Corrosion Behavior of Amorphous Sol–Gel TiO2–ZrO2 Nano Thickness Film on Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate
2.2. Chemicals and Reagents
2.3. Preparation of Sol–Gel TiO2–ZrO2 Film
- 0.5 mol of titanium isopropoxide and 0.5 mol of zirconium butoxide (ratio 1:1) as the precursors;
- 3.75 mmol yttrium acetate hydrate for the ZrO2 stabilization;
- 0.8 mol acetylacetone as the chelating agent;
- 40 mol of i-propanol as the solvent;
- 0.05 mol of nitric acid as the catalyst;
- 5 mol of distilled water for the hydrolysis.
2.4. Characterization Methods
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization
3.2. Corrosion Behavior
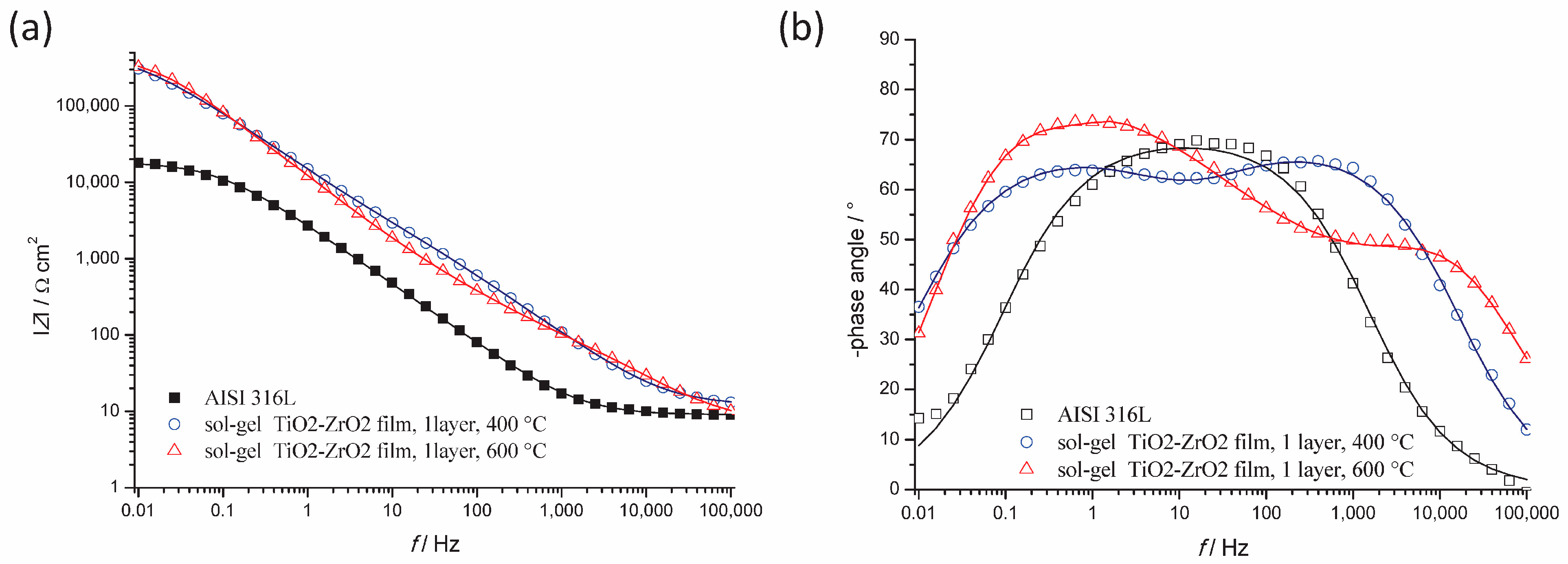
3.2.1. EIS Measurements
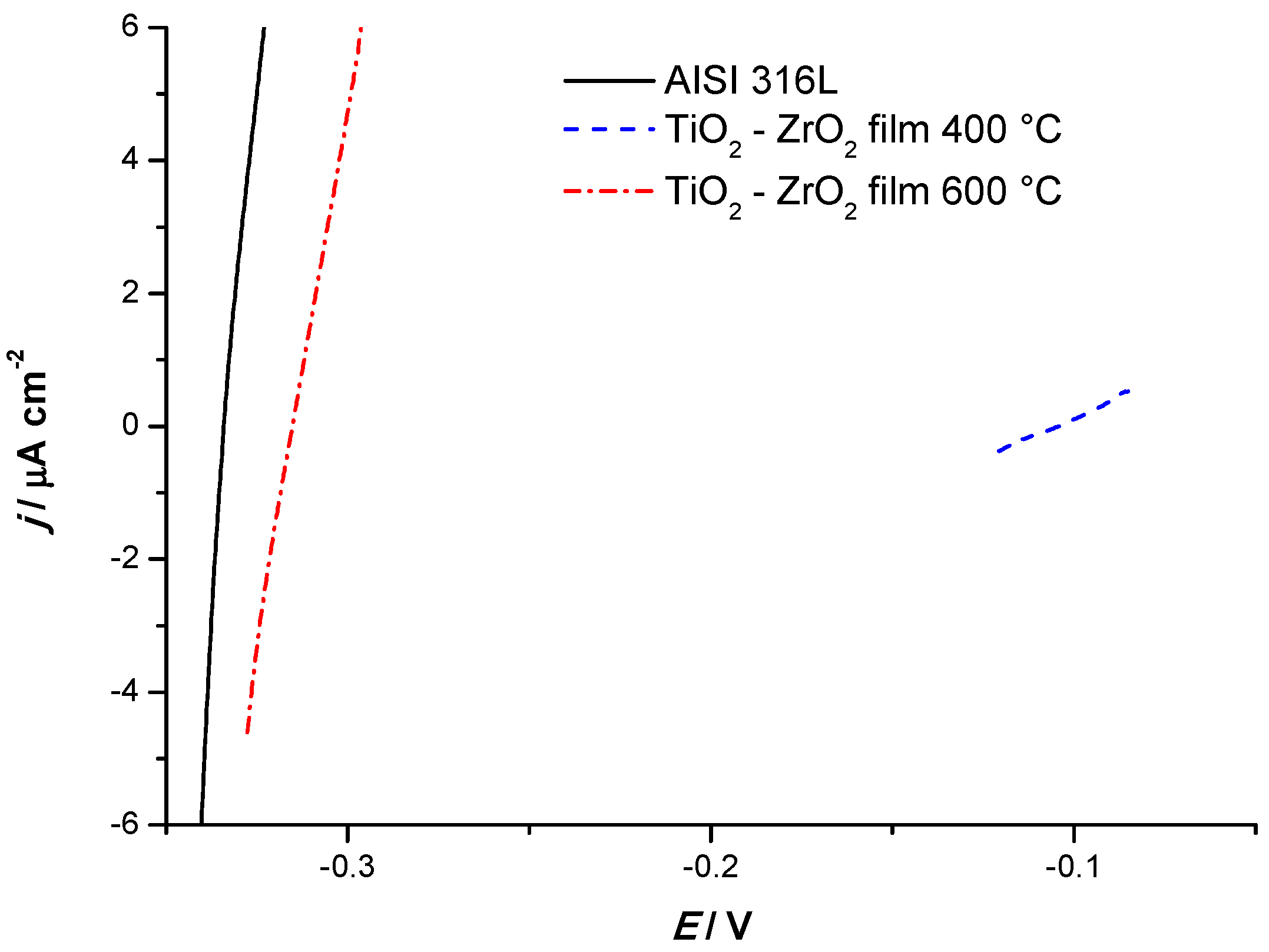
