Dopants for Enhanced Performance of Tin-Based Perovskite Solar Cells—A Short Review
Abstract
:1. Introduction
2. Additive Engineering
2.1. Inorganic Materials
2.2. Organic Materials
3. Crystal Structure Engineering
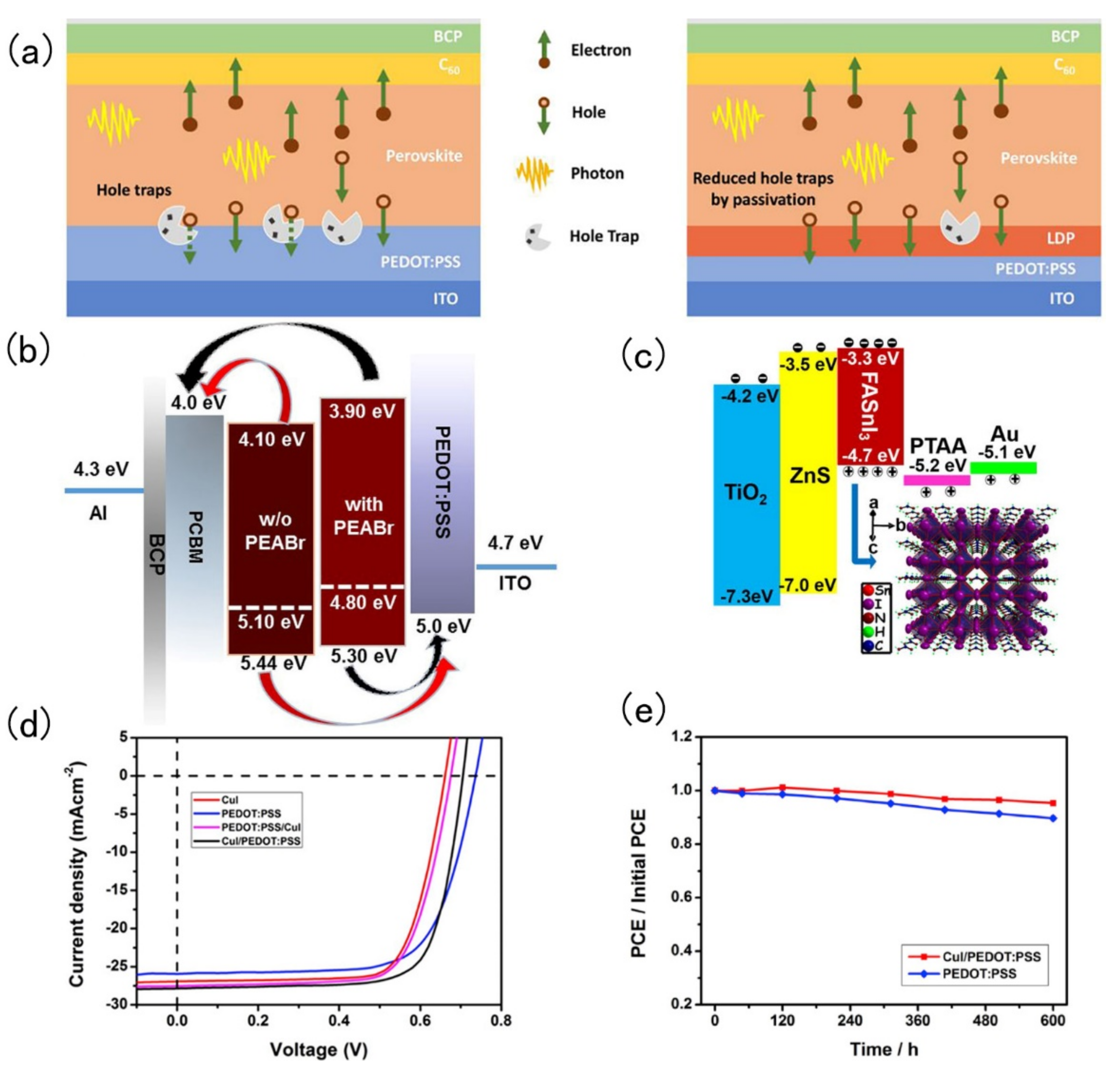
4. Interfacial Layer Engineering
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- NREL Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.20200406.pdf (accessed on 19 September 2020).
