Three-Dimensional Carbon-Coated LiFePO4 Cathode with Improved Li-Ion Battery Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Characterization
2.3. Electrochemical Measurements
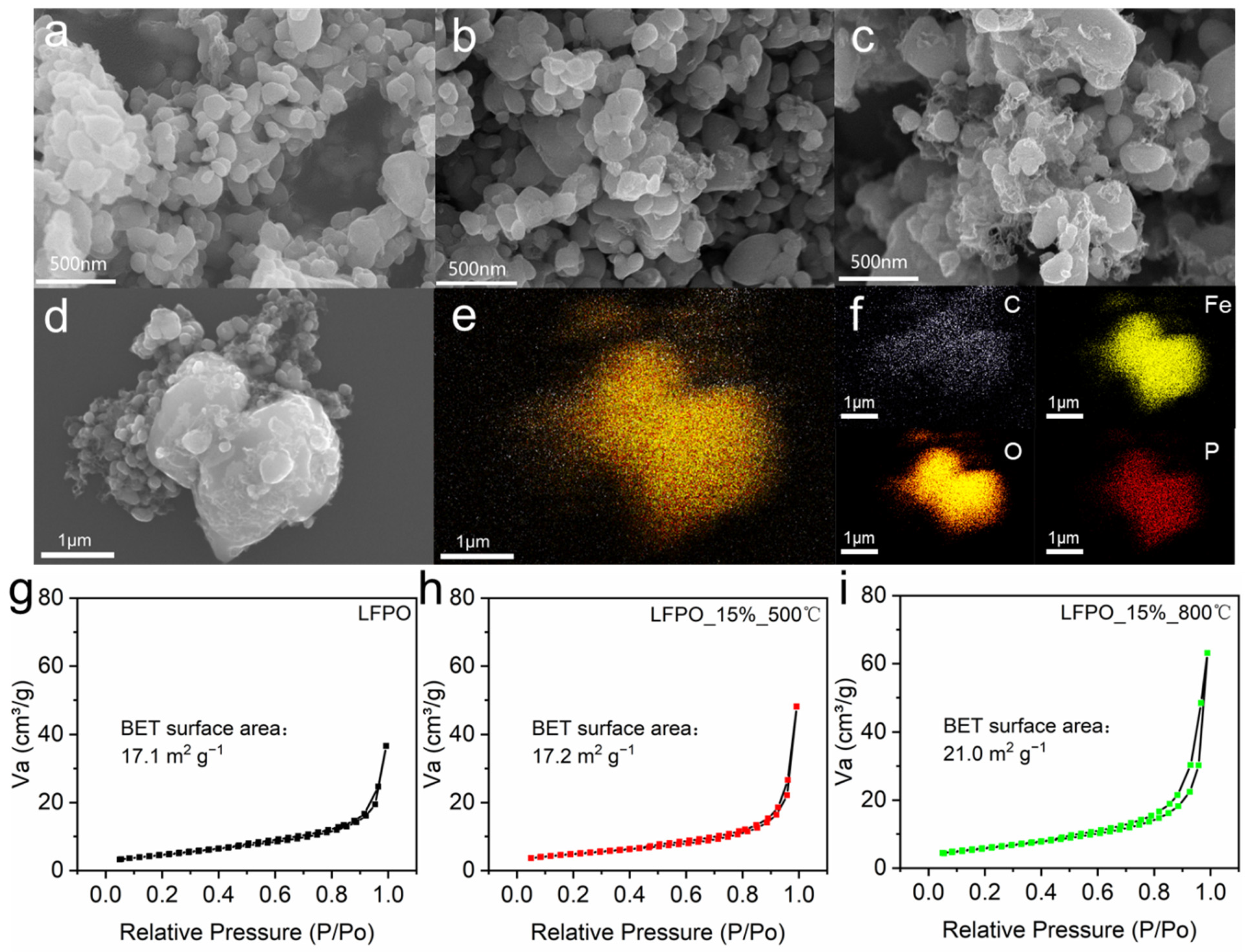
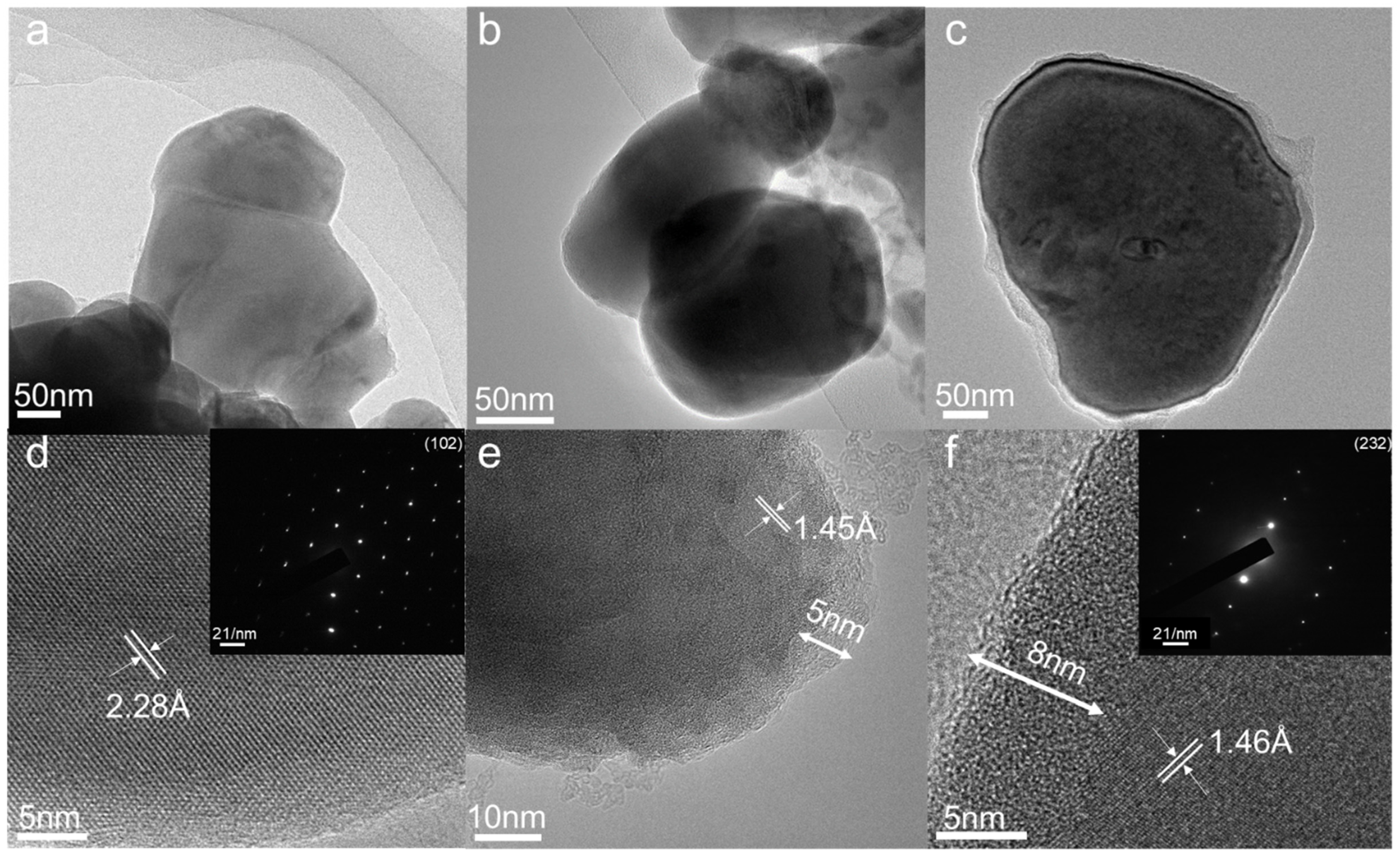
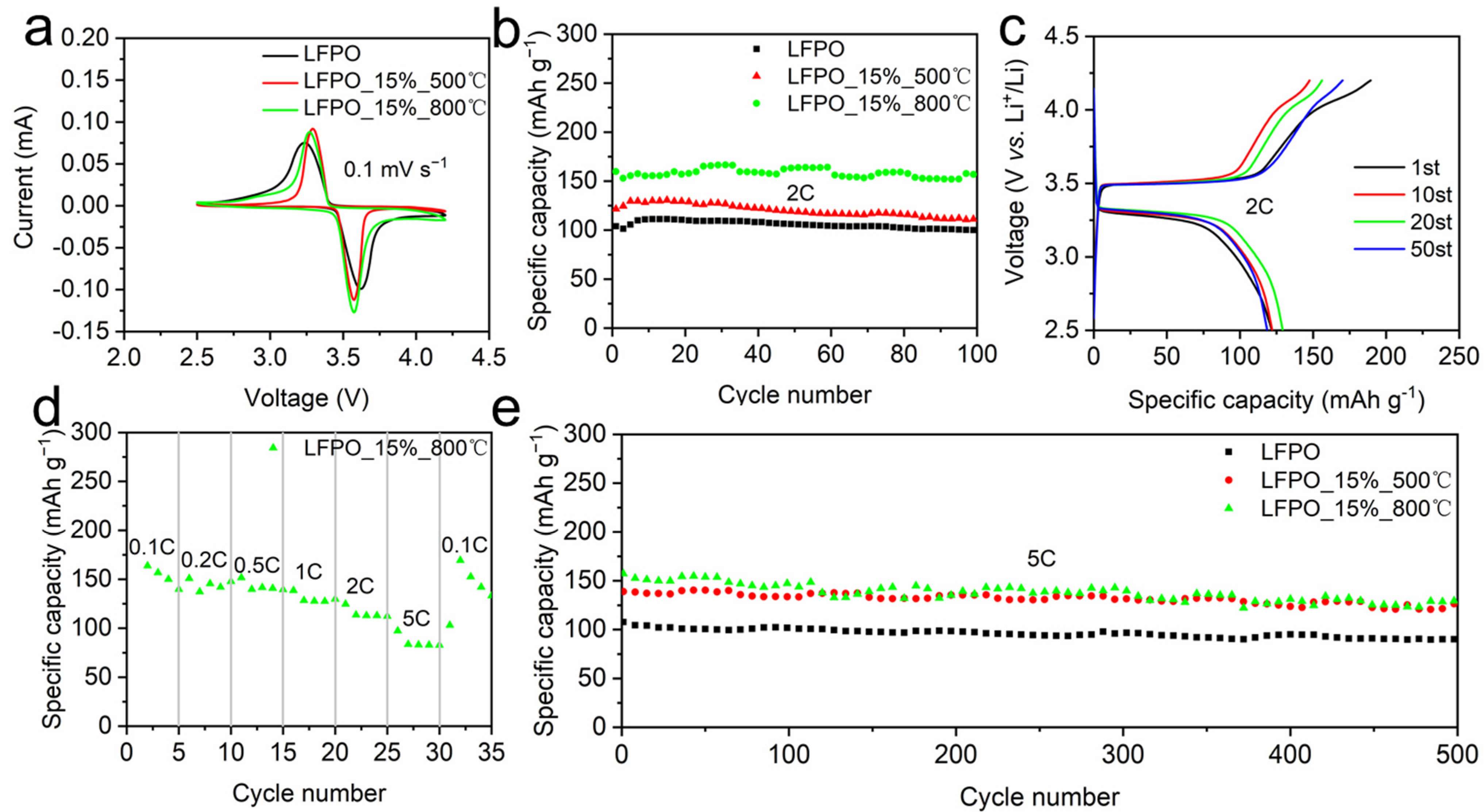
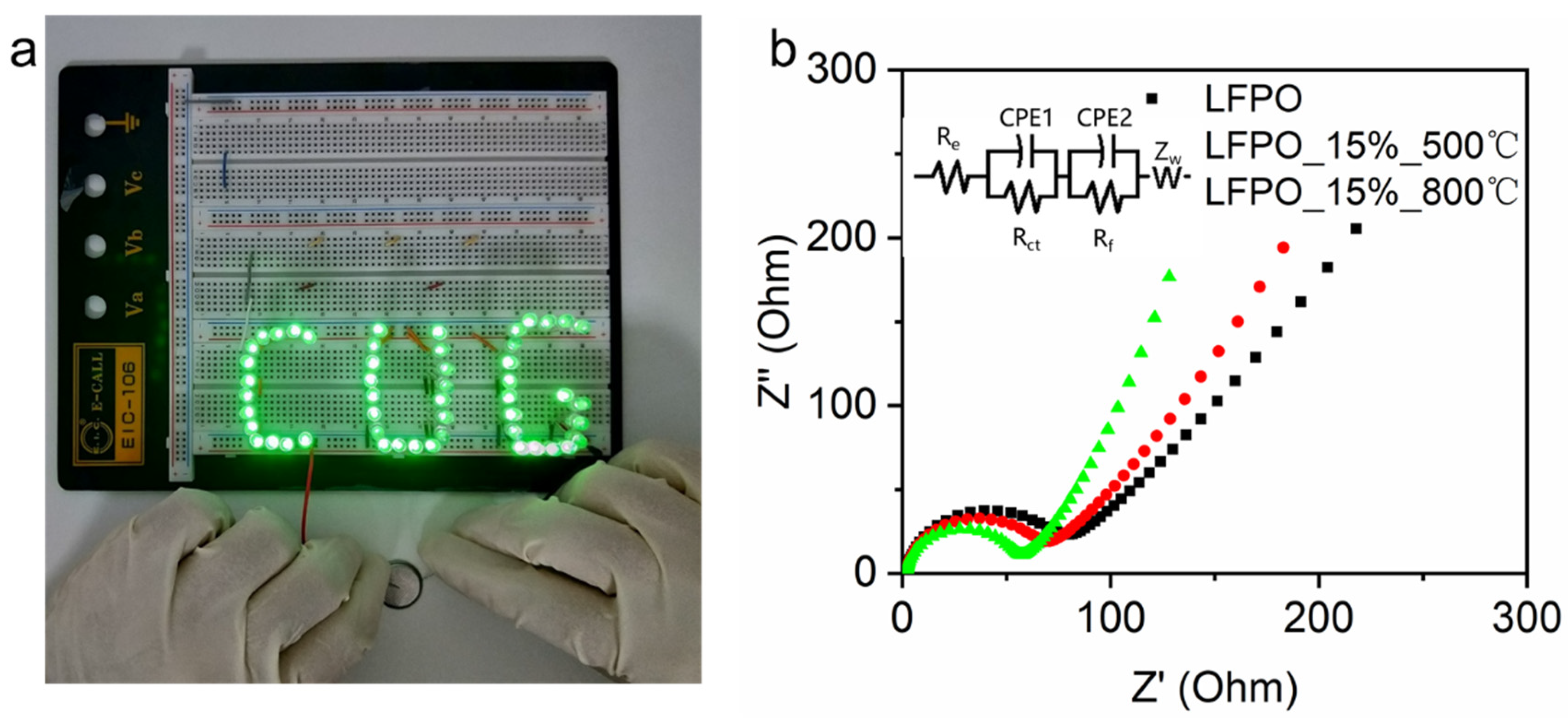
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.; Wu, T.P.; Amine, K. State-of-the-art characterization techniques for advanced lithium-ion batteries. Nat. Energy 2017, 2, 17011. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Tarascon, J.-M. Na-ion versus Li-ion batteries: Complementarity rather than competitiveness. Joule 2020, 4, 1616–1620. [Google Scholar] [CrossRef]
- Gao, X.-P.; Yang, H.-X. Multi-electron reaction materials for high energy density batteries. Energy Environ. Sci. 2010, 3, 174–189. [Google Scholar] [CrossRef]
- Tian, X.; Zhou, K. 3D printing of cellular materials for advanced electrochemical energy storage and conversion. Nanoscale 2020, 12, 7416–7432. [Google Scholar] [CrossRef]
- Haji Akhoundzadeh, M.; Panchal, S.; Samadani, E.; Raahemifar, K.; Fowler, M.; Fraser, R. Investigation and simulation of electric train utilizing hydrogen fuel cell and lithium-ion battery. Sustain. Energy Technol. Assess. 2021, 46, 101234. [Google Scholar]
- Duan, J.; Zhao, J.; Li, X.; Panchal, S.; Yuan, J.; Fraser, R.; Fowler, M. Modeling and analysis of heat dissipation for liquid cooling lithium-ion batteries. Energies 2021, 14, 4187. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Ma, Z.; Pan, F.; Curtiss, L.A.; Amine, K. The role of nanotechnology in the development of battery materials for electric vehicles. Nat. Nanotechnol. 