Push-Pull Heterocyclic Dyes Based on Pyrrole and Thiophene: Synthesis and Evaluation of Their Optical, Redox and Photovoltaic Properties
Abstract
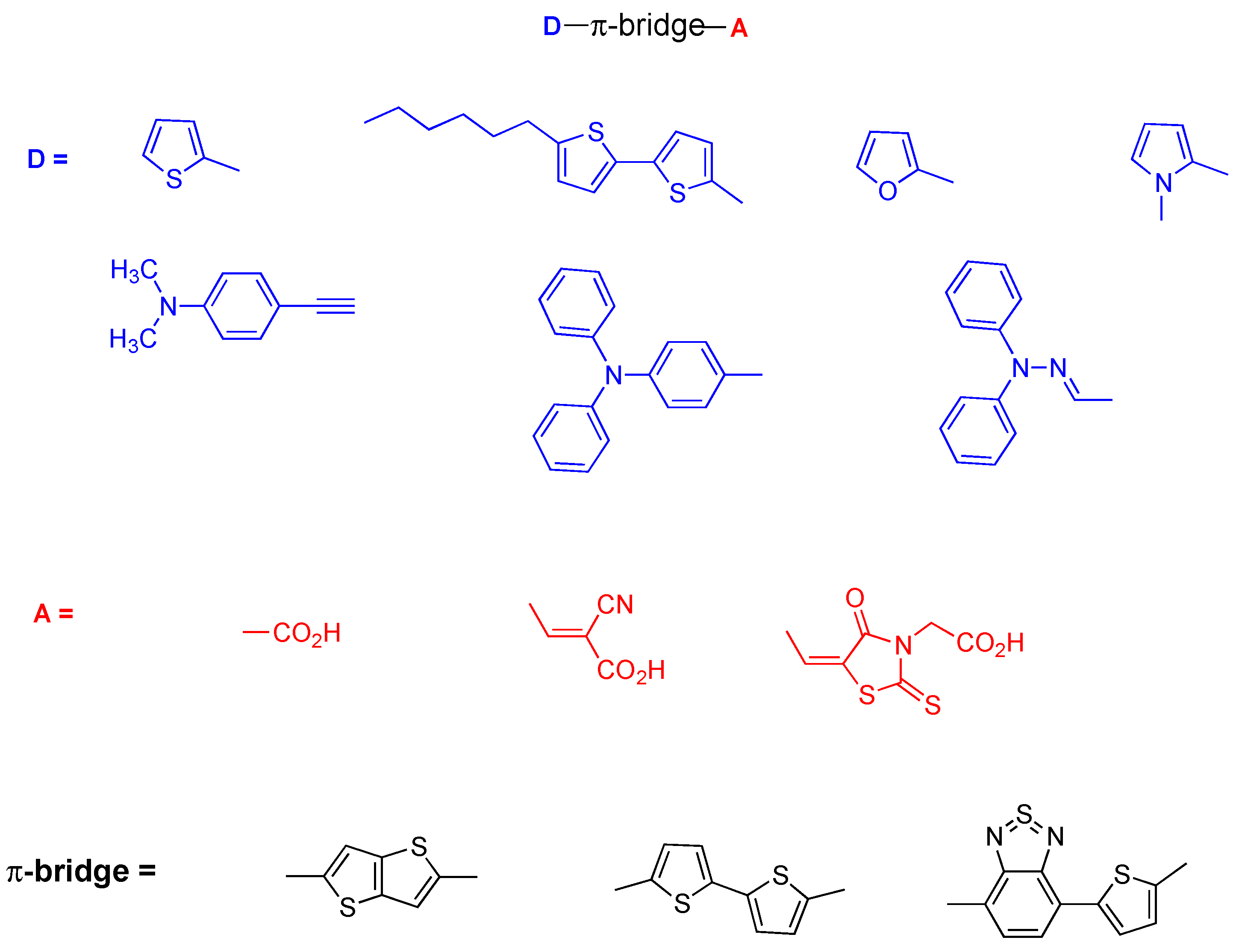
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis
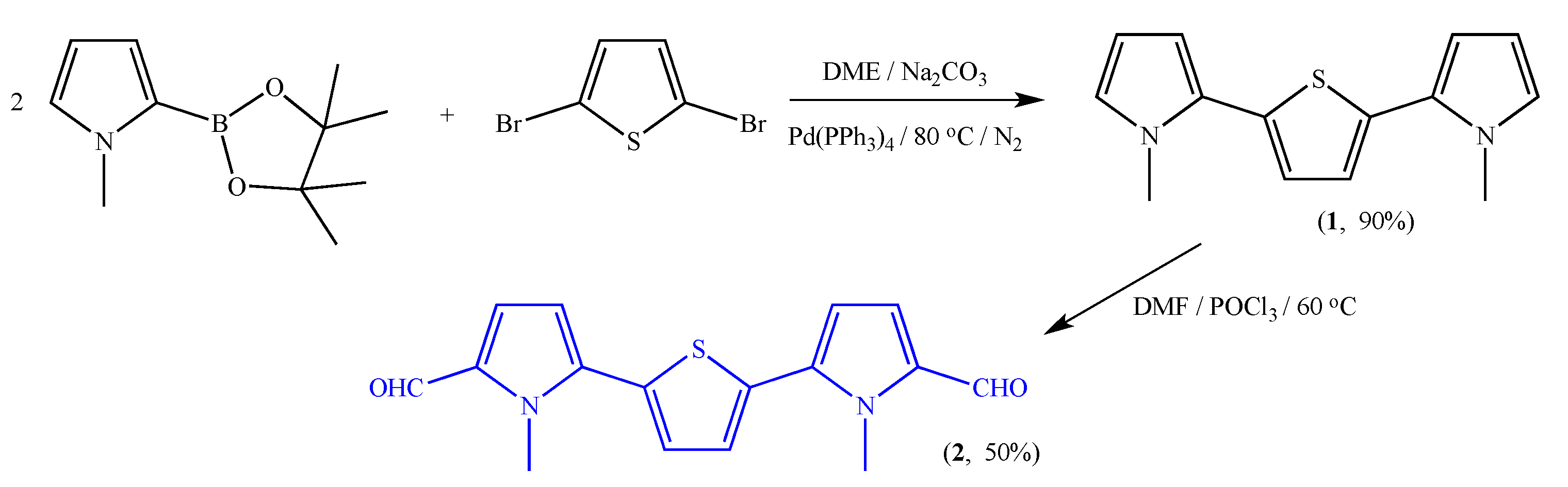
2.2.1. General Procedure for the Synthesis of Precursor 1 through Suzuki-Miyaura Cross-Coupling
2.2.2. General Procedure for the Synthesis of Aldehyde 2 by Vilsmeier-Haack Formylation
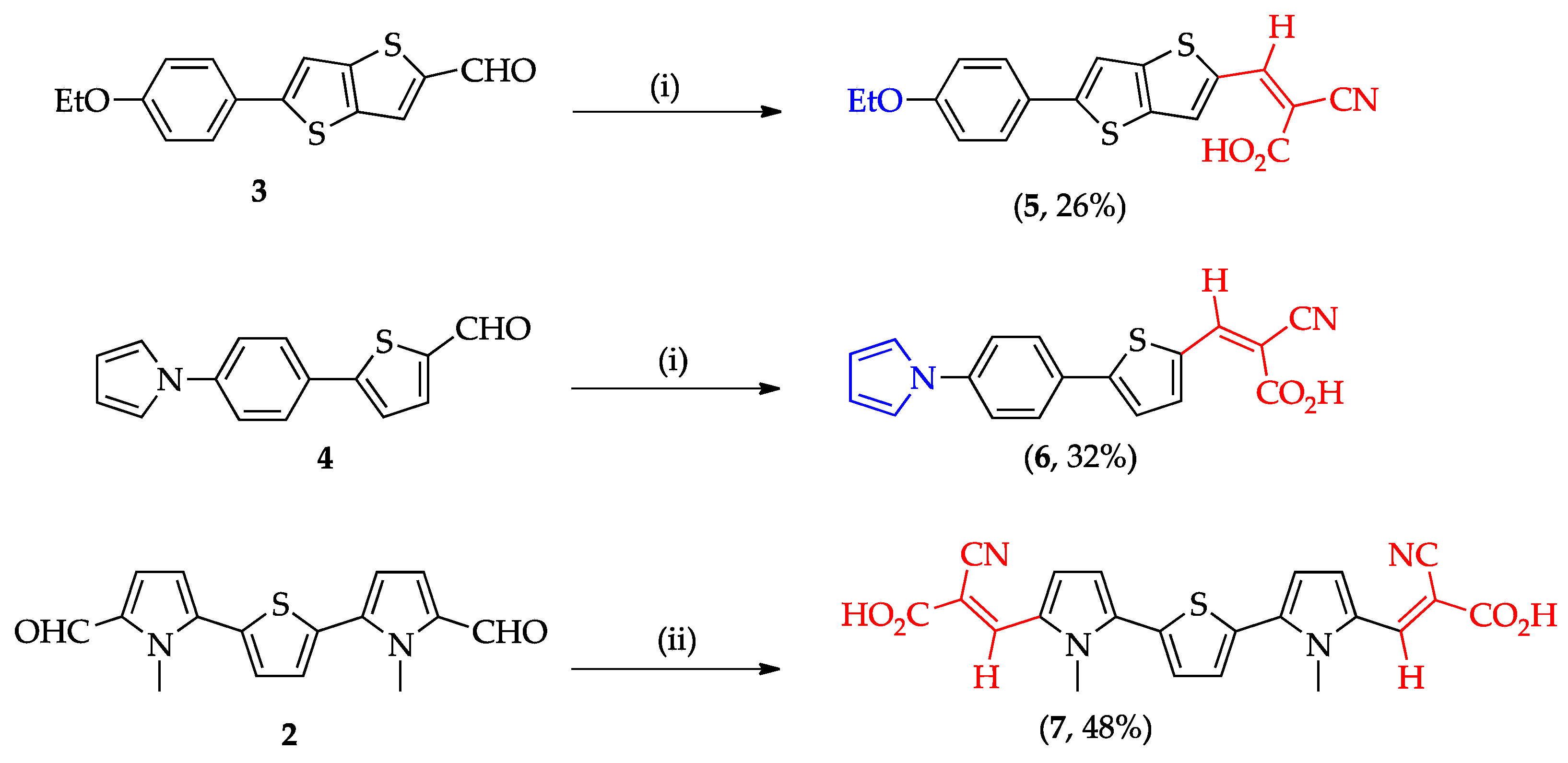
2.2.3. General Procedure for the Synthesis of Dyes 5–7 by Knoevenagel Condensation of Aldehydes 2–4 with 2-Cyanoacetic Acid
2.3. Preparation and Characterization of DSSCs
3. Results and Discussion
3.1. Design, Synthesis and Characterization of Dyes 5–7 and for the Aldehyde Precursor 2
3.2. Electrochemical Study
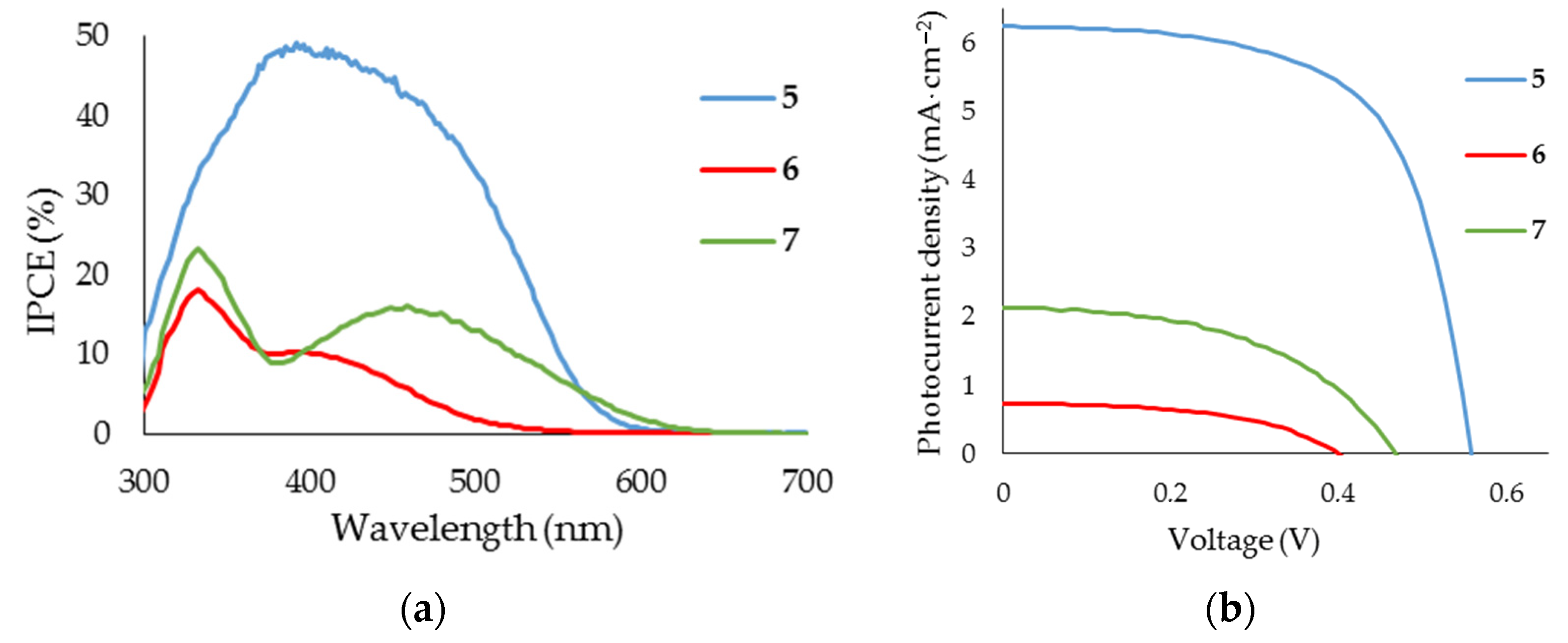
3.3. Evaluation of DSSCs Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grätzel, M. Conversion of sunlight to electric power by nanocrystalline dye-sensitized solar cells. J. Photochem. Photobiol. A 2004, 164, 3–14. [Google Scholar] [CrossRef]
- Gonçalves, L.M.; Bermudez, V.Z.; Ribeiro, H.A.; Mendes, A.M. Dye-sensitized solar cells: A safe bet for the future. Energy Environ. Sci. 2008, 1, 655–667. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-García, A.B.; Benesperi, I.; Boschloo, G.; Concepcion, J.J.; Delcamp, J.H.; Gibson, E.A.; Meyer, G.J.; Pavone, M.; Pettersson, H.; Hagfeldt, A.; et al. Dye-sensitized solar cells strike back. Chem. Soc. Rev. 2021, 50, 12450–12550. [Google Scholar] [CrossRef]
- Zhang, D.; Stojanovic, M.; Ren, Y.; Cao, Y.; Eickemeyer, F.T.; Socie, E.; Vlachopoulos, N.; Moser, J.E.; Zakeeruddin, S.M.; Hagfeldt, A.; et al. A molecular photosensitizer achieves a Voc of 1.24 V enabling highly efficient and stable dye-sensitized solar cells with copper(II/I)-based electrolyte. Nat. Commun. 2021, 12, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Dunlop, E.D.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (Version 58). Prog. Photovolt. Res. Appl. 2021, 29, 657–667. [Google Scholar] [CrossRef]
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced research trends in dye-sensitized solar cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef] [PubMed]
- Robertson, N. Optimizing dyes for dye-sensitized solar cells. Angew. Chem. Int. Ed. 2006, 45, 2338–2345. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-free organic dyes for dye-sensitized solar cells: From structure: Property relationships to design rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef] [PubMed]