3.2.2. Linear Polarization Resistance Measurements
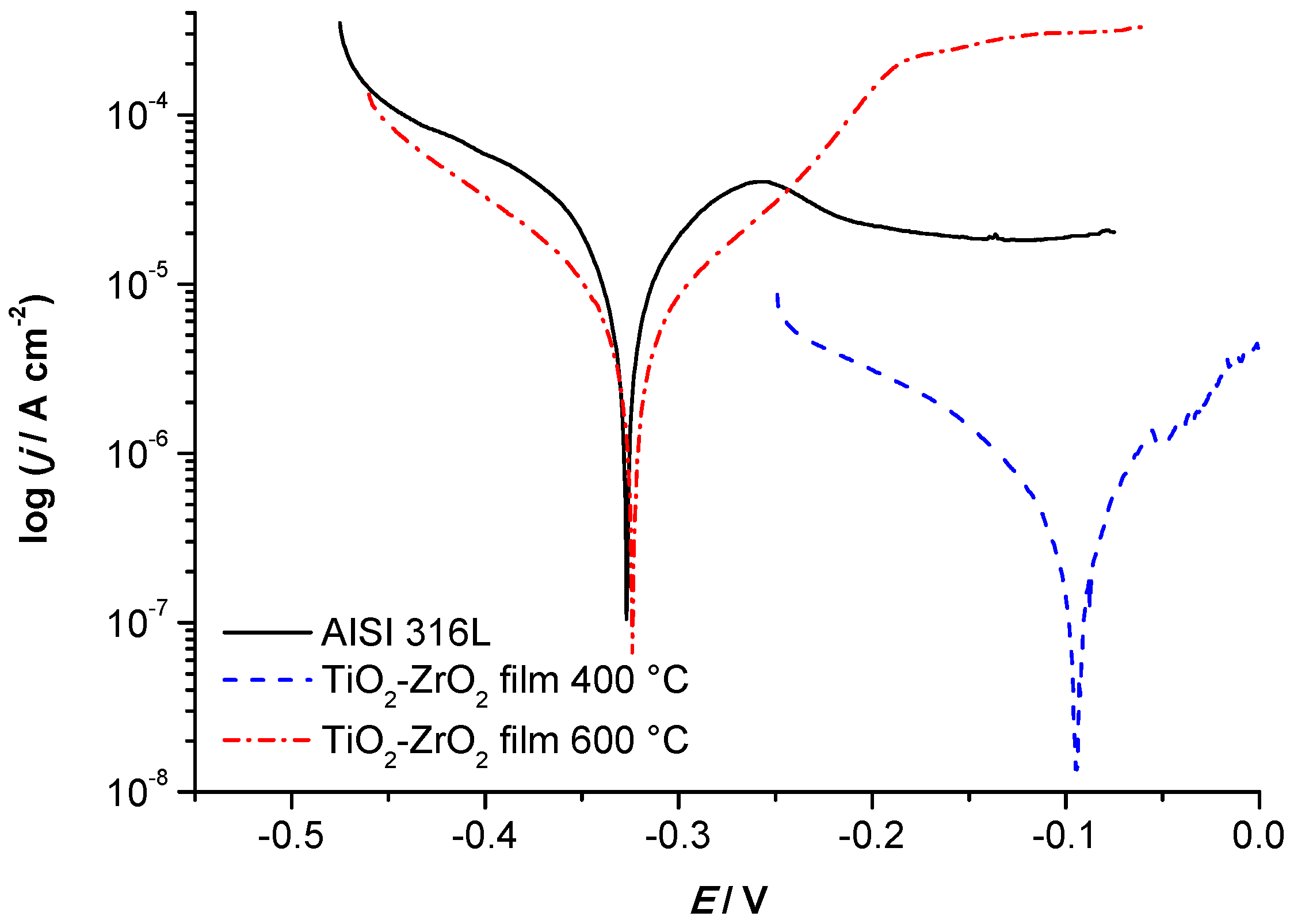
3.2.3. Tafel Extrapolation Method
4. Conclusions
- Nano-thickness of TiO2–ZrO2 films after thermal treatment at 400 °C or 600 °C are 8 nm and 10 nm, respectively.
- XRD analysis confirms that the sol–gel TiO2–ZrO2 powdered samples have an amorphous structure.