- Yang, W.F.; Igbari, F.; Lou, Y.H.; Wang, Z.K.; Liao, L.S. Tin halide perovskites: Progress and challenges. Adv. Energy Mater. 2019, 10, 1902584. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.K.; Zhuo, M.P.; Hu, Y.; Hu, K.H.; Ye, Q.Q.; Jain, S.M.; Yang, Y.G.; Gao, X.Y.; Liao, L.S. Pb–Sn–Cu ternary organometallic halide perovskite solar cells. Adv. Mater. 2018, 30, e1800258. [Google Scholar] [CrossRef]
- Singh, T.; Miyasaka, T. Stabilizing the efficiency beyond 20% with a mixed cation perovskite solar cell fabricated in ambient air under controlled humidity. Adv. Energy Mater. 2018, 8, 1700677. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, X.; Feng, X.; Lu, S.; Chen, Z.; Yong, X.; Redfern, S.A.T.; Wei, H.; Wang, H.; Shen, H.; et al. Polymer-passivated inorganic cesium lead mixed-halide perovskites for stable and efficient solar cells with high open-circuit voltage over 1.3 V. Adv. Mater. 2018, 30, 1705393. [Google Scholar] [CrossRef] [PubMed]
- Valerio, L.; Rosa, A.; Rodriguez, V.; Enriquez, C.; Telles, A.; Ramirez, Y.; Rivera, D.; Hierro, J.; Bustamante, L.; Tong, X.; et al. Characterization and analysis of FAxCs(1−x) Pb(IyBr(1−y))3 perovskite solar cells with thickness controlled transport layers for performance optimization. AIP Adv. 2019, 9, 105006. [Google Scholar] [CrossRef]
- Bai, S.; Da, P.; Li, C.; Wang, Z.; Yuan, Z.; Fu, F.; Kawecki, M.; Liu, X.; Sakai, N.; Wang, J.T.; et al. Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature 2019, 571, 245–250. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.G.; Wang, Z.K.; Kang, T.; Wang, Q.; Turren-Cruz, S.H.; Gao, X.Y.; Hsu, C.S.; Liao, L.S.; Abate, A. Perovskite grains embraced in a soft fullerene network make highly efficient flexible solar cells with superior mechanical stability. Adv. Mater. 2019, 31, e1901519. [Google Scholar] [CrossRef]
- Li, M.; Zuo, W.W.; Wang, Q.; Wang, K.L.; Zhuo, M.P.; Köbler, H.; Halbig, C.E.; Eigler, S.; Yang, Y.G.; Gao, X.Y.; et al. Ultrathin nanosheets of oxo-functionalized graphene inhibit the ion migration in perovskite solar cells. Adv. Energy Mater. 2019, 10, 1902653. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Long, M.; Qin, M.; Lu, X.; Chen, S.; Xie, F.; Gong, L.; Chen, J.; Chu, M.; Miao, Q.; et al. Stable and efficient 3D-2D perovskite-perovskite planar heterojunction solar cell without organic hole transport layer. Joule 2018, 2, 2706–2721. [Google Scholar] [CrossRef] [Green Version]
- Hartono, N.T.P.; Sun, S.; Gélvez-Rueda, M.C.; Pierone, P.J.; Erodici, M.P.; Yoo, J.; Wei, F.; Bawendi, M.; Grozema, F.C.; Sher, M.-J.; et al. The effect of structural dimensionality on carrier mobility in lead-halide perovskites. J. Mater. Chem. A 2019, 7, 23949–23957. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.-K.; Kang, T.; Yang, Y.; Gao, X.; Hsu, C.-S.; Li, Y.; Liao, L.-S. Graphdiyne-modified cross-linkable fullerene as an efficient electron-transporting layer in organometal halide perovskite solar cells. Nano Energy 2018, 43, 47–54. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Xu, J.; Liu, H.; Qin, R.; Zhai, H.; Chen, S.; Yuan, M. All-inorganic perovskite solar cells based on CsPbIBr2 and metal oxide transport layers with improved stability. Nanomaterials 2019, 9, 1666. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cao, H.L.; Jiao, W.B.; Wang, Q.; Wei, M.; Cantone, I.; Lu, J.; Abate, A. Biological impact of lead from halide perovskites reveals the risk of introducing a safe threshold. Nat. Commun. 2020, 11, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Cryst. 1976, 32, 751. [Google Scholar] [CrossRef]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B.; et al. Lead-free organic–inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar] [CrossRef]
- Mare, S.D.; Menossi, D.; Salavei, A.; Artegiani, E.; Piccinelli, F.; Kumar, A.; Mariotto, G.; Romeo, A. SnS thin film solar cells: Perspectives and limitations. Coatings 2017, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Mare, S.D.; Salavei, A.; Menossi, D.; Piccinelli, F.; Bernardi, P.; Artegiani, E.; Kumar, A.; Mariotto, G.; Romeo, A. A study of SnS recrystallization by post deposition treatment. In Proceedings of the IEEE 43rd Photovoltaic Specialists Conference 2016, Portland, OR, USA, 5–10 June 2016; p. 16468525. [Google Scholar]
- Mare, S.D.; Salavei, A.; Menossi, D.; Piccinelli, F.; Artegiani, E.; Kumar, A.; Mariotto, G.; Bernardi, P.; Romeo, A. Effects of temperature and post deposition annealing on SnS polycrystalline thin film growth. In Proceedings of the 32nd European Photovoltaic Solar Energy Conference and Exhibition 2016, Munich, Germany, 20–24 June 2016; pp. 263–266. [Google Scholar]
- Takahashi, Y.; Obara, R.; Lin, Z.Z.; Takahashi, Y.; Naito, T.; Inabe, T.; Ishibashi, S.; Terakura, K. Charge-transport in tin-iodide perovskite CH3NH3SnI3: Origin of high conductivity. Dalton Trans. 2011, 40, 5563–5568. [Google Scholar] [CrossRef] [Green Version]
- Koh, T.M.; Krishnamoorthy, T.; Yantara, N.; Shi, C.; Leong, W.L.; Boix, P.P.; Grimsdale, A.C.; Mhaisalkar, S.G.; Mathews, N. Formamidinium tin-based perovskite with low Eg for photovoltaic applications. J. Mater. Chem. A 2015, 3, 14996–15000. [Google Scholar] [CrossRef]
- Shi, T.; Zhang, H.-S.; Meng, W.; Teng, Q.; Liu, M.; Yang, X.; Yan, Y.; Yip, H.-L.; Zhao, Y.-J. Effects of organic cations on the defect physics of tin halide perovskites. J. Mater. Chem. A 2017, 5, 15124–15129. [Google Scholar] [CrossRef]
- Bansode, U.; Naphade, R.; Game, O.; Agarkar, S.; Ogale, S. Hybrid Perovskite films by a new variant of pulsed excimer laser deposition: A room-temperature dry process. J. Phys. Chem. C 2015, 119, 9177–9185. [Google Scholar] [CrossRef]
- Song, T.-B.; Yokoyama, T.; Aramaki, S.; Kanatzidis, M.G. Performance enhancement of lead-free tin-based perovskite solar cells with reducing atmosphere-assisted dispersible additive. ACS Energy Lett. 2017, 2, 897–903. [Google Scholar] [CrossRef]
- Lee, S.J.; Shin, S.S.; Kim, Y.C.; Kim, D.; Ahn, T.K.; Noh, J.H.; Seo, J.; Seok, S.I. Fabrication of efficient formamidinium tin iodide perovskite solar cells through SnF2-pyrazine complex. J. Am. Chem. Soc. 2016, 138, 3974–3977. [Google Scholar] [CrossRef]
