Protective Efficiency of ZrO2/Chitosan “Sandwich” Coatings on Galvanized Low-Carbon Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Sample Types
2.2. Preparation of Chitosan Solution
2.3. Preparation of ZrO2 Coatings on the CS Underlayer
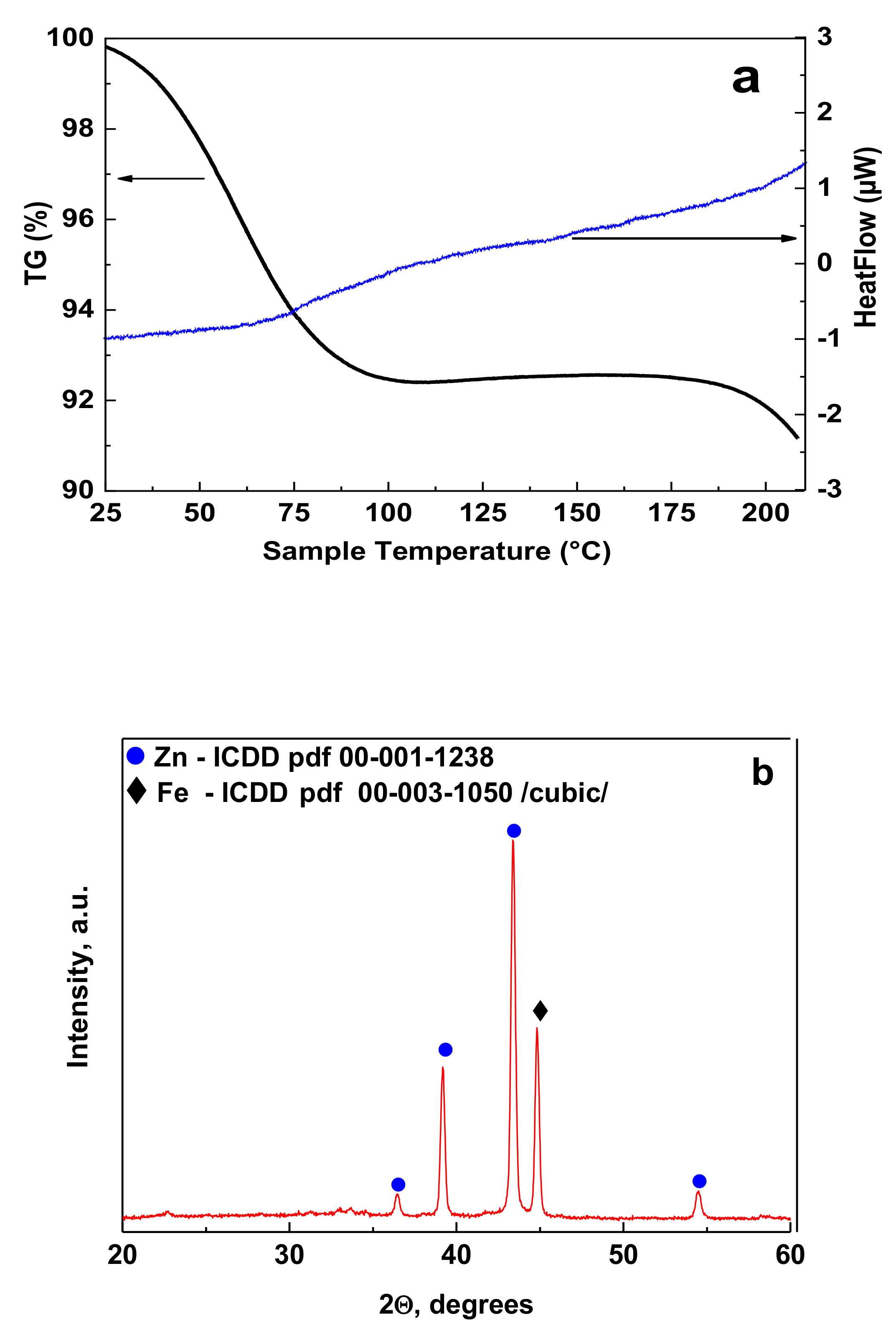
2.4. Differential Thermal (DTA)/Thermogravimetric (TG) Analyses
2.5. AFM Studies
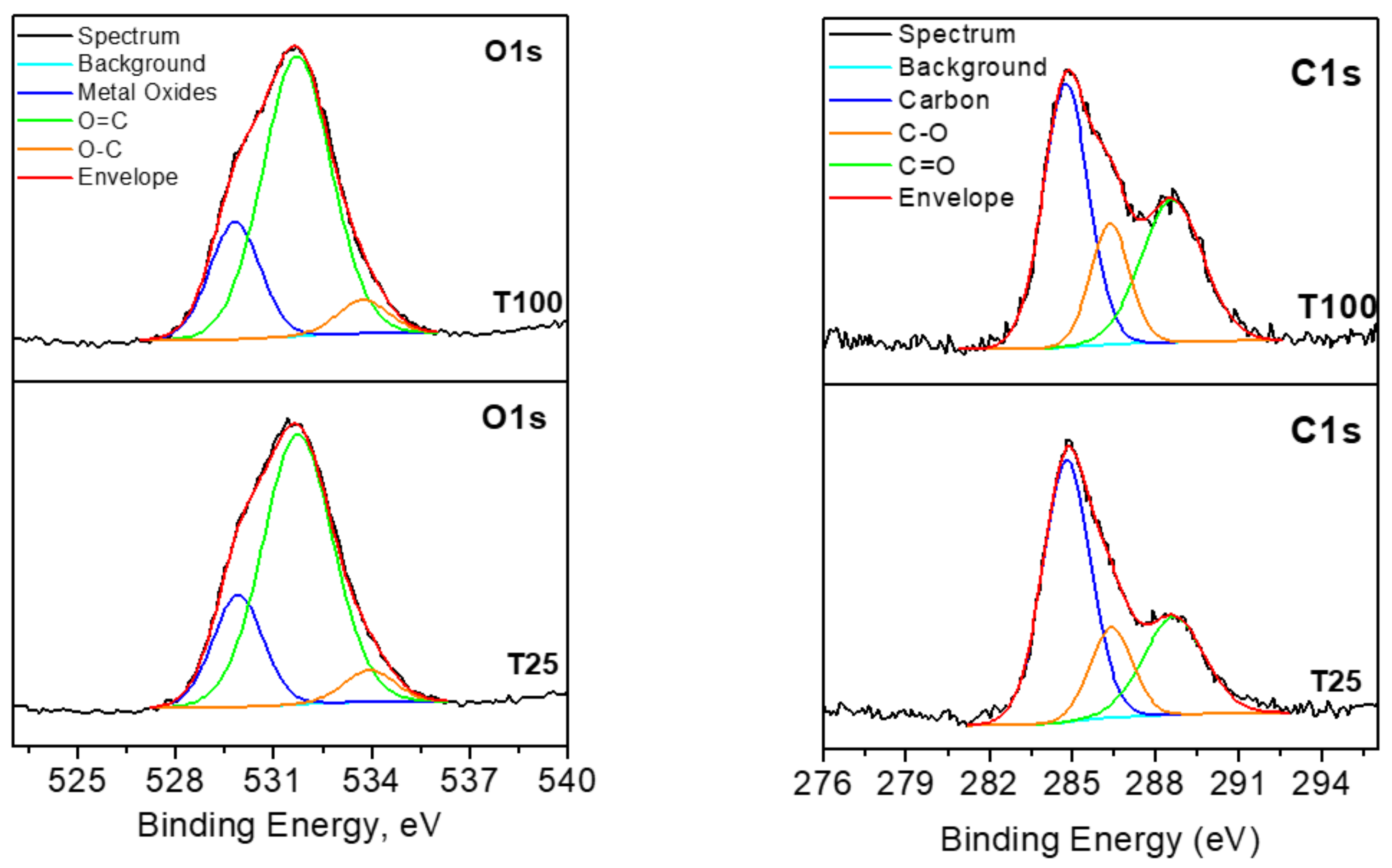
2.6. XPS Studies
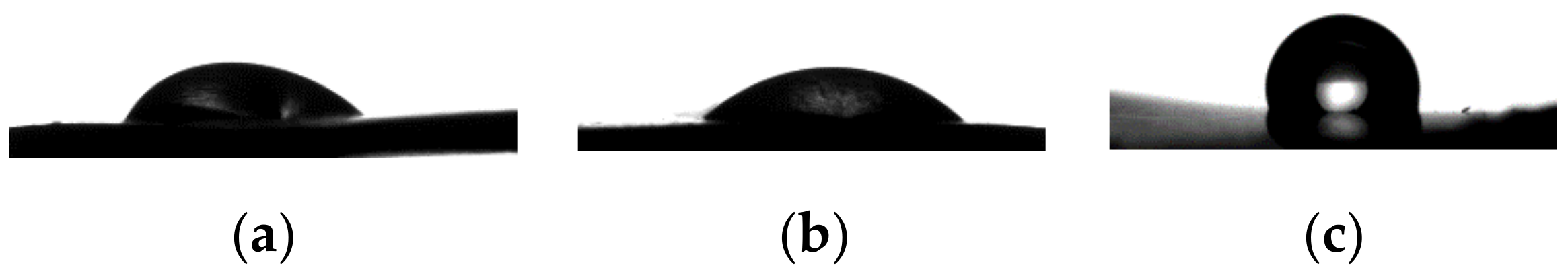
2.7. Contact Angle Measurements
2.8. Electrochemical Tests—Potentiodynamic Polarization (PDP) and Electrochemical Impedance Spectroscopy (EIS) Investigations
2.9. Reproducibility
3. Results
3.1. Differential Thermal (DTA)/Thermogravimetric (TG) and XRD Analyses
3.2. AFM Studies
3.3. XPS Investigations
3.4. Contact Angle Measurements
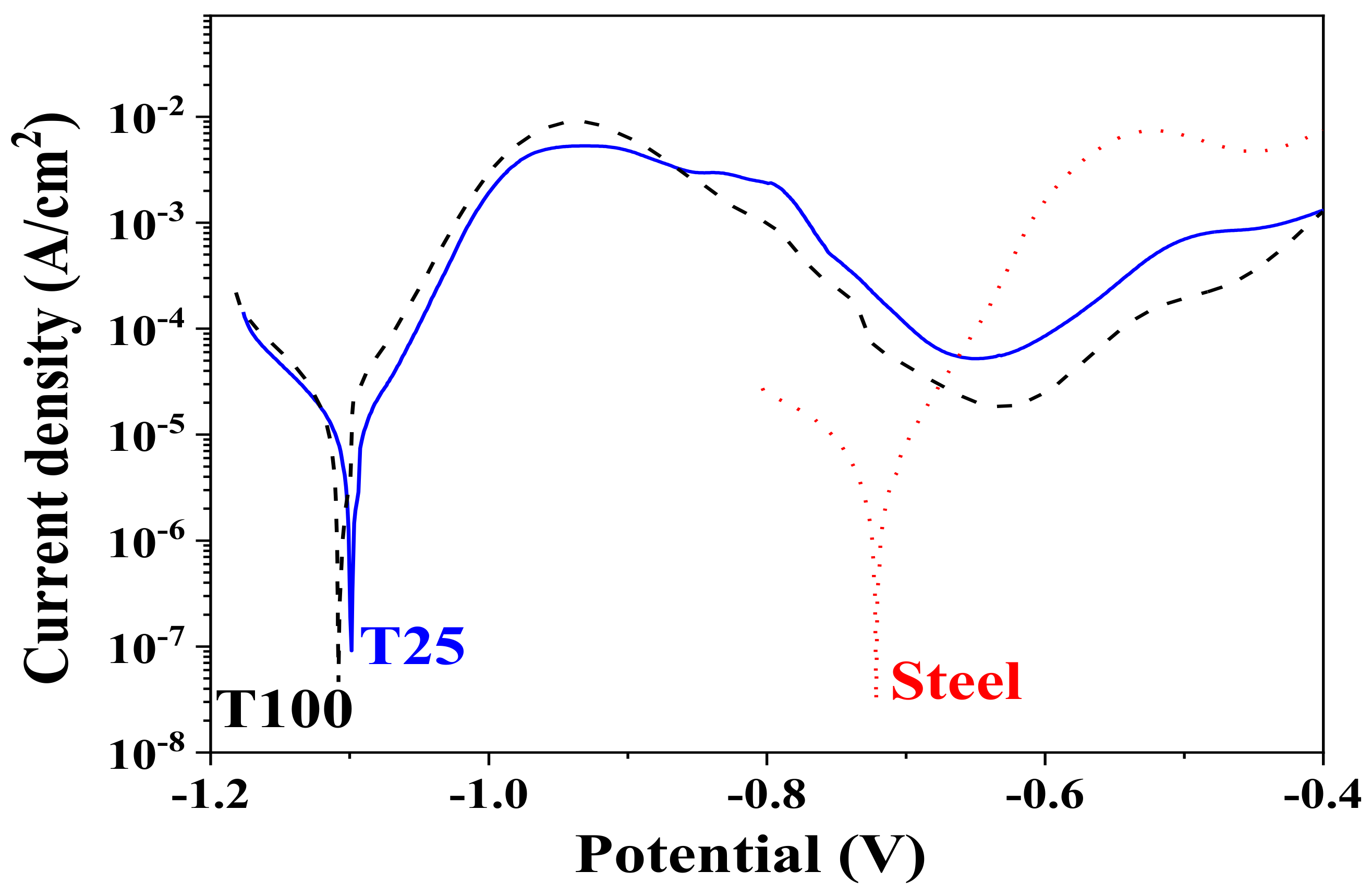
3.5. Potentiodynamic Investigations
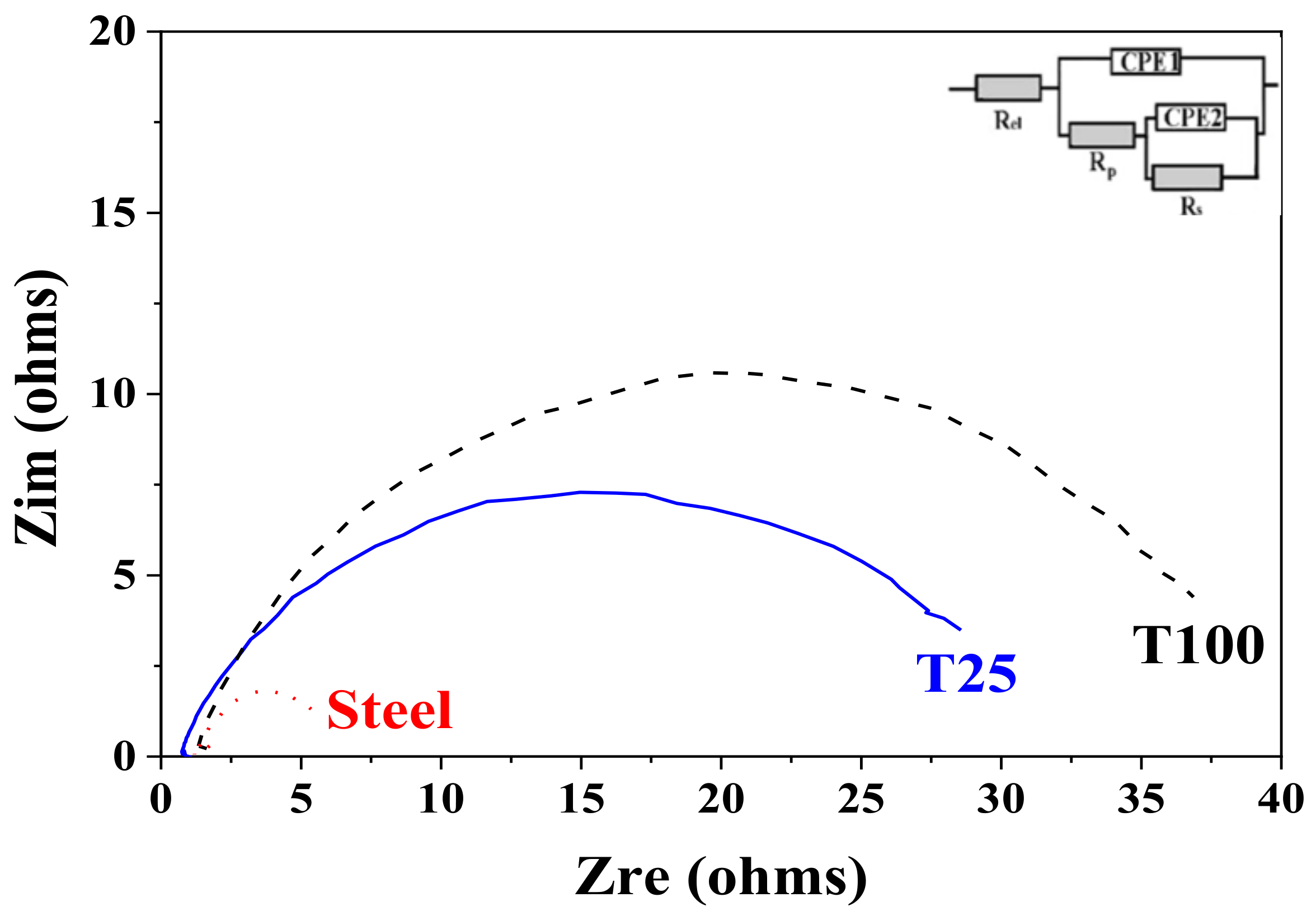
3.6. EIS Studies
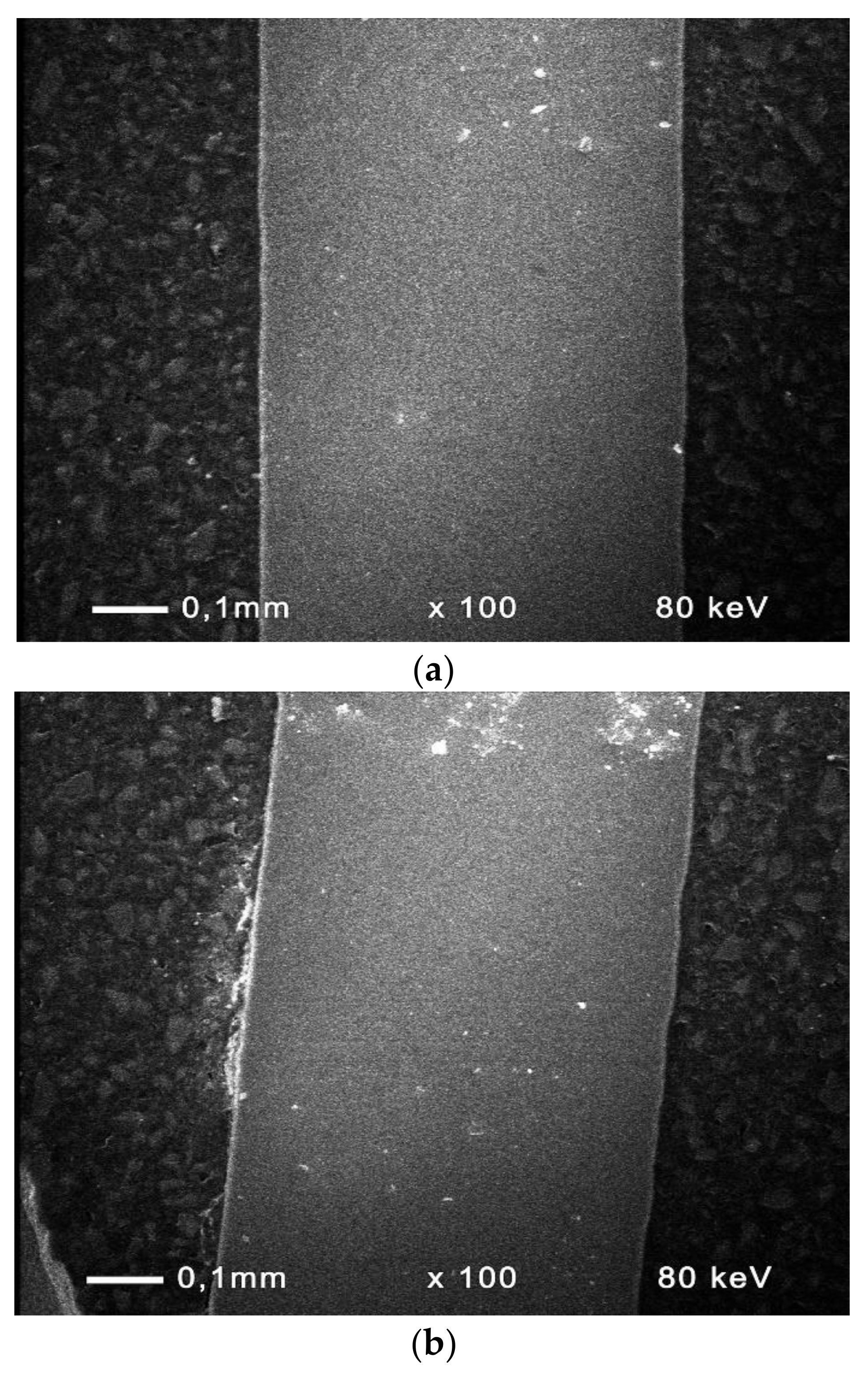
3.7. Cross-Sections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.P.; Wang, S.L.; Wang, J.Q. Relationship between amorphous structure and corrosion behaviour in a Zr–Ni metallic glass. Corros. Sci. 2012, 59, 88–95. [Google Scholar] [CrossRef]