- Ooyama, Y.; Harima, Y. Molecular designs and synthesis of organic dyes for dye-sensitized solar cells. Eur. J. Org. Chem. 2009, 18, 2903–2934. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Ning, Z.; Fu, Y.; Tian, H. Improvement of dye-sensitized solar cells: What we know and what we need to know. Energy Environ. Sci. 2010, 3, 1170–1181. [Google Scholar] [CrossRef]
- Clifford, J.N.; Martinez-Ferrero, E.; Viterisi, A.; Palomares, E. Sensitizer molecular structure-device efficiency relationship in dye sensitized solar cells. Chem. Soc. Rev. 2011, 40, 1635–1646. [Google Scholar] [CrossRef]
- Ooyama, Y.; Harima, Y. Photophysical and electrochemical properties, and molecular structures of organic dyes for dye-sensitized solar cells. ChemPhysChem 2012, 13, 4032–4080. [Google Scholar] [CrossRef]
- Yen, Y.-S.; Chou, H.-H.; Chen, Y.-C.; Hsu, C.-Y.; Lin, J.T. Recent developments in molecule-based organic materials for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 8734–8747. [Google Scholar] [CrossRef]
- Kanaparthi, R.K.; Kandhadi, J.; Giribabu, L. Metal-free organic dyes for dye-sensitized solar cells: Recent advances. Tetrahedron 2012, 68, 8383–8393. [Google Scholar] [CrossRef]
- Kim, B.-G.; Chung, K.; Kim, J. Molecular design principle of all-organic dyes for dye-sensitized solar cells. Chem. Eur. J. 2013, 19, 5220–5230. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhu, W. Organic sensitizers from D-π-A to D-A-π-A: Effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances. Chem. Soc. Rev. 2013, 42, 2039–2058. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Guillen, E.; Kavan, L.; Grätzel, M.; Nazeeruddin, M.K. Metal free sensitizer and catalyst for dye sensitized solar cells. Energy Environ. Sci. 2013, 6, 3439–3466. [Google Scholar] [CrossRef]
- Sugathan, V.; John, E.; Sudhakar, K. Recent improvements in dye sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2015, 52, 54–64. [Google Scholar] [CrossRef]
- Obotowo, I.N.; Obot, I.B.; Ekpe, U.J. Organic sensitizers for dye-sensitized solar cell (DSSC): Properties from computation, progress and future perspectives. J. Mol. Struct. 2016, 1122, 80–87. [Google Scholar] [CrossRef]
- Maçaira, J.; Andrade, L.; Mendes, A. Modeling, simulation and design of dye sensitized solar cells. RSC Adv. 2014, 4, 2830–2844. [Google Scholar] [CrossRef] [Green Version]