- Yokoyama, T.; Song, T.-B.; Cao, D.H.; Stoumpos, C.C.; Aramaki, S.; Kanatzidis, M.G. The origin of lower hole carrier concentration in methylammonium tin halide films grown by a vapor-assisted solution process. ACS Energy Lett. 2017, 2, 22–28. [Google Scholar] [CrossRef]
- Chen, L.J.; Lee, C.R.; Chuang, Y.J.; Wu, Z.H.; Chen, C. Synthesis and optical properties of lead-free cesium tin halide perovskite quantum rods with high-performance solar cell application. J. Phys. Chem. Lett. 2016, 7, 5028–5035. [Google Scholar] [CrossRef] [PubMed]
- Jokar, E.; Chien, C.H.; Tsai, C.M.; Fathi, A.; Diau, E.W. Robust tin-based perovskite solar cells with hybrid organic cations to attain efficiency approaching 10%. Adv. Mater. 2019, 31, e1804835. [Google Scholar] [CrossRef]
- Nishimura, K.; Kamarudin, M.A.; Hirotani, D.; Hamada, K.; Shen, Q.; Iikubo, S.; Minemoto, T.; Yoshino, K.; Hayase, S. Lead-free tin-halide perovskite solar cells with 13% efficiency. Nano Energy 2020, 74, 104858. [Google Scholar] [CrossRef]
- Cao, J.; Tai, Q.; You, P.; Tang, G.; Wang, T.; Wang, N.; Yan, F. Enhanced performance of tin-based perovskite solar cells induced by an ammonium hypophosphite additive. J. Mater. Chem. A 2019, 7, 26580–26585. [Google Scholar] [CrossRef]
- Tai, Q.; Guo, X.; Tang, G.; You, P.; Ng, T.W.; Shen, D.; Cao, J.; Liu, C.K.; Wang, N.; Zhu, Y.; et al. Antioxidant grain passivation for air-stable tin-based perovskite solar cells. Angew. Chem. Int. Ed. Engl. 2019, 58, 806–810. [Google Scholar] [CrossRef]
- Kayesh, M.E.; Chowdhury, T.H.; Matsuishi, K.; Kaneko, R.; Kazaoui, S.; Lee, J.-J.; Noda, T.; Islam, A. Enhanced photovoltaic performance of FASnI3-based perovskite solar cells with hydrazinium chloride coadditive. ACS Energy Lett. 2018, 3, 1584–1589. [Google Scholar] [CrossRef]
- Chung, I.; Lee, B.; He, J.; Chang, R.P.; Kanatzidis, M.G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 2012, 485, 486–489. [Google Scholar] [CrossRef]
- Kumar, M.H.; Dharani, S.; Leong, W.L.; Boix, P.P.; Prabhakar, R.R.; Baikie, T.; Shi, C.; Ding, H.; Ramesh, R.; Asta, M.; et al. Lead-free halide perovskite solar cells with high photocurrents realized through vacancy modulation. Adv. Mater. 2014, 26, 7122–7127. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Stoumpos, C.C.; Guo, P.; Zhou, N.; Marks, T.J.; Chang, R.P.; Kanatzidis, M.G. Solvent-mediated crystallization of CH3NH3SnI3 films for heterojunction depleted perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 11445–11452. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, D.; Yu, Y.; Grice, C.R.; Wang, C.; Cimaroli, A.J.; Schulz, P.; Meng, W.; Zhu, K.; Xiong, R.G.; et al. Lead-free inverted planar formamidinium tin triiodide perovskite solar cells achieving power conversion efficiencies up to 6.22%. Adv. Mater. 2016, 28, 9333–9340. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hao, F.; Stoumpos, C.C.; Phelan, B.T.; Wasielewski, M.R.; Kanatzidis, M.G. Carrier diffusion lengths of over 500 nm in lead-free perovskite CH3NH3SnI3 films. J. Am. Chem. Soc. 2016, 138, 14750–14755. [Google Scholar] [CrossRef] [PubMed]