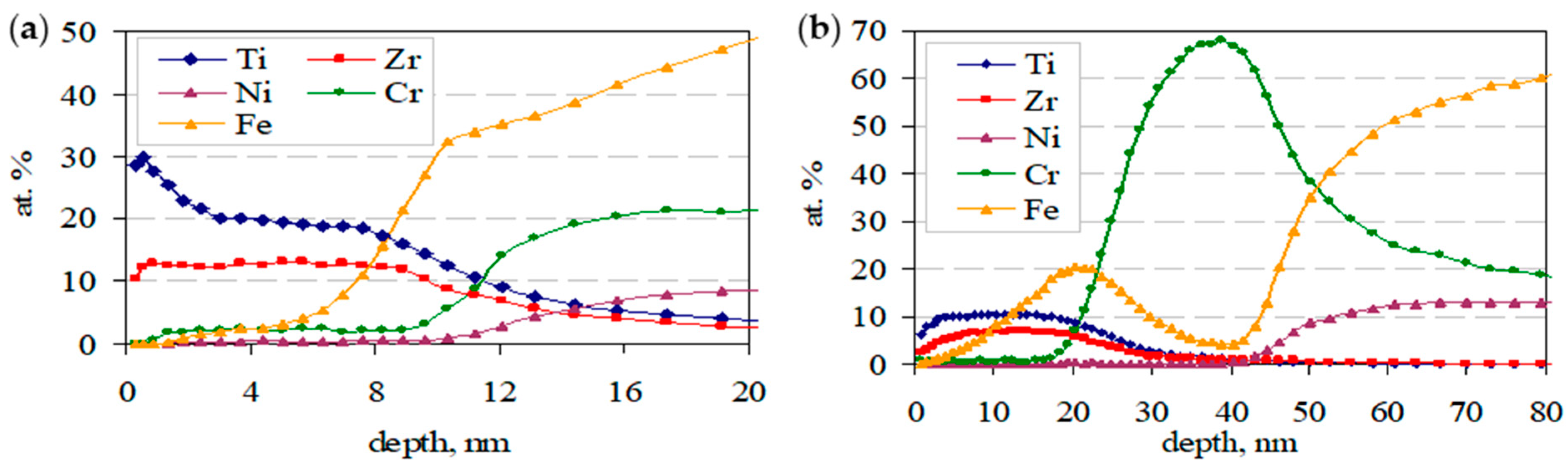
- The results of the quantitative depth profile analysis of the sol–gel TiO2–ZrO2 films deposited on the stainless steel, obtained by glow-discharge optical emission spectrometry (GD-OES), indicate that the thickness of the deposited film increases by increasing the calcination temperature. It is also found that the diffusion of some chemical elements (Cr and Fe) from the substrate into the deposited films increases by increasing the heat treatment temperature. GD-OES, as a fast, easy-to-use analytical technique, is proven to be a powerful tool for a rapid bulk chemical analysis, as well as for the determination of elemental concentrations as a function of the depth, measured in the nanoscale.
- The results of the electrochemical tests (AC and DC methods) show that the sol–gel TiO2−ZrO2 film annealed at 400 °C significantly improves the corrosion resistance of the austenitic stainless steel AISI 316L in acidic and neutral chloride medium, compared to bare steel and to films annealed at 600 °C.
- Composite TiO2–ZrO2 coating provides better corrosion protection than monolithic TiO2 films that were examined in previous work [24] on AISI 304 in the same media. A previous study in 3% NaCl solution showed an increase in the charge transfer resistance from 3.37 kΩ cm2 for uncoated AISI 304 to 32.2 kΩ cm2 for TiO2 film-protected steel. In this work, Rct is increased from 18.54 kΩ cm2 for bare AISI 316L to 537 kΩ cm2 or 459 kΩ cm2 in TiO2–ZrO2 films calcined at 400 or 600 °C, all measured in 3% NaCl solution, which simulates the marine environment. This shows that single-layered sol–gel TiO2–ZrO2 coating significantly improves the corrosion resistance of AISI 316L stainless steel for the application in marine engineering.
- Previous study [24] of corrosion resistance of AISI 304, protected with TiO2 film, measured in 0.5 mol dm−3 HCl solution, showed a reduction in corrosion current density from 64.85 μA cm−2 to 7.77 μA cm−2. Presented research shows an extensive reduction in corrosion current from 42.11 μA cm−2 for unprotected AISI 316L to 0.68 μA cm−2 for AISI 316L coated with a single-layer of TiO2−ZrO2 calcined at 400 °C. When the TiO2−ZrO2 is calcined at 600 °C, the corrosion current is also significantly lower, 8.05 μA cm−2. These findings suggest that although the stainless steel AISI 316L is typically not recommended for the application where any hydrochloric acid media is present, its corrosion resistance may be significantly improved by TiO2−ZrO2 sol–gel coating calcined at 400 °C.