- Chaurasia, S.; Lin, J.T. Metal-free sensitizers for dye-sensitized solar cells. Chem. Rec. 2016, 16, 1311–1336. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.; Harris, N. Powering the environmental internet of things. Sensors 2019, 19, 1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishu, M.K.; Rokonuzzaman, M.; Pasupuleti, J.; Shakeri, M.; Rahman, K.S.; Hamid, F.A.; Tiong, S.K.; Amin, N. Prospective efficient ambient energy harvesting sources for IoT-equipped sensor applications. Electronics 2020, 9, 1345. [Google Scholar] [CrossRef]
- Michaels, H.; Rinderle, M.; Freitag, R.; Benesperi, I.; Edvinsson, T.; Socher, R.; Gagliardi, A.; Freitag, M. Dye-sensitized solar cells under ambient light powering machine learning: Towards autonomous smart sensors for the Internet of things. Chem. Sci. 2020, 11, 2895–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslam, A.; Mehmood, U.; Arshad, M.H.; Ishfaq, A.; Zaheer, J.; Ul Haq Khan, A.; Sufyan, M. Dye-sensitized solar cells (DSSCs) as a potential photovoltaic technology for the self-powered internet of things (IoTs) applications. Sol. Energy 2020, 207, 874–892. [Google Scholar] [CrossRef]
- Mathews, I.; Kantareddy, S.N.; Buonassisi, T.; Peters, I.M. Technology and market perspective for indoor photovoltaic cells. Joule 2019, 3, 1415–1426. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Grätzel, M. Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)–based redox electrolyte exceed 12% efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Bignozzi, C.A.; Argazzi, R.; Boaretto, R.; Busatto, E.; Carli, S.; Ronconi, F.; Caramori, S. The role of transition metal complexes in dye sensitized solar devices. Coord. Chem. Rev. 2013, 257, 1472–1492. [Google Scholar] [CrossRef]
- Pashaei, B.; Shahroosvand, H.; Grätzel, M.; Nazeeruddin, M.K. Influence of ancillary ligands in dye-sensitized solar cells. Chem. Rev. 2016, 116, 9485–9564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cole, J.M. Anchoring groups for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Chang, D.M.; Kim, Y.S. Effect of electron withdrawing unit for dye-sensitized solar cell based on D-A-π-A organic dyes. Mater. Res. Bull. 2014, 58, 93–96. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Wang, B.; Li, X.; Liu, Q.; Naruta, Y.; Zhu, W. Influence of different anchoring groups in indoline dyes for dye-sensitized solar cells: Electron injection, impedance and charge recombination. J. Power Sources 2013, 234, 139–146. [Google Scholar] [CrossRef]