2016, 11, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Driscoll, D.J.; Fisher, C.A.J.; Slater, P.R. Atomic-Scale Investigation of defects, dopants, and lithium transport in the LiFePO4 olivine-type battery material. Chem. Mater. 2005, 17, 5085–5092. [Google Scholar] [CrossRef]
- Chen, G.; Song, X.; Richardson, T. Electron microscopy study of the LiFePO4 to FePO4 phase transition. J. Electrochem. Soc. 2006, 9, A295–A298. [Google Scholar]
- Arumugam, D.V.M.; Theivanayagam, M.G.; Manthiram, A. Comparison of microwave assisted solvothermal and hydrothermal syntheses of LiFePO4/C nanocomposite cathodes for lithium ion batteries. J. Phys. Chem. C 2008, 112, 14665–14671. [Google Scholar]
- Tran, M.-K.; DaCosta, A.; Mevawalla, A.; Panchal, S.; Fowler, M. Comparative study of equivalent circuit models performance in four common lithium-ion batteries: LFP, NMC, LMO, NCA. Batteries 2021, 7, 51. [Google Scholar] [CrossRef]
- Xu, L.; Tang, S.; Cheng, Y.; Wang, K.; Liang, J.; Liu, C.; Cao, Y.-C.; Wei, F.; Mai, L. Interfaces in solid-state lithium batteries. Joule 2018, 2, 1991–2015. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Zhang, K.; Hu, Z.; Tao, Z.; Mai, L.; Kang, Y.-M.; Chou, S.-L.; Chen, J. Recent developments on and prospects for electrode materials with hierarchical structures for lithium-ion batteries. Adv. Energy Mater. 2017, 8, 1701415. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Zhao, K.; Luo, W.; Li, S.; Zhou, L.; Mai, L. Interconnected LiCuVO4 networks with in situ Cu generation as high-performance lithium-ion battery anode. Phys. Chem. Chem. Phys. 2017, 19, 13341–13347. [Google Scholar] [CrossRef]