- Electrochemical studies show that the differences in corrosion protection level provided by films annealed at 400 °C or 600 °C can be related to the increased porosity of the coating treated at 600 °C. Thus, an increase in the annealing temperature is not recommended for TiO2–ZrO2 films preparation. Further research should, rather, focus on the possibility of application of several layers of TiO2–ZrO2 films as a means of additional improvement of the stainless steel corrosion protection.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rezaee, S.; Arman, A.; Jurečka, S.; Grayeli Korpi, A.; Mwema, F.; Luna, C.; Sobola, D.; Kulesza, S.; Shakoury, R.; Bramowicz, M.; et al. Effect of annealing on the micromorphology and corrosion properties of Ti/SS thin films. Superlattices Microstruct. 2020, 146, 106681. [Google Scholar] [CrossRef]
- Yu, J.; Ji, G.; Liu, Q.; Zhang, J.; Shi, Z. Effect of sol–gel ZrO2 films on corrosion behavior of the 304 stainless steel in coal-gases environment at high temperature. Surf. Coat. Technol. 2017, 331, 21–26. [Google Scholar] [CrossRef]
- Coelho, L.B.; Kossman, S.; Mejias, A.; Noirfalise, X.; Montagne, A.; Van Gorp, A.; Poorteman, M.; Olivier, M.G. Mechanical and corrosion characterization of industrially treated 316L stainless steel surfaces. Surf. Coat. Technol. 2020, 382, 125175. [Google Scholar] [CrossRef]
- Kim, J.J.; Young, M.Y. Study on the Passive Film of Type 316 Stainless Steel. Int. J. Electrochem. Sci. 2013, 8, 11847–11859. [Google Scholar]
- Bačić, I.; Otmačić Ćurković, H.; Ćurković, L.; Mandić, V.; Šokčević, Z. Corrosion Protection of AISI 316L Stainless Steel with the Sol–Gel Yttria Stabilized ZrO2 Films: Effects of Sintering Temperature and Doping. Int. J. Electrochem. Sci. 2016, 11, 9192–9205. [Google Scholar] [CrossRef]
- Kossman, S.; Bertolucci Coelho, L.; Montagne, A.; Mejias, A.; Van Gorp, A.; Coorevits, T.; Touzin, M.; Druart, M.-E.; Staia, M.H.; Poorteman, M.; et al. The effect of the substrate surface state on the morphology, topography and tribocorrosion behavior of Si/Zr sol–gel coated 316L stainless steel. Surf. Coat. Technol. 2021, 406, 12666. [Google Scholar] [CrossRef]
- Saman Khosravi, H.; Veerapandiyan, K.V.; Vallant, R.; Reichmann, K. Effect of processing conditions on the structural properties and corrosion behavior of TiO2–SiO2 multilayer coatings derived via the sol–gel method. Ceram. Int. 2020, 46, 17741–17751. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Mazur, A.; Szczurek, A.; Chęcmanowski, J.G.; Szczygieł, B. Corrosion resistance and bioactivity of SiO2-Y2O3 coatings deposited on 316L steel. Surf. Coat. Technol. 2018, 50, 502–510. [Google Scholar] [CrossRef]
- Adachi, S.; Ueda, N. Wear and corrosion properties of cold-sprayed AISI 316L coatings treated by combined plasma carburizing and nitriding at low temperature. Coatings 2018, 8, 456. [Google Scholar] [CrossRef] [Green Version]