- Bureš, F. Fundamental aspects of property tuning in push-pull molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef] [Green Version]
- Albert, I.D.L.; Marks, T.J.; Ratner, M.A. Large molecular hyperpolarizabilities: Quantitative analysis of aromaticity and auxiliary donor-acceptor effects. J. Am. Chem. Soc. 1997, 119, 6575–6582. [Google Scholar] [CrossRef]
- Raposo, M.M.M.; Sousa, A.M.R.C.; Kirsch, G.; Cardoso, P.; Belsley, M.; de Matos Gomes, E.; Fonseca, A.M.C. Synthesis and characterization of dicyanovinyl-substituted thienylpyrroles as new nonlinear optical chromophores. Org. Lett. 2006, 8, 3681–3684. [Google Scholar] [CrossRef] [PubMed]
- Pina, J.; Seixas de Melo, J.S.; Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M. The influence of the relative position of the thiophene and pyrrole rings in donor-acceptor thienylpyrrolyl-benzothiazole derivatives. A photophysical and theoretical investigation. Phys. Chem. Chem. Phys. 2010, 12, 9719–9725. [Google Scholar] [CrossRef] [Green Version]
- Castro, M.C.R.; Belsley, M.; Fonseca, A.M.C.; Raposo, M.M.M. Synthesis and characterization of novel second-order NLO-chromophores bearing pyrrole as an electron donor group. Tetrahedron 2012, 68, 8147–8155. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Amóros, J.; Reig, M.; Castro, M.C.R.; Cuadrado, A.; Raposo, M.M.M.; Velasco, D. Molecular photo-oscillators based on highly accelerated heterocyclic azo dyes in nematic liquid crystals. Chem. Commun. 2014, 50, 6704–6706. [Google Scholar] [CrossRef] [Green Version]
- Castro, M.C.R.; Belsley, M.; Raposo, M.M.M. Synthesis and characterization of push–pull bithienylpyrrole NLOphores with enhanced hyperpolarizabilities. Dyes Pigments 2016, 131, 333–339. [Google Scholar] [CrossRef]
- Castro, M.C.R.; Belsley, M.; Raposo, M.M.M. Push–pull second harmonic generation chromophores bearing pyrrole and thiazole heterocycles functionalized with several acceptor moieties: Syntheses and characterization. Dyes Pigments 2016, 128, 89–95. [Google Scholar] [CrossRef]
- Fernandes, S.S.M.; Belsley, M.; Ciarrocchi, C.; Licchelli, M.; Raposo, M.M.M. Terpyridine derivatives functionalized with (hetero)aromatic groups and the corresponding Ru complexes: Synthesis and characterization as SHG chromophores. Dyes Pigments 2018, 150, 49–58. [Google Scholar] [CrossRef]