- Diegle, R.B.; Sorensen, N.R.; Tsuru, T.; Latanision, R.M. 2-The Corrosion Resistance of Glassy Alloys. Treatise Mater. Sci. Technol. 1983, 23, 59–102. [Google Scholar] [CrossRef]
- Taheri, P.; Laha, P.; Terryn, H.; Mol, J.M.C. An in situ study of zirconium-based conversion treatment on zinc surfaces. Appl. Surf. Sci. 2015, 356, 837–843. [Google Scholar] [CrossRef]
- Rosalbino, S.F.; Scavino, G.; Mortarino, G.; Angelini, E.; Lunazzi, G. EIS study on the corrosion performance of a Cr(III)-conversion coating on zinc galvanized steel for the automotive industry. J. Solid State Electrochem. 2011, 15, 703–709. [Google Scholar] [CrossRef]
- Di Sarli, A.R.; Culcasi, J.D.; Tomachuk, C.R.; Elsner, C.I.; Ferreira, J.M.; Costa, I. A conversion layer based on trivalent chromium and cobalt for the corrosion protection of electrogalvanized steel. Surf. Coat. Technol. 2014, 258, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Stambolova, I.; Stoyanova, D.; Shipochka, M.; Boshkova, N.; Eliyas, A.; Simeonova, S.; Grozev, N.; Boshkov, N. Surface morphological and chemical features of anticorrosion ZrO2–TiO2 coatings: Impact of zirconium precursor. Coatings 2021, 11, 703. [Google Scholar] [CrossRef]