- Adachi, S.; Egawa, M.; Yamaguchi, T.; Ueda, N. Low-temperature plasma nitriding for austenitic stainless steel layers with various nickel contents fabricated via direct laser metal deposition. Coatings 2020, 10, 365. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Wang, J.; Jiang, R.; Xiang, S. Anticorrosion Properties of the Low-Temperature Glow Plasma Nitriding Layer on AISI 904L Austenitic Stainless Steel in Hydrofluoric Acid Obtained at Various NH3 Pressures. Coatings 2020, 12, 1156. [Google Scholar] [CrossRef]
- Grilec, K.; Ćurković, L.; Žmak, I. Wear rate evaluation of sol–gel TiO2-ZrO2 films by quantitative depth profile analysis. Trans. FAMENA 2020, 44, 1–11. [Google Scholar] [CrossRef]
- Sakoman, M.; Ćorić, D.; Aleksandrov Fabijanić, T.; Kovačić, S. Tribological Properties of Coatings Applied on Near-Nano and Nanostructured WC-Co Hardmetals by Using Plasma- Assisted Chemical Vapour Deposition Technique. Trans. FAMENA 2020, 44, 29–42. [Google Scholar] [CrossRef]
- Metroke, T.L.; Parkhill, R.L.; Knobbe, E.T. Passivation of metal alloys using sol–gel-derived materials—A review. Prog. Org. Coat. 2001, 41, 233–238. [Google Scholar] [CrossRef]
- Gheriani, R.; Chtourou, R. Preparation of Nanocrystalline Titanium Dioxide (TiO2) Thin Films by the Sol–Gel Dip Coating Method. J. Nano Res. 2012, 16, 105–111. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Xue, B.; Luo, J. Effect of Sol–Gel Film on the Corrosion Resistance of Low Carbon Steel Plate. Mater. Sci. Forum 2020, 984, 174–177. [Google Scholar] [CrossRef]
- Seah, M.P.; Mulcahy, C.P.A.; Biswas, S. Nonlinearities in depth profiling nanometer layers. J. Vac. Sci. Technol. B 2010, 28, 1215–1221. [Google Scholar] [CrossRef]
- Escobar, R.G.; Gago, R.; Duday, D.; Palacio, C. Towards nanometric resolution in multilayer depth profiling: A comparative study of RBS, SIMS, XPS and GDOES. Anal. Bioanal. Chem. 2010, 396, 2725–2740. [Google Scholar] [CrossRef] [PubMed]
- Xhoffer, C.; Dillen, H. The Versatility of GDOES: From Bulk Analysis to Thin Film and Surface Analysis. BHM Berg-Und Hüttenmännische Mon. 2007, 152, 18–23. [Google Scholar] [CrossRef]
- Nelis, T.; Pallosi, J. Glow Discharge as a Tool for Surface and Interface Analysis. Appl. Spectrosc. Rev. 2006, 41, 227–258. [Google Scholar] [CrossRef]
- Manriquez, M.E.; Picquart, M.; Bokhimi, X.; López, T.; Quintana, P.; Coronado, J.M. X-Ray Diffraction, and Raman Scattering Study of Nanostructured ZrO2−TiO2 Oxides Prepared by Sol–Gel. J. Nanosci. Nanotechnol. 2008, 8, 6623–6629. [Google Scholar] [CrossRef]
- Liang, L.; Sheng, Y.; Xu, Y.; Wu, D.; Sun, Y. Optical properties of sol gel derived ZrO2−TiO2 composite films. Thin Solid Films 2007, 515, 7765–7771. [Google Scholar] [CrossRef]
- Ćurković, L.; Otmačić Ćurković, H.; Salopek, S.; Majić Renjo, M.; Šegota, S. Enhancement of corrosion protection of AISI 304 stainless steel by nanostructured sol–gel TiO2 films. Corros. Sci. 2013, 77, 176–184. [Google Scholar] [CrossRef] [Green Version]
