kajitvichyanukul, P.; Sirivisoot, S.; Termsuksawad, P. Hydroxyapatite electrodeposition on anodized titanium nanotubes for orthopedic applications. Appl. Surf. Sci. 2014, 311, 54–61. [Google Scholar] [CrossRef]
- Dey, T.; Roy, P.; Fabry, B.; Schmuki, P. Anodic mesoporous TiO2 layer on Ti for enhanced formation of biomimetic hydroxyapatite. Acta Biomaterialia 2011, 7, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Park, J.; Faltenbacher, J.; Berger, S.; von der Mark, K.; Schmuki, P. Size selective behavior of mesenchymal stem cells on ZrO2 and TiO2 nanotube arrays. Integr. Biol. 2009, 1, 525–532. [Google Scholar] [CrossRef]




| Sample Codification | Chemical Composition (mM) | Ratio (Ca + Zn)/P | ||
|---|---|---|---|---|
| Ca(NO3)2∙4H2O | NH4H2PO4 | Zn(NO3)2∙6H2O | ||
| H | 10 | 6 mM | - | Ca/P = 1.67 |
| H-Zn1 | 9.985 | 0.015 | (Ca + Zn)/P = 1.67 | |
| H-Zn2 | 8.980 | 0.020 | ||
| Material | Elemental Composition | Ca/P Ratio | (Ca + Zn)/P Ratio | |||||
|---|---|---|---|---|---|---|---|---|
| Ca | P | Zn | ||||||
| (at.%) | (wt.%) | (at.%) | (wt.%) | (at.%) | (wt.%) | |||
| H | 61.46 | 67.36 | 38.54 | 32.64 | - | - | 1.59 | 1.59 |
| H-Zn1 | 60.21 | 65.71 | 39.01 | 32.90 | 0.78 | 1.39 | 1.54 | 1.56 |
| H-Zn2 | 59.54 | 64.77 | 39.16 | 32.93 | 1.30 | 2.30 | 1.52 | 1.55 |
| Material | a = b (Å) | c (Å) | 2θ (002) (°) | Crystallite Dimension (nm) | Crystallinity (%) |
|---|---|---|---|---|---|
| HAp, ICDD #09-0432 | 9.418 | 6.884 | 25.88 | - | - |
| H | 9.439 | 6.873 | 26.04 | 19.96 | 20.90 |
| H-Zn1 | 9.421 | 6.860 | 26.12 | 25.60 | 44.30 |
| H-Zn2 | 9.422 | 6.852 | 26.14 | 25.47 | 43.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vranceanu, D.M.; Ungureanu, E.; Ionescu, I.C.; Parau, A.C.; Kiss, A.E.; Vladescu, A.; Cotrut, C.M. Electrochemical Surface Biofunctionalization of Titanium through Growth of TiO2 Nanotubes and Deposition of Zn Doped Hydroxyapatite. Coatings 2022, 12, 69. https://doi.org/10.3390/coatings12010069
Vranceanu DM, Ungureanu E, Ionescu IC, Parau AC, Kiss AE, Vladescu A, Cotrut CM. Electrochemical Surface Biofunctionalization of Titanium through Growth of TiO2 Nanotubes and Deposition of Zn Doped Hydroxyapatite. Coatings. 2022; 12(1):69. https://doi.org/10.3390/coatings12010069
Chicago/Turabian StyleVranceanu, Diana Maria, Elena Ungureanu, Ionut Cornel Ionescu, Anca Constantina Parau, Adrian Emil Kiss, Alina Vladescu, and Cosmin Mihai Cotrut. 2022. "Electrochemical Surface Biofunctionalization of Titanium through Growth of TiO2 Nanotubes and Deposition of Zn Doped Hydroxyapatite" Coatings 12, no. 1: 69. https://doi.org/10.3390/coatings12010069
APA StyleVranceanu, D. M., Ungureanu, E., Ionescu, I. C., Parau, A. C., Kiss, A. E., Vladescu, A., & Cotrut, C. M. (2022). Electrochemical Surface Biofunctionalization of Titanium through Growth of TiO2 Nanotubes and Deposition of Zn Doped Hydroxyapatite. Coatings, 12(1), 69. https://doi.org/10.3390/coatings12010069






.jpg)