- Stambolova, I.; Dimitrov, O.; Vassilev, S.; Yordanov, S.; Blaskov, V.; Boshkov, N.; Shipochka, M. Preparation of newly developed CeO2/ZrO2 multilayers: Effect of the treatment temperature on the structure and corrosion performance of stainless steel. J. Alloy. Compd. 2019, 806, 1357–1367. [Google Scholar] [CrossRef]
- Amaraki, K. Treatment of zinc surface with cerium (III) nitrate to prevent zinc corrosion in aerated 0.5 M NaCl. Corros. Sci. 2001, 43, 2201–2215. [Google Scholar] [CrossRef]
- Biculcius, G.; Ricunsckiene, A.; Sudavicius, A.; Burocas, V.; Gruguceviciene, A. Cerium-permanganate conversion coatings for a Zn-Co alloy. Surf. Coat. Technol. 2008, 203, 115–120. [Google Scholar] [CrossRef]
- Hosseini, M.; Ashassi-Sorkhabi, H.; Ghiasvand, H.A.Y. Corrosion protection of electrogalvanized steel by green conversion coatings. J. Rare Earths 2007, 25, 537–543. [Google Scholar] [CrossRef]
- Andreatta, F.; Paussa, L.; Aldighieri, P.; Lanzutti, A.; Ondratschek, D.; Fedrizzi, L. ZrO2 films deposited with the sol–gel technique improved the corrosion resistance of AA 2024. Surf. Interface Anal. 2010, 42, 293. [Google Scholar] [CrossRef]
- Pareja, R.; López, I.; Martín, F.; Ramos-Barrado, J.R.; Leinen, D. Corrosion behaviour of zirconia barrier coatings on galvanized steel. Surf. Coat. Technol. 2006, 200, 6606–6610. [Google Scholar] [CrossRef]