- Handa, T.; Yamada, T.; Kubota, H.; Ise, S.; Miyamoto, Y.; Kanemitsu, Y. Photocarrier recombination and injection dynamics in long-term stable lead-free CH3NH3SnI3 perovskite thin films and solar cells. J. Phys. Chem. C 2017, 121, 16158–16165. [Google Scholar] [CrossRef]
- Milot, R.L.; Klug, M.T.; Davies, C.L.; Wang, Z.; Kraus, H.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. The effects of doping density and temperature on the optoelectronic properties of formamidinium tin triiodide thin films. Adv. Mater. 2018, 30, e1804506. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, C.; Gupta, S.; Bendikov, T.; Kozina, X.; Kunze, T.; Félix, R.; Hodes, G.; Wilks, R.G.; Cahen, D.; Bär, M. Interfaces, impact of SnF2 addition on the chemical and electronic surface structure of CsSnBr3. ACS Appl. Mater. Interfaces 2020, 12, 12353–12361. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Ran, C.; Li, J.; Dong, H.; Jiao, B.; Zhang, L.; Lan, X.; Hou, X.; Wu, Z. Robust stability of efficient lead-free formamidinium tin iodide perovskite solar cells realized by structural regulation. J. Phys. Chem. Lett. 2018, 9, 6999–7006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, W.; Stoumpos, C.C.; Spanopoulos, I.; Chen, M.; Wasielewski, M.R.; Kanatzidis, M.G. Diammonium cations in the FASnI3 perovskite structure lead to lower dark currents and more efficient solar cells. ACS Energy Lett. 2018, 3, 1470–1476. [Google Scholar] [CrossRef]
- Jokar, E.; Chien, C.-H.; Fathi, A.; Rameez, M.; Chang, Y.-H.; Diau, E.W.-G. Slow surface passivation and crystal relaxation with additives to improve device performance and durability for tin-based perovskite solar cells. Energy Environ. Sci. 2018, 11, 2353–2362. [Google Scholar] [CrossRef]
- Kayesh, M.E.; Matsuishi, K.; Kaneko, R.; Kazaoui, S.; Lee, J.-J.; Noda, T.; Islam, A. Coadditive engineering with 5-ammonium valeric acid iodide for efficient and stable sn perovskite solar cells. ACS Energy Lett. 2018, 4, 278–284. [Google Scholar] [CrossRef]
- Ran, C.; Gao, W.; Li, J.; Xi, J.; Li, L.; Dai, J.; Yang, Y.; Gao, X.; Dong, H.; Jiao, B.; et al. Conjugated organic cations enable efficient self-healing FASnI3 solar cells. Joule 2019, 3, 3072–3087. [Google Scholar] [CrossRef]
- Yu, B.B.; Xu, L.; Liao, M.; Wu, Y.; Liu, F.; He, Z.; Ding, J.; Chen, W.; Tu, B.; Lin, Y.; et al. Synergy effect of both 2,2,2-trifluoroethylamine hydrochloride and SnF2 for highly stable FASnI3−xClx perovskite solar cells. Solar RRL 2019, 3, 1800290. [Google Scholar] [CrossRef]
- He, X.; Wu, T.; Liu, X.; Wang, Y.; Meng, X.; Wu, J.; Noda, T.; Yang, X.; Moritomo, Y.; Segawa, H.; et al. Highly efficient tin perovskite solar cells achieved in a wide oxygen concentration range. J. Mater. Chem. A 2020, 8, 2760–2768. [Google Scholar] [CrossRef]
- Boehm, A.M.; Liu, T.; Park, S.M.; Abtahi, A.; Graham, K.R. Influence of surface ligands on energetics at FASnI3/C60 interfaces and their impact on photovoltaic performance. ACS Appl. Mater. Interfaces 2020, 12, 5209–5218. [Google Scholar] [CrossRef]