- Fernandes, S.S.M.; Castro, M.C.R.; Mesquita, I.; Andrade, L.; Mendes, A.; Raposo, M.M.M. Synthesis and characterization of novel thieno[3,2-b]thiophene based metal-free organic dyes with different heteroaromatic donor moieties as sensitizers for dye-sensitized solar cells. Dyes Pigments 2017, 136, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, S.S.M.; Mesquita, I.; Andrade, L.; Mendes, A.; Justino, L.L.G.; Burrows, H.D.; Raposo, M.M.M. Synthesis and characterization of push-pull bithiophene and thieno[3,2-b]thiophene derivatives bearing an ethyne linker as sensitizers for dye-sensitized solar cells. Org. Electron. 2017, 49, 194–205. [Google Scholar] [CrossRef]
- Fernandes, S.S.M.; Castro, M.C.R.; Pereira, A.I.; Mendes, A.; Serpa, C.; Pina, J.; Justino, L.L.G.; Burrows, H.D.; Raposo, M.M.M. Optical and photovoltaic properties of thieno[3,2-b]thiophene-based push–pull organic dyes with different anchoring groups for dye-sensitized solar cells. ACS Omega 2017, 2, 9268–9279. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Pereira, A.; Ivanou, D.; Mendes, A.; Raposo, M. Benzothiadiazole derivatives functionalized with two different (hetero)aromatic donor groups: Synthesis and evaluation as TiO2 sensitizers for DSSCs. Dyes Pigments 2018, 151, 89–94. [Google Scholar] [CrossRef]
- Fernandes, S.S.M.; Belsley, M.; Pereira, A.; Ivanou, D.; Mendes, A.; Justino, L.L.G.; Burrows, H.D.; Raposo, M.M.M. Push-pull N,N-diphenylhydrazones bearing bithiophene or thienothiophene spacers as NLO second harmonic generators and as photosensitizers for nanocrystalline TiO2 DSSCs. ACS Omega 2018, 3, 12893–12904. [Google Scholar] [CrossRef] [Green Version]
- Abbotto, A.; Beverina, L.; Manfredi, N.; Pagani, G.A.; Archetti, G.; Kuball, H.-G.; Wittenburg, C.; Heck, J.; Holtmann, J. Second-order nonlinear optical activity of dipolar chromophores based on pyrrole-hydrazono donor moieties. Chem. Eur. J. 2009, 15, 6175–6185. [Google Scholar] [CrossRef] [PubMed]
- Herbivo, C.; Comel, A.; Kirsch, G.; Fonseca, A.M.C.; Belsley, M.; Raposo, M.M.M. Synthesis and characterization of novel, thermally sTable 2-aryl-5-dicyanovinylthiophenes and 5-aryl-5′-dicyanovinyl-2,2′-bithiophenes as potentially promising non-linear optical materials. Dyes Pigments 2010, 86, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, S.S.M.; Herbivo, C.; Aires-de-Sousa, J.; Comel, A.; Belsley, M.; Raposo, M.M.M. Theoretical and experimental studies of aryl-bithiophene based push-pull π-conjugated heterocyclic systems bearing cyanoacetic or rhodanine-3-acetic acid acceptors for SHG nonlinear optical applications. Dyes Pigments 2018, 149, 566–573. [Google Scholar] [CrossRef]