- Al Lateef, A.; Khalaf, M. Corrosion resistance of ZrO2–TiO2 nanocomposite multilayer thin films coated on carbon steel in hydrochloric acid solution. Mater. Charact. 2015, 108, 29–41. [Google Scholar] [CrossRef]
- Sahnesarayi, M.; Sarpoolaky, H.; Rastegari, S. Effect of heat treatment temperature on the performance of nano-TiO2 coating in protecting 316L stainless steel against corrosion under UV illumination and dark conditions. Surf. Coat. Technol. 2014, 258, 861–870. [Google Scholar] [CrossRef]
- O’Keefe, M.J.; Geng, S.; Joshi, S. Cerium-Based Conversion Coatings as Alternatives to Hex Chrome. Met. Finish. 2007, 105, 25–28. [Google Scholar] [CrossRef]
- Creus, J.; Brezault, F.; Rebere, C.; Gadouleau, M. Synthesis and characterisation ofthin cerium oxide coatings elaborated by cathodic electrolytic deposition on steel substrate. Surf. Coat. Technol. 2006, 2004, 636–4645. [Google Scholar]
- Ershov, S.; Druart, M.E.; Poelman, M.; Cossement, D.; Snyders, R.; Olivier, M.-G. Deposition of cerium oxide thin films by reactive magnetron sputtering for the development of corrosion protective coatings. Corros. Sci. 2013, 75, 158–168. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Chacko, F. Development of nano CeO2-incorporated high performance hot-dip zinc coating. Surf. Coat. Technol. 