- Dhanabalan, A.; Wang, C. Lithium-ion batteries: Three-dimensional porous core-shell Sn@carbon composite anodes for high-performance lithium-ion battery applications. Adv. Energy Mater. 2012, 2, 174. [Google Scholar]
- Meng, J.; Liu, X.; Niu, C.; Pang, Q.; Li, J.; Liu, F.; Liu, Z.; Mai, L. Advances in metal–organic framework coatings: Versatile synthesis and broad applications. Chem. Soc. Rev. 2020, 49, 3142–3186. [Google Scholar] [CrossRef]
- Meng, J.; He, Q.; Xu, L.; Zhang, X.; Liu, F.; Wang, X.; Li, Q.; Xu, X.; Zhang, G.; Niu, C.; et al. Identification of phase control of carbon-confined Nb2O5 nanoparticles toward high-performance lithium storage. Adv. Energy Mater. 2019, 9, 1802695. [Google Scholar] [CrossRef]
- Meng, J.; Liu, X.; Li, J.; Li, Q.; Zhao, C.; Xu, L.; Wang, X.; Liu, F.; Yang, W.; Xu, X.; et al. General oriented synthesis of precise carbon-confined nanostructures by low-pressure vapor superassembly and controlled pyrolysis. Nano Lett. 2017, 17, 7773–7781. [Google Scholar] [CrossRef]
- Wang, S.; Fang, Y.; Wang, X.; Lou, X.W. Hierarchical microboxes constructed by SnS nanoplates coated with nitrogen-doped carbon for efficient sodium storage. Angew. Chem. Int. Edit. 2019, 58, 760–763. [Google Scholar] [CrossRef]
- Sarigul, G.; Chamorro-Mena, I.; Linares, N.; García-Martínez, J.; Serrano, E. Hybrid amino acid-TiO2 materials with tuneable crystalline structure and morphology for photocatalytic applications. Adv. Sustain. Syst. 2021, 2100076. [Google Scholar] [CrossRef]
- Zhou, L.; Zhuang, Z.; Zhao, H.; Lin, M.; Zhao, D.; Mai, L. Intricate hollow structures: Controlled synthesis and applications in energy storage and conversion. Adv. Mater. 2017, 29, 1602914. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, M.; Ganapathy, S.; Li, C.; Li, Z.; Zhang, X.; He, P.; Zhou, H.; Wagemaker, M. Revealing the impact of space-charge layers on the Li-ion transport in all-solid-state batteries. Joule 2020, 4, 1311–1323. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Wang, D.; Li, X.; Geng, D.; Liang, G.; Gauthier, M.; Li, R.; Sun, X. 3D porous LiFePO4/graphene hybrid cathodes with enhanced performance for Li-ion batteries. J. Power Sources 2012, 208, 340–344. [Google Scholar] [CrossRef]
- Shi, Y.; Chou, S.-L.; Wang, J.-Z.; Wexler, D.; Li, H.-J.; Liu, H.-K.; Wu, Y. Graphene wrapped LiFePO4/C composites as cathode materials for Li-ion batteries with enhanced rate capability. J. Mater. Chem. 2012, 22, 16465–16470. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Wang, F.; Zhu, Y.; Liu, Z. Graphene modified LiFePO4 cathode materials for high power lithium ion batteries. J. Mater. Chem. 2011, 21, 3353–3358. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.-W.; Hong, J.; Lim, H.-D.; Kim, H.S.; Yoo, J.-K.; Kang, K. Graphene-based hybrid electrode material for high-power lithium-ion batteries. J. Electrochem. Soc. 2011, 158, A930–A935. [Google Scholar] [CrossRef]
- Lung-Hao Hu, B.; Wu, F.-Y.; Lin, C.-T.; Khlobystov, A.N.; Li, L.-J. Graphene-modified LiFePO4 cathode for lithium ion battery beyond theoretical capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Lee, J.T.; Li, P.; Kang, B.; Kim, J.H.; Yi, G.R.; Park, J.H. Conformal coating strategy comprising N-doped carbon and conventional graphene for achieving ultrahigh power and cyclability of LiFePO4. Nano Lett. 2015, 15, 6756–6763. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, N.; Lee, H.-W.; Zhao, J.; Li, W.; Li, Y.; Cui, Y. Nonfilling carbon coating of porous silicon micrometer-sized particles for high-performance lithium battery anodes. ACS Nano 2015, 9, 2540–2547. [Google Scholar] [CrossRef]