- Zeng, W.; Cui, D.; Li, Z.; Tang, Y.; Yu, X.; Li, Y.; Deng, Y.; Ye, R.; Niu, Q.; Xia, R.; et al. Surface optimization by poly(α-methylstyrene) as additive in the anti-solution to enhance lead-free Sn-based perovskite solar cells. Sol. Energy 2019, 194, 272–278. [Google Scholar] [CrossRef]
- Meng, X.; Lin, J.; Liu, X.; He, X.; Wang, Y.; Noda, T.; Wu, T.; Yang, X.; Han, L. Highly stable and efficient FASnI3-based perovskite solar cells by introducing hydrogen bonding. Adv. Mater. 2019, 31, e1903721. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, C.; Liu, G.; Yang, J.; Duan, X.; Tan, L.; Chen, Y. Preparation of efficient inverted tin-based perovskite solar cells via the bidentate coordination effect of 8-hydroxyquinoline. Chem. Commun. 2020, 56, 4006–4010. [Google Scholar] [CrossRef]
- Wu, T.; Liu, X.; He, X.; Wang, Y.; Meng, X.; Noda, T.; Yang, X.; Han, L. Efficient and stable tin-based perovskite solar cells by introducing π-conjugated Lewis base. Sci. China Chem. 2019, 63, 107–115. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, H.; Zhou, W.; Yang, D.; Shang, Y.; Shi, Z.; Li, B.; Jiang, X.; Zhang, L.; Quan, L.N.; et al. Highly oriented low-dimensional tin halide perovskites with enhanced stability and photovoltaic performance. J. Am. Chem. Soc. 2017, 139, 6693–6699. [Google Scholar] [CrossRef]
- Ke, W.; Stoumpos, C.C.; Zhu, M.; Mao, L.; Spanopoulos, I.; Liu, J.; Kontsevoi, O.Y.; Chen, M.; Sarma, D.; Zhang, Y.; et al. Enhanced photovoltaic performance and stability with a new type of hollow 3D perovskite {en}FASnI3. Sci. Adv. 2017, 3, e1701293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, C.; Xi, J.; Gao, W.; Yuan, F.; Lei, T.; Jiao, B.; Hou, X.; Wu, Z. Bilateral interface engineering toward efficient 2D–3D bulk heterojunction tin halide lead-free perovskite solar cells. ACS Energy Lett. 2018, 3, 713–721. [Google Scholar] [CrossRef]
- Ng, C.H.; Hamada, K.; Kapil, G.; Kamarudin, M.A.; Wang, Z.; Iikubo, S.; Shen, Q.; Yoshino, K.; Minemoto, T.; Hayase, S. Reducing Traps Density and Carriers Concentration by Ge Additive for An Efficient Quasi 2D/3D Perovskite Solar Cell. J. Mater. Chem. A 2020, 8, 2962–2968. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, X.; Chen, H.; Shang, Y.; Liu, H.; Wei, J.; Zhou, W.; He, H.; Liu, W.; Ning, Z. 2D-quasi-2D-3D hierarchy structure for tin perovskite solar cells with enhanced efficiency and stability. Joule 2018, 2, 2732–2743. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zuo, W.-W.; Yang, Y.-G.; Aldamasy, M.H.; Wang, Q.; Cruz, S.H.T.; Feng, S.-L.; Saliba, M.; Wang, Z.-K.; Abate, A. Tin halide perovskite films made of highly oriented 2D crystals enable more efficient and stable lead-free perovskite solar cells. ACS Energy Lett. 2020, 5, 1923–1929. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Y.; He, T.; Li, S.; Wang, H.; Chen, Y.; Yuan, M.; Chen, J. Orientation regulation of tin-based reduced-dimensional perovskites for highly efficient and stable photovoltaics. Adv. Funct. Mater. 2019, 29, 1907696. [Google Scholar] [CrossRef]
- Tsai, C.-M.; Lin, Y.-P.; Pola, M.K.; Narra, S.; Jokar, E.; Yang, Y.-W.; Diau, E.W.-G. Control of crystal structures and optical properties with hybrid formamidinium and 2-hydroxyethylammonium cations for mesoscopic carbon-electrode tin-based perovskite solar cells. ACS Energy Lett. 2018, 3, 2077–2085. [Google Scholar] [CrossRef]