2008, 202, 4971–4975. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partenzana, J.M. Multilayer (TiN, TiAlN) ceramic coatings for nuclear fuel cladding. J. Nucl. Mater. 2016, 478, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Marin, E.; Guzman, L.; Lanzutti, A.; Ensinger, W.; Fedrizzi, L. Multilayer Al2O3/TiO2 Atomic Layer Deposition coatings for the corrosion protection of stainless steel. Thin Solid Film. 2012, 522, 283–288. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, Q.; Zhu, X.; Ma, D.; Ma, F.; Song, Z.; Fu, Y.Q. Corrosion performance of ZrN/ZrO2 multilayer coatings deposited on 304 stainless steel using multi-arc ion plating. Appl. Surf. Sci. 2018, 431, 170–176. [Google Scholar] [CrossRef]
- Mariello, M.; Guido, F.; Mastronardi, V.M.; De Donato, F.; Salbini, M.; Brunetti, V.; Qualtieri, A.; Rizzi, F.; De Vittorio, M. Captive-air-bubble aerophobicity measurements of antibiofouling coatings for underwater MEMS devices. Nanomater. Nanotechnol. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Petousis, M.; Lazaros Tzounis, L.; Papageorgiou, D.; Vidakis, N. Decoration of SiO2 and Fe3O4 Nanoparticles onto the Surface of MWCNT-Grafted Glass Fibers: A SimpleApproach for the Creation of Binary Nanoparticle Hierarchical and Multifunctional Composite Interphases. Nanomaterials 2020, 10, 2500. [Google Scholar] [CrossRef]