- Ji, Q.; Gao, X.; Zhang, Q.; Jin, L.; Wang, D.; Xia, Y.; Yin, S.; Xia, S.; Hohn, N.; Zuo, X.; et al. Dental resin monomer enables unique NbO2/carbon lithium-ion battery negative electrode with exceptional performance. Adv. Funct. Mater. 2019, 29, 1904961. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Dai, Z.; Liu, S.; Bao, J.; Guo, Y.-G. Ultra-uniform SnOx/carbon nanohybrids toward advanced lithium-ion battery anodes. Adv. Mater. 2014, 26, 3943–3949. [Google Scholar] [CrossRef]
- An, Q.; Xiong, F.; Wei, Q.; Sheng, J.; He, L.; Ma, D.; Yao, Y.; Mai, L. Nanoflake-assembled hierarchical Na3V2(PO4)3/C microflowers: Superior Li storage performance and insertion/extraction mechanism. Adv. Energy Mater. 2015, 5, 1401963. [Google Scholar] [CrossRef]
- Chen, Z.; Dahn, J.R. Improving the capacity retention of LiCoO2 cycled to 4.5 V by heat-treatment. Electrochem. Solid-State Lett. 2004, 7, A11–A14. [Google Scholar] [CrossRef]
- Shim, J.-H.; Lee, K.-S.; Missyul, A.; Lee, J.; Linn, B.; Lee, E.C.; Lee, S. Characterization of spinel LixCo2O4-coated LiCoO2 prepared with post-thermal treatment as a cathode material for lithium ion batteries. Chem. Mater. 2015, 27, 3273–3279. [Google Scholar] [CrossRef]
- Kalluri, S.; Yoon, M.; Jo, M.; Park, S.; Myeong, S.; Kim, J.; Dou, S.X.; Guo, Z.; Cho, J. Surface engineering strategies of layered LiCoO2 cathode material to realize high-energy and high-voltage Li-ion cells. Adv. Energy Mater. 2017, 7, 601507. [Google Scholar]