- Dalton, L.R.; Sullivan, P.A.; Bale, D.H. Electric field poled organic electro-optic materials: State of the art and future prospects. Chem. Rev. 2010, 110, 25–55. [Google Scholar] [CrossRef]
- Ohmori, Y. Development of organic light-emitting diodes for electro-optical integrated devices. Laser Photon. Rev. 2010, 4, 300–310. [Google Scholar] [CrossRef]
- Allard, S.; Forster, M.; Souharce, B.; Thiem, H.; Scherf, U. Organic semiconductors for solution-processable field-effect transistors (OFETs). Angew. Chem. Int. Ed. 2008, 47, 4070–4098. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Wang, Z.Y. Near-infrared organic compounds and emerging applications. Chem. Asian J. 2010, 5, 1006–1029. [Google Scholar] [CrossRef]
- Cinar, M.E.; Ozturk, T. Thienothiophenes, dithienothiophenes, and thienoacenes: Syntheses, oligomers, polymers, and properties. Chem. Rev. 2015, 115, 3036–3140. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-W.; Kim, J.-Y.; Lee, D.-H.; Ko, M.J. Novel D-π-A organic dyes with thieno[3,2-b]thiophene-3,4-ethylenedioxythiophene unit as a π-bridge for highly efficient dye-sensitized solar cells with long-term stability. ACS Appl. Mater. Interfaces 2014, 6, 4102–4108. [Google Scholar] [CrossRef]
- Liu, H.; Wu, F.; Zhao, B.; Meng, L.; Wang, G.; Zhang, J.; Shen, P.; Tan, S. Synthesis and photovoltaic properties of the acceptor pended push–pull conjugated polymers incorporating thieno[3,2–b] thiophene in the backbone chain or side chains. Dyes Pigments 2015, 120, 44–51. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Otsuka, T.; Kyomen, T.; Unno, M.; Hanaya, M. An achievement of over 12 percent efficiency in an organic dye-sensitized solar cell. Chem. Commun. 2014, 50, 6379–6381. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Yen, Y.-S.; Hsu, Y.-C.; Lin, J.T.; Chang, C.-W.; Hsu, C.-P.; Yin, D.-J. Pyrrole-based organic dyes for dye-sensitized solar cells. J. Phys. Chem. C 2008, 112, 12557–12567. [Google Scholar] [CrossRef]
- Li, Q.; Lu, L.; Zhong, C.; Huang, J.; Huang, Q.; Shi, J.; Jin, X.; Peng, T.; Qin, J.; Li, Z. New pyrrole-based organic dyes for dye-sensitized solar cells: Convenient synthesis and high efficiency. Chem. Eur. J. 2009, 15, 9664–9668. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lu, L.; Zhong, C.; Shi, J.; Huang, Q.; Jin, X.; Peng, T.; Qin, J.; Li, Z. New indole-based metal-free organic dyes for dye-sensitized solar cells. J. Phys. Chem. B 2009, 113, 14588–14595. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Roy, M.S.; Sharma, G.D. Synthesis of new low band gap dyes with BF2–azopyrrole complex and their use for dye-sensitized solar cells. J. Power Sources 2010, 195, 5391–5398. [Google Scholar] [CrossRef]