- Georgieva, V.; Zvezdova, D.; Vlaev, L. No-isothermal kinetics of thermal degradation of chitosan. Chem. Cent. J. 2012, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, J.; Tedim, J.; Ferreira, M.G.S. Chitosan as a smart coating for corrosion protection of aluminum alloy 2024: A review. Progr. Org. Coat. 2015, 89, 348–356. [Google Scholar] [CrossRef]
- Salam, J.A.; Baby, A.M.; Joseph, A. Corrosion inhibition of mild steel using chitosan/TiO2 nanocomposite coatings. Progr. Org. Coat. 2019, 122, 254–259. [Google Scholar]
- Sanmugam, A.; Vikraman, D.; Karuppasamy, K.; Lee, J.Y.; Kim, H.-S. Evaluation of the corrosion resistance properties of electroplated chitosan-Zn1−X CuxO composite thin films. Nanomaterials 2017, 7, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: A review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Cui, L.; Gao, S.; Song, X.; Huang, L.; Dong, H.; Liu, J.; Chen, F.; Yu, S. Preparation and characterization of chitosan membranes. RSC Adv. 2018, 8, 28433–28439. [Google Scholar] [CrossRef] [Green Version]
- Gyliene, O.; Nivinskiene, O.; Vengris, T. Sorption of tartrate, citrate, and EDTA onto chitosan and its regeneration applying electrolysis. Carbohydr. Res. 2008, 343, 1324–1353. [Google Scholar] [CrossRef] [PubMed]
- Eaton, P.; West, P. Atomic Force Microscopy; OXFORD University Press: Oxford, UK, 2010. [Google Scholar]
- Zawadzki, J.; Kaczmarek, H. Thermal treatment of chitosan in various conditions. Carbohydr. Polym. 2010, 80, 394–400. [Google Scholar] [CrossRef]
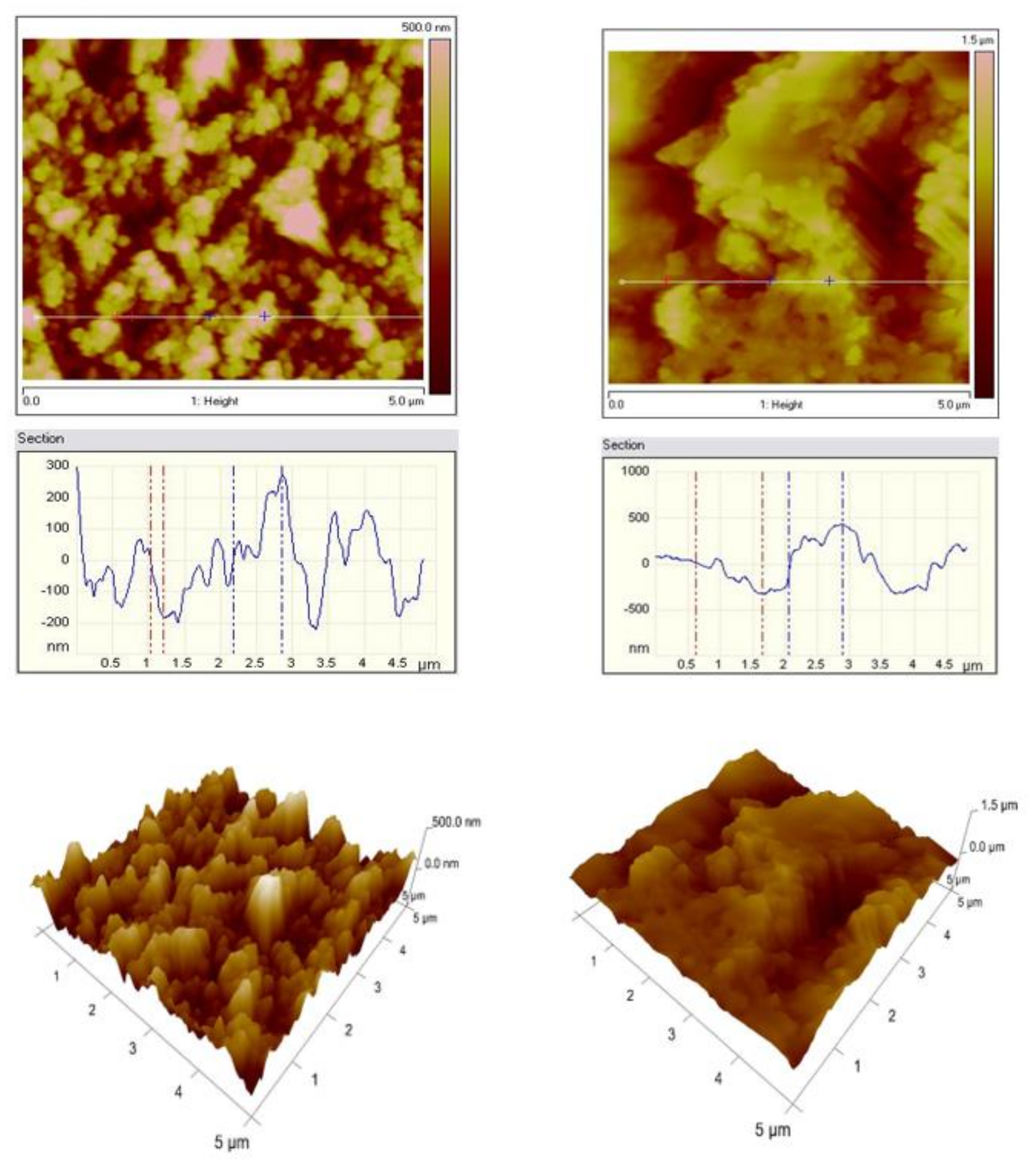


| Roughness (nm) | Galvanized Steel | T25 | T100 |
|---|---|---|---|
| Scan 5 µm | Scan 5 µm | Scan 5 µm | |
| Rq | 66 | 119 | 230 |
| Ra | 52 | 95.7 | 178 |
| Binding Energy (eV) | Chemical Bond | Concentration (%) |
|---|---|---|
| C1s | T25 | |
| 284.8 | C-C | 56.1 |
| 286.4 | C-O | 17.1 |
| 288.7 | C=O | 26.8 |
| C1s | T100 | |
| 284.8 | C-C | 47.0 |
| 286.3 | C-O | 18.5 |
| 288.6 | C=O | 34.5 |
| Binding Energy (eV) | Chemical Bond | Concentration (%) |
|---|---|---|
| O1s | T25 | |
| 529.9 | O-Me | 21.5 |
| 531.7 | O=C | 72.2 |
| 533.9 | O-C | 6.3 |
| O1s | T100 | |
| 529.8 | O-Me | 22.3 |
| 531.7 | O=C | 71.3 |
| 533.8 | O-C | 6.4 |
| Sample | Ecorr, V | Icorr, A/cm2 | Ipass, A/cm2 | mm y−1 |
|---|---|---|---|---|
| T25 | −1.10 | 9.4 × 10−6 | 5.2 × 10−5 | 0.141 |
| T100 | −1.11 | 1.3 × 10−5 | 1.8 × 10−5 | 0.196 |