- Li, P.; Liu, X.; Zhang, Y.; Liang, C.; Chen, G.; Li, F.; Su, M.; Xing, G.; Tao, X.; Song, Y. Low-dimensional dion–jacobson-phase lead-free perovskites for high-performance photovoltaics with improved stability. Angew. Chem. Int. Ed. Engl. 2020, 59, 6909–6914. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Z.; Shiu, M.; Ma, J.H.; Alpert, M.R.; Zhang, D.; Foley, B.J.; Smilgies, D.M.; Lee, S.H.; Choi, J.J. Origin of vertical orientation in two-dimensional metal halide perovskites and its effect on photovoltaic performance. Nat. Commun. 2018, 9, 1336. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Wu, P.; Yang, W.; Su, R.; Luo, D.; Yang, X.; Tu, Y.; Zhu, R.; Gong, Q. Low-dimensional perovskite interlayer for highly efficient lead-free formamidinium tin iodide perovskite solar cells. Nano Energy 2018, 49, 411–418. [Google Scholar] [CrossRef]
- Liao, M.; Yu, B.B.; Jin, Z.; Chen, W.; Zhu, Y.; Zhang, X.; Yao, W.; Duan, T.; Djerdj, I.; He, Z. Efficient and stable FASnI3 perovskite solar cells with effective interface modulation by low-dimensional perovskite layer. ChemSusChem 2019, 12, 5007–5014. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Stoumpos, C.C.; Logsdon, J.L.; Wasielewski, M.R.; Yan, Y.; Fang, G.; Kanatzidis, M.G. TiO2-ZnS cascade electron transport layer for efficient formamidinium tin iodide perovskite solar cells. J. Am. Chem. Soc. 2016, 138, 14998–15003. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhang, L.; She, S.; Wu, J.; Zhou, X.; Li, X.; Wang, D.; Miao, J.; Mi, G.; Chen, H.; et al. Electron transporting bilayer of SnO2 and TiO2 nanocolloid enables highly efficient planar perovskite solar cells. Solar RRL 2019, 4, 1900331. [Google Scholar] [CrossRef]
- Song, J.; Hu, W.; Li, Z.; Wang, X.-F.; Tian, W. A double hole-transport layer strategy toward efficient mixed tin-lead iodide perovskite solar cell. Sol. Energy Mater. Sol. Cells 2020, 207, 110351. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, Z.; Yang, F.; Yang, J.; Grace, A.N.; Li, J.; Tripathi, S.; Jain, S.M. Dopants for Enhanced Performance of Tin-Based Perovskite Solar Cells—A Short Review. Coatings 2021, 11, 1045. https://doi.org/10.3390/coatings11091045
Liu H, Zhang Z, Yang F, Yang J, Grace AN, Li J, Tripathi S, Jain SM. Dopants for Enhanced Performance of Tin-Based Perovskite Solar Cells—A Short Review. Coatings. 2021; 11(9):1045. https://doi.org/10.3390/coatings11091045
Chicago/Turabian StyleLiu, Hairui, Zuhong Zhang, Feng Yang, Jien Yang, Andrews Nirmala Grace, Junming Li, Sapana Tripathi, and Sagar M. Jain. 2021. "Dopants for Enhanced Performance of Tin-Based Perovskite Solar Cells—A Short Review" Coatings 11, no. 9: 1045. https://doi.org/10.3390/coatings11091045
APA StyleLiu, H., Zhang, Z., Yang, F., Yang, J., Grace, A. N., Li, J., Tripathi, S., & Jain, S. M. (2021). Dopants for Enhanced Performance of Tin-Based Perovskite Solar Cells—A Short Review. Coatings, 11(9), 1045. https://doi.org/10.3390/coatings11091045