- Wu, Y.; Wen, Z.; Li, J. Hierarchical carbon-coated LiFePO4 nanoplate microspheres with high electrochemical performance for Li-ion batteries. Adv. Mater. 2011, 23, 1126–1129. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, J.; Zhang, J.; Yu, F.; Dong, L.; Nie, N.; Li, W. Metal organic frameworks derived porous lithium iron phosphate with continuous nitrogen-doped carbon networks for lithium ion batteries. J. Power Sources 2016, 304, 42–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Wang, X.; Guo, Y.; Jia, D.; Tang, X. Improved electrochemical performance of lithium iron phosphate in situ coated with hierarchical porous nitrogen-doped graphene-like membrane. J. Power Sources 2016, 305, 122–127. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Tang, Y.; Wang, D.; Li, X.; Hu, Y.; Li, R.; Liang, G.; Sham, T.-K.; Sun, X. LiFePO4–graphene as a superior cathode material for rechargeable lithium batteries: Impact of stacked graphene and unfolded graphene. Energy Environ. Sci. 2013, 6, 1521. [Google Scholar] [CrossRef]
- Belharouak, I.; Johnson, C.; Amine, K. Synthesis and electrochemical analysis of vapor-deposited carbon-coated LiFePO4. Electrochem. Commun. 2005, 7, 983–988. [Google Scholar] [CrossRef]
- Lou, X.; Zhang, Y. Synthesis of LiFePO4/C cathode materials with both high-rate capability and high tap density for lithium-ion batteries. J. Mater. Chem. 2011, 21, 4156. [Google Scholar] [CrossRef]
- Dong, Y.; Li, S.; Zhao, K.; Han, C.; Chen, W.; Wang, B.; Wang, L.; Xu, B.; Wei, Q.; Zhang, L.; et al. Hierarchical zigzag Na1.25V3O8 nanowires with topotactically encoded superior performance for sodium-ion battery cathodes. Energy Environ. Sci. 2015, 8, 1267–1275. [Google Scholar] [CrossRef]