- De Lima Neto, P.; Atik, M.; Avaca, L.A.; Aegerter, M.A. Sol-Gel Coatings for Chemical Protection of Stainless Steel. J. Sol-Gel Sci. Technol. 1994, 2, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Ruhi, G.; Modi, O.P.; Sinha, A.S.K.; Singh, I.B. Effect of sintering temperatures on corrosion and wear properties of sol–gel alumina coatings on surface pre-treated mild steel. Corros. Sci. 2008, 50, 639–649. [Google Scholar] [CrossRef]
- Atik, M.; Kha, C.R.; de Lima Neto, P.; Avaca, L.A.; Aegerter, M.A.; Zarzycki, J. Protection of 316L stainless steel by zirconia sol–gel coatings in 15% H2SO4 solutions. J. Mater. Sci. Lett. 1995, 14, 178–181. [Google Scholar] [CrossRef]




| wt% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | P | S | Si | Mn | Cu | Cr | Ni | Mo | V | Fe |
| 0.026 | 0.0287 | 0.0021 | 0.37 | 1.42 | 0.345 | 16.38 | 10.53 | 2.17 | 0.1 | rest |
| Sample | AISI 316L | TiO2–ZrO2, 400 °C | TiO2–ZrO2, 600 °C |
|---|---|---|---|
| Eoc/V vs. SCE | −0.365 ± 0.015 | −0.175 ± 0.008 | −0.280 ± 0.013 |
| Sample | AISI 316L | TiO2–ZrO2, 400 °C | TiO2–ZrO2, 600 °C |
|---|---|---|---|
| Rf (Ω cm2) | - | 7485 ± 1147 | 136 ± 44 |
| Qf(μSsn cm−2) | - | 12.1 ± 3.28 | 8.89 ± 2.17 |
| nf | - | 0.77 ± 0.12 | 0.75 ± 0.09 |
| Y0 (μS s1/2 cm−2) | - | - | 90.1 ± 20.2 |
| KD (s1/2) | - | - | 0.77 ± 0.22 |
| Rct (kΩ cm2) | 18.54 ± 2.30 | 537.0 ± 123.2 | 459.0 ± 90.6 |
| Qdl (μS sn cm−2) | 82.6 ± 10.2 | 5.96 ± 2.32 | 8.88 ± 3.61 |
| ndl | 0.79 ± 0.05 | 0.75 ± 0.09 | 0.98 ± 0.02 |
| Sample | Ecorr vs. SCE, mV | jcorr, μA cm−2 | Rp, kΩ cm2 |
|---|---|---|---|
| Bare AISI 316L | −351 ± 35 | 42.11 ± 4.08 | 1.28 ± 0.16 |
| TiO2–ZrO2 film, 400 °C | −98 ± 20 | 0.68 ± 0.18 | 42.30 ± 12.0 |
| TiO2–ZrO2 film, 600 °C | −305 ± 31 | 8.05 ± 1.39 | 3.03 ± 0.55 |
| Sample | Ecorr vs. SCE, mV | jcorr, μA/cm2 | ba, mV/dec | −bc, mV/dec |
|---|---|---|---|---|
| Bare AISI 316L | −336 ± 38 | 40 ± 3.15 | 280 ± 44 | 180 ± 31 |
| TiO2–ZrO2 film, 400 °C | −95 ± 24 | 0.65 ± 0.10 | 130 ± 20 | 140 ± 27 |
| TiO2–ZrO2 film, 600 °C | −304 ± 40 | 9.35 ± 1.51 | 105 ± 19 | 140 ± 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćurković, L.; Otmačić Ćurković, H.; Žmak, I.; Mustafa, M.K.; Gabelica, I. Corrosion Behavior of Amorphous Sol–Gel TiO2–ZrO2 Nano Thickness Film on Stainless Steel. Coatings 2021, 11, 988. https://doi.org/10.3390/coatings11080988
Ćurković L, Otmačić Ćurković H, Žmak I, Mustafa MK, Gabelica I. Corrosion Behavior of Amorphous Sol–Gel TiO2–ZrO2 Nano Thickness Film on Stainless Steel. Coatings. 2021; 11(8):988. https://doi.org/10.3390/coatings11080988
Chicago/Turabian StyleĆurković, Lidija, Helena Otmačić Ćurković, Irena Žmak, Mihone Kerolli Mustafa, and Ivana Gabelica. 2021. "Corrosion Behavior of Amorphous Sol–Gel TiO2–ZrO2 Nano Thickness Film on Stainless Steel" Coatings 11, no. 8: 988. https://doi.org/10.3390/coatings11080988
APA StyleĆurković, L., Otmačić Ćurković, H., Žmak, I., Mustafa, M. K., & Gabelica, I. (2021). Corrosion Behavior of Amorphous Sol–Gel TiO2–ZrO2 Nano Thickness Film on Stainless Steel. Coatings, 11(8), 988. https://doi.org/10.3390/coatings11080988