| Steel | −0.72 | 4.5 × 10−6 | − | 0.068 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanova, D.; Stambolova, I.; Shipochka, M.; Boshkova, N.; Simeonova, S.; Grozev, N.; Avdeev, G.; Dimitrov, O.; Boshkov, N. Protective Efficiency of ZrO2/Chitosan “Sandwich” Coatings on Galvanized Low-Carbon Steel. Coatings 2021, 11, 1103. https://doi.org/10.3390/coatings11091103
Stoyanova D, Stambolova I, Shipochka M, Boshkova N, Simeonova S, Grozev N, Avdeev G, Dimitrov O, Boshkov N. Protective Efficiency of ZrO2/Chitosan “Sandwich” Coatings on Galvanized Low-Carbon Steel. Coatings. 2021; 11(9):1103. https://doi.org/10.3390/coatings11091103
Chicago/Turabian StyleStoyanova, Daniela, Irina Stambolova, Maria Shipochka, Nelly Boshkova, Silviya Simeonova, Nikolay Grozev, Georgi Avdeev, Ognian Dimitrov, and Nikolai Boshkov. 2021. "Protective Efficiency of ZrO2/Chitosan “Sandwich” Coatings on Galvanized Low-Carbon Steel" Coatings 11, no. 9: 1103. https://doi.org/10.3390/coatings11091103
APA StyleStoyanova, D., Stambolova, I., Shipochka, M., Boshkova, N., Simeonova, S., Grozev, N., Avdeev, G., Dimitrov, O., & Boshkov, N. (2021). Protective Efficiency of ZrO2/Chitosan “Sandwich” Coatings on Galvanized Low-Carbon Steel. Coatings, 11(9), 1103. https://doi.org/10.3390/coatings11091103