- Li, Q.; Shi, J.; Li, H.; Li, S.; Zhong, C.; Guo, F.; Peng, M.; Hua, J.; Qin, J.; Li, Z. Novel pyrrole-based dyes for dye-sensitized solar cells: From rod-shape to “H” type. J. Mater. Chem. 2012, 22, 6689–6696. [Google Scholar] [CrossRef]
- Li, H.; Yang, L.; Tang, R.; Hou, Y.; Yang, Y.; Wang, H.; Han, H.; Qin, J.; Li, Q.; Li, Z. Organic dyes incorporating N-functionalized pyrrole as conjugated bridge for dye-sensitized solar cells: Convenient synthesis, additional withdrawing group on the π-bridge and the suppressed aggregation. Dyes Pigments 2013, 99, 863–870. [Google Scholar] [CrossRef]
- Kim, B.H.; Freeman, H.S. New N-methyl pyrrole and thiophene based D–π–A systems for dye-sensitized solar cells. Dyes Pigments 2013, 96, 313–318. [Google Scholar] [CrossRef]
- Tejada, R.P.; Pelleja, L.; Palomares, E.; Franco, S.; Orduna, J.; Garin, J.; Andreu, R. Novel 4H-pyranylidene organic dyes for dye-sensitized solar cells: Effect of different heteroaromatic rings on the photovoltaic properties. Org. Electron. 2014, 15, 3237–3250. [Google Scholar] [CrossRef]
- Manfredi, N.; Cecconi, B.; Abbotto, A. Multi-branched multi-anchoring metal-free dyes for dye-sensitized solar cells. Eur. J. Org. Chem. 2014, 2014, 7069–7086. [Google Scholar] [CrossRef]
- Raposo, M.M.M.; Herbivo, C.; Hugues, V.; Clermont, G.; Castro, M.C.R.; Comel, A.; Blanchard-Desce, M. Synthesis, fluorescence, and two-photon absorption properties of push-pull 5-arylthieno[3,2-b]thiophene derivatives. Eur. J. Org. Chem. 2016, 31, 5263–5273. [Google Scholar] [CrossRef] [Green Version]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef]
- Hallensleben, M.L.; van Hooren, M.; Peters, M. Alternating copolymers based on 1-methylpyrrole and thiophene—Synthesis and electrochemical studies. Polymer Bull. 1998, 40, 167–172. [Google Scholar] [CrossRef]
- Raposo, M.M.M.; Fonseca, A.M.C.; Castro, M.C.R.; Belsley, M.; Cardoso, M.F.S.; Carvalho, L.M.; Coelho, P.J. Synthesis and characterization of novel diazenes bearing pyrrole, thiophene and thiazole heterocycles as efficient photochromic and nonlinear optical (NLO) materials. Dyes Pigments 2011, 91, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Leonat, L.; Sbârcea, G.; Branzoi, I.V. Cyclic voltammetry for energy levels estimation of organic materials. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2013, 75, 111–118. [Google Scholar]