- Pei, C.; Yin, Y.; Liao, X.; Xiong, F.; An, Q.; Jin, M.; Zhao, Y.; Mai, L. Structural properties and electrochemical performance of different polymorphs of Nb2O5 in magnesium-based batteries. J. Energy Chem. 2021, 58, 586–592. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, J.; Chen, M.; Guo, Y.; Zhou, G.; Li, N.; Zhou, S.; Wong, C.-P. Self-assembled NaV6O15 flower-like microstructures for high-capacity and long-life sodium-ion battery cathode. Nano Energy 2020, 68, 104357. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, X.; Li, S.; Han, C.; Zhao, K.; Zhang, L.; Niu, C.; Huang, Z.; Mai, L. Inhibiting effect of Na+ pre-intercalation in MoO3 nanobelts with enhanced electrochemical performance. Nano Energy 2015, 15, 145–152. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Yuan, X.; Tan, H.; Jian, S.; Ma, Z.; Zhao, J.; Wang, X.; Chen, D.; Dong, Y. Three-Dimensional Carbon-Coated LiFePO4 Cathode with Improved Li-Ion Battery Performance. Coatings 2021, 11, 1137. https://doi.org/10.3390/coatings11091137
Wang C, Yuan X, Tan H, Jian S, Ma Z, Zhao J, Wang X, Chen D, Dong Y. Three-Dimensional Carbon-Coated LiFePO4 Cathode with Improved Li-Ion Battery Performance. Coatings. 2021; 11(9):1137. https://doi.org/10.3390/coatings11091137
Chicago/Turabian StyleWang, Can, Xunlong Yuan, Huiyun Tan, Shuofeng Jian, Ziting Ma, Junjie Zhao, Xuewen Wang, Dapeng Chen, and Yifan Dong. 2021. "Three-Dimensional Carbon-Coated LiFePO4 Cathode with Improved Li-Ion Battery Performance" Coatings 11, no. 9: 1137. https://doi.org/10.3390/coatings11091137
APA StyleWang, C., Yuan, X., Tan, H., Jian, S., Ma, Z., Zhao, J., Wang, X., Chen, D., & Dong, Y. (2021). Three-Dimensional Carbon-Coated LiFePO4 Cathode with Improved Li-Ion Battery Performance. Coatings, 11(9), 1137. https://doi.org/10.3390/coatings11091137