- Khalid, M.; Hussain, R.; Hussain, A.; Ali, B.; Jaleel, F.; Imran, M.; Assiri, M.A.; Khan, M.U.; Ahmed, S.; Abid, S.; et al. Electron donor and acceptor influence on the nonlinear optical response of diacetylene-functionalized organic materials (DFOMs): Density functional theory calculations. Molecules 2019, 24, 2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagfeldt, A.; Grätzel, M. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]
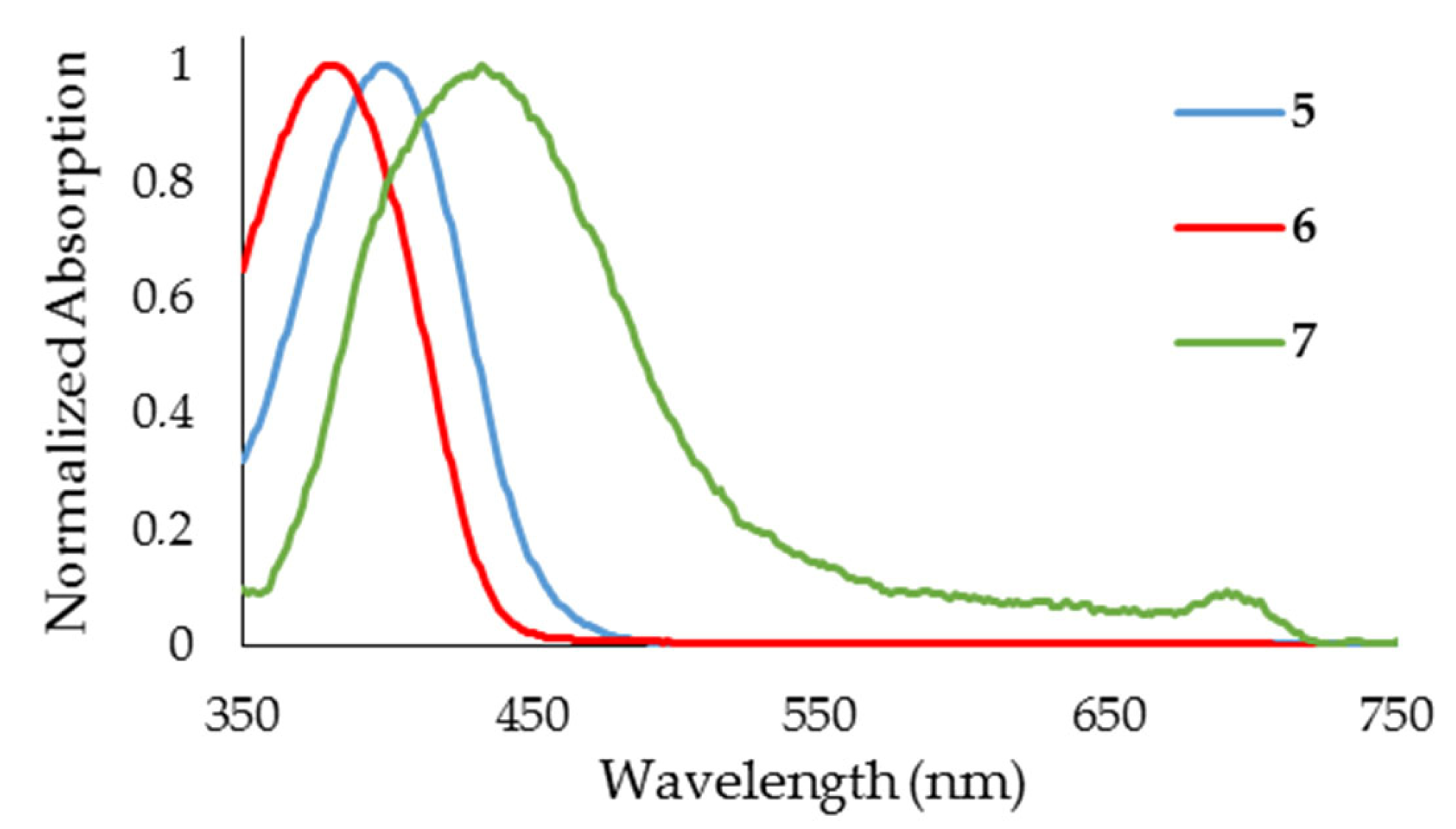
| Dye | Yield (%) | UV-Vis 1 | 1H NMR 2 | |
|---|---|---|---|---|
| λmax (nm) | ε (M−1cm−1) | Hvinylic (ppm) | ||
| 5 | 20 | 399 | 31,370 | 8.11 |
| 6 | 32 | 379 | 26,858 | 8.07 |
| 7 | 48 | 432 | 17,517 | 8.00 |
| Dye | Reduction 1 (V) | Oxidation 1 (V) | EHOMO 2 (eV) | ELUMO 2 (eV) | Band Gap 3 (eV) |
|---|---|---|---|---|---|
| 5 | −1.65 | 0.83 | −5.22 | −2.74 | 2.48 |
| 6 | −1.96 | 0.53 | −4.92 | −2.43 | 2.49 |
| 7 | −1.91 | 0.25 | −4.64 | −2.48 | 2.16 |
| Dye | VOC (V) | JSC (mA·cm−2) | FF (%) | η (%) |
|---|---|---|---|---|
| 5 | 0.56 | 6.24 | 0.63 | 2.21 |
| 6 | 0.37 | 0.64 | 0.50 | 0.13 |
| 7 | 0.44 | 1.74 | 0.49 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, S.S.M.; Castro, M.C.R.; Ivanou, D.; Mendes, A.; Raposo, M.M.M. Push-Pull Heterocyclic Dyes Based on Pyrrole and Thiophene: Synthesis and Evaluation of Their Optical, Redox and Photovoltaic Properties. Coatings 2022, 12, 34. https://doi.org/10.3390/coatings12010034
Fernandes SSM, Castro MCR, Ivanou D, Mendes A, Raposo MMM. Push-Pull Heterocyclic Dyes Based on Pyrrole and Thiophene: Synthesis and Evaluation of Their Optical, Redox and Photovoltaic Properties. Coatings. 2022; 12(1):34. https://doi.org/10.3390/coatings12010034
Chicago/Turabian StyleFernandes, Sara S. M., Maria Cidália R. Castro, Dzmitry Ivanou, Adélio Mendes, and Maria Manuela M. Raposo. 2022. "Push-Pull Heterocyclic Dyes Based on Pyrrole and Thiophene: Synthesis and Evaluation of Their Optical, Redox and Photovoltaic Properties" Coatings 12, no. 1: 34. https://doi.org/10.3390/coatings12010034
APA StyleFernandes, S. S. M., Castro, M. C. R., Ivanou, D., Mendes, A., & Raposo, M. M. M. (2022). Push-Pull Heterocyclic Dyes Based on Pyrrole and Thiophene: Synthesis and Evaluation of Their Optical, Redox and Photovoltaic Properties. Coatings, 12(1), 34. https://doi.org/10.3390/coatings12010